Abstract
Despite the early discovery of caspase-2, its physiological function has long remained an enigma. A number of recent publications now suggest not just one, but multiple functions, including roles in apoptosis, DNA repair and tumor suppression. How can one enzyme have so many talents? Considering the diversity of interaction partners and the specific mode of pro-apoptotic action proposed in these studies, caspase-2 might in fact represent a primordial protease serving numerous pathways, established before the advent of a more elaborate functionally diversified caspases system.
Keywords: caspase, apoptosis, DNA repair, tumor suppression
Most members of the family of cystein-dependent aspartate-specific proteases, known as caspases, are involved in killing damaged, harmful or no-longer wanted cells (caspases-3, -6, -7, -8, -9 and -10). Others serve to fire up the innate immune response upon host–pathogen interaction by processing and thus activating inflammatory mediators, such as interleukin (IL)-1β and IL-18, a function served by the inflammatory caspases-1, -4 and -5 (Li and Yuan, 2008). In a few special cases, caspases are also involved in cell differentiation, mostly by exerting what has been called limited apoptosis during maturation of distinct cell types. This has been well documented during spermatid individualization in Drosophila (Arama et al., 2003) or, in mice and men, during keratinocyte maturation, regulated by caspase-14 (Lamkanfi et al., 2007), as well as during platelet formation in megakaryocytes (De Botton et al., 2002). Surprisingly, optimal proliferation of lymphocytes upon mitogen stimulation relies on the presence of caspase-8, and maybe also caspase-3, indicating that limited and selective substrate cleavage by or simple scaffold functions of caspases are critical for normal physiology (Lamkanfi et al., 2007). Finally, some evidence exists that caspases may also be critical in preventing tumor formation (Zhivotovsky and Orrenius, 2006).
Recent studies now suggest that one member of this family, caspase-2, maybe even more versatile as previously thought by mediating such opposing functions as either killing (Sidi et al., 2008; Olsson et al., 2009) or saving a cell after DNA damage (Shi et al., 2009) and, subsequently, even protecting the whole organism from developing cancer (Ho et al., 2009). Therefore, caspase-2 may represent the archetype member of this protease family that still unifies many of the above-mentioned functions in a single enzyme (Figure 1).
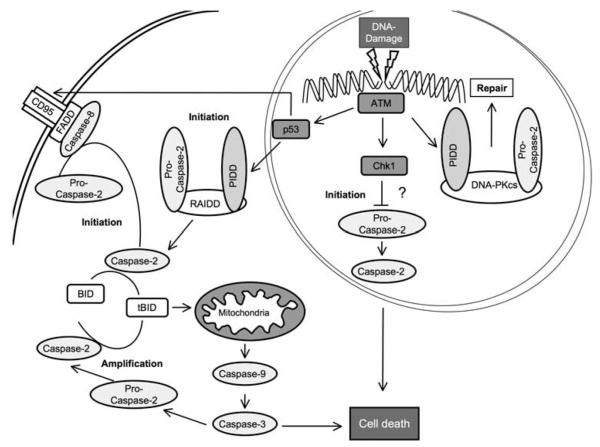
Figure 1.
Depending on the severity and type of genotoxic stress, as well as cell type, caspase-2 can be induced to either serve a pro-apoptotic function or support DNA repair. DNA repair is supported by caspase-2 activity in a nuclear platform, recruiting components of the nonhomologs end-joining machinery (catalytic subunit of DNA-PK (DNA-PKcs)), and contributes to G2/M checkpoint maintenance. Caspase-2-dependent cell death can be initiated in cytosolic (PIDDosome) or membrane-based activation platforms (DISC), and subsequent mitochondrial membrane permeabilization, involving BH3-interacting domain (Bid) processing, leads to apoptosis. A number of publications also provide evidence that caspase-2 can contribute to a mitochondrial amplification loop, downstream of caspse-3 activation. Alternatively, in the absence of p53 and a simultaneous inhibition of checkpoint kinase 1 (Chk1), caspase-2 may mediate apoptosis directly through a primordial mitochondria-independent death pathway. ATM, ataxia telangiectasia-mutated protein kinase, FADD, Fas-associated protein with death domain, PIDD, P53-induced protein with death domain, RAIDD, RIP-associated Ich-1/CED homologous protein with death domain.
In principle, this may not be entirely surprising, as caspase-2 shows the highest sequence homology with the archetypical Caenorhabditis elegans caspase, Ced-3 (Kumar et al., 1992). As such, it structurally looks like an initiator caspase, with a long pro-domain for binding to and auto-activation in multimolecular platforms, whereas its in vitro cleavage activity resembles that of the death effectors, caspase-3 and -7. This apparent functional ambiguity, multiple ill-defined interaction partners and the lack of an overt phenotype in caspase-2 knockout mice (Bergeron et al., 1998) have long masked its true functions. Furthermore, the fact that putative caspase-2 substrates, with the exception of Golgin160 (Mancini et al., 2000), are also avidly cleaved by the highly active caspase-3 or caspase-8 still renders the inference of caspase-2 function by substrate identification difficult (Krumschnabel et al., 2009).
After the identification of a putative activation platform for caspase-2, dubbed the PIDDosomoe, which supported its assumed role as an initiator caspase of apoptosis (Tinel and Tschopp, 2004), a study last year indicated that it might be specifically involved in an ATM/ATR-dependent cell death pathway controlled by checkpoint kinase-1 on a p53-deficient background and activated in response to DNA damage (Sidi et al., 2008). Most strikingly, this killing function seemed independent from the participation of classical apoptosis-related molecules, such as caspase-3 or Bcl-2. This was proposed to represent a perhaps primordial alternative cell death pathway, acting independently of the p53 network upon DNA damage. Considering the relative simplicity of this pathway, one might indeed tend to interpret this to reflect a more general and possibly ancient mechanism, in which one caspase, as in C.elegans, contains all the killing activity required to eliminate damaged cells, before the invention of complex signaling networks and multiple caspase-cascades.
Another more elaborate but possibly derived mechanism for caspase-2 activation and apoptosis induction, involving components of the intrinsic and extrinsic pathway of apoptosis alike, and considered before by others (Duan and Dixit, 1997; Lavrik et al., 2006), has been reinvestigated in a study published recently in Oncogene (Olsson et al., 2009). As above, the initiating stimulus is DNA damage, but this is then transmitted in a p53-dependent manner to induce activation of the Fas receptor at the cell membrane, followed by recruitment of FADD (Fas-associated protein with death domain) and caspase-8 and, surprisingly, also caspase-2. The function of caspase-2 is then to cleave the BH3-only protein, Bid, together with caspase-8, to form active tBid, which subsequently leads to the induction of mitochondrial outer membrane permeabilization and the well-described downstream consequences of this event. What remains unclear here is why only a particular anticancer agent, namely, 5-fluorouracil, and not other chemotherapeutics, triggers this response and why endogenous caspase-8 on its own is unable to cleave sufficient amounts of Bid under these circumstances. Maybe, cardiolipin-mediated activation of caspase-8 at mitochondria, shown to be compromised in type II cells from Barth Syndrome patients (Gonzalvez et al., 2008), is also compromised in these colon cancer cells, but can be compensated for by the activation of caspase-2 to process Bid, triggering full mitochondrial damage and apoptosis.
Although these studies eventually provided strong evidence of an apoptotic function of caspase-2, findings presented more recently suggested that it may not only act as a killer but is in fact involved in mediating just the opposite, that is, in rescuing cells after induction of DNA damage (Shi et al., 2009). Specifically, it was observed that, on low-dose γ-irradiation, caspase-2 was phosphorylated in its pro-domain and this in turn led to its activation. For this phosphorylation-mediated activation to occur, caspase-2 associated with the catalytic subunit of DNA-PK, a key nuclear serine/threonine protein kinase involved in DNA repair and nonhomologous end joining, which actually mediated the phosphorylation of caspase-2. This observation is unique inasmuch as all previously described phosphorylation events on caspases seem to be inhibitory in nature (Li and Yuan, 2008). In addition, the catalytic subunit of DNA-PK recruited the p53-inducible death-domain-containing protein, PIDD, with all three proteins together forming a constitutively assembled high-molecular mass complex in the nucleus that some-how senses DNA damage. The downstream targets of caspase-2 processed on activation in this complex were not identified, but experiments with catalytically inactive caspase-2 suggested that its proteolytic activity was indeed required to mediate efficient nonhomologous end joining of DNA double-strand breaks and that this complex contributed to the maintenance of the G2/M checkpoint and cell-cycle arrest. Although providing strong biochemical evidence that caspase-2 can act as a savior by facilitating DNA repair, no evidence was provided that caspase-2-deficient cells show reduced clonal survival upon DNA damage or increased transformation potential.
However, indirect support for the importance of caspase-2 in the maintenance of genomic integrity was reported in a study published in Proceedings of the National Academy of Sciences of the United States of America (Ho et al., 2009), in which it was shown that both primary as well as E1A/Ras-transformed mouse embryonic fibroblasts lacking caspase-2 potently resisted apoptosis induced by DNA damage and also grew significantly faster than did wild-type mouse embryonic fibroblasts in culture. Furthermore, the authors noted an increased colony formation potential in soft agar assays, which also translated into accelerated and more aggressive tumor formation when such cells were injected into athymic nude mice. Notably, when Eμ-myc transgenic mice, predisposed for the development of spontaneous B-cell lymphomas, were crossed with caspase-2-deficient mice, a further acceleration of tumor formation was observed, with the loss of a single allele being sufficient to cause a significant effect. The molecular basis for accelerated B-cell lymphomagenesis, however, remains unresolved at present, but may be accounted for by a general resistance of caspase-2-deficient B cells to oncogenic stress, as observed in mouse embryonic fibroblasts. Clearly, a more thorough analysis of premalignant mice and a workup of tumor mutational status will be required to understand the basis of caspase-2-mediated inhibition of B-cell lymphomagenesis, as its closest mammalian homolog, caspase-9, is unable to cause a similar effect in the very same disease model (Scott et al., 2004). Keeping this in mind, caspase-2-dependent apoptosis in response to Myc overexpression is less likely to be critical for its tumor suppressor function, but the enzyme may well contribute to the elimination of DNA lesions, usually accumulating on oncogene-driven replication stress.
Regardless of the mechanism involved, both these studies are well in line with an involvement of caspase-2 in aging processes, as caspase-2 deficiency in mice was reportedly associated with a diminished maximum life span and concurrently enhanced oxidative stress damage markers (Zhang et al., 2007), whereas at the same time, a positive correlation between the expression of caspase-2 and age was seen in wild-type mice (Braga et al., 2008). Notably here, caspase-2 has also been implied to act as a sensor of nutritional state in oocytes, being kept in check by an inhibitory phosphorylation in normal conditions and getting activated by de-repression on metabolic stress (Nutt et al., 2005).
Together, these studies clearly indicate important novel and previously unrecognized functions for caspase-2, virtually accompanying the organism from the cradle (oocyte development) to the grave (aging control), and in between taking care of genomic integrity (DNA damage repair) to prevent cancer. One is tempted to speculate that, during evolution, a split of tasks has been fulfilled by which caspase-9 ended up with the job as the prime killer, whereas caspase-2 acts often redundantly in apoptosis signaling (Samraj et al., 2007), but maintained the function to protect cells from the possible consequences of carrying damaged DNA. All these functions are coordinated by complex pre- or posttranslational modifications of the enzyme, involving differential splicing or phosphorylation at several sites, respectively, by translocation between compartments and/or recruitment into different activation complexes, thereby serving multiple clients, possibly reflecting an ancient protease-based control system established before the advent of a diversified system of functionally more specialized caspases. Given that accumulating evidence suggests that limited proteolytic activity of caspases, controlled, for example, by IAP (inhibitor of apoptosis)-mediated nondegradative polyubiquitylation (Ditzel et al., 2008) or by strict subcellular compartmentalization (Yi and Yuan, 2009), may allow caspases to serve presently unknown functions in healthy cells, the example of caspase-2 functional versatility may thus be only a first glimpse into a more general and widespread involvement of caspases in normal physiology than previously expected.
Acknowledgements
The work in our laboratory is supported by fellowships and grants from the Austrian Science Fund (Y212-B13, SFB021, MCBO), EU-FP6 (ApopTrain) and the Tyrolean Science Fund (TWF). We apologize to the many scientists in this field whose excellent research was not cited but was only referred to indirectly through reviews.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- Arama E, Agapite J, Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev Cell. 2003;4:687–697. doi: 10.1016/s1534-5807(03)00120-5. [DOI] [PubMed] [Google Scholar]
- Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev. 1998;12:1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga M, Hikim AP Sinha, Datta S, Ferrini MG, Brown D, Kovacheva EL, et al. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008;13:822–832. doi: 10.1007/s10495-008-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Botton S, Sabri S, Daugas E, Zermati Y, Guidotti JE, Hermine O, et al. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- Ditzel M, Broemer M, Tenev T, Bolduc C, Lee TV, Rigbolt KT, et al. Inactivation of effector caspases through nondegradative polyubiquitylation. Mol Cell. 2008;32:540–553. doi: 10.1016/j.molcel.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Dixit VM. RAIDD is a new ‘death’ adaptor molecule. Nature. 1997;385:86–89. doi: 10.1038/385086a0. [DOI] [PubMed] [Google Scholar]
- Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, et al. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S. A tumor suppressor function for caspase-2. Proc Natl Acad Sci USA. 2009;106:5336–5341. doi: 10.1073/pnas.0811928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumschnabel G, Sohm B, Bock F, Manzl C, Villunger A. The enigma of caspase-2: the laymen’s view. Cell Death Differ. 2009;16:195–207. doi: 10.1038/cdd.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tomooka Y, Noda M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem Biophys Res Commun. 1992;185:1155–1161. doi: 10.1016/0006-291x(92)91747-e. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- Lavrik IN, Golks A, Baumann S, Krammer PH. Caspase-2 is activated at the CD95 death-inducing signaling complex in the course of CD95-induced apoptosis. Blood. 2006;108:559–565. doi: 10.1182/blood-2005-07-007096. [DOI] [PubMed] [Google Scholar]
- Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, Casciola-Rosen LA, et al. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J Cell Biol. 2000;149:603–612. doi: 10.1083/jcb.149.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, et al. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell. 2005;123:89–103. doi: 10.1016/j.cell.2005.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Vakifahmetoglu H, Abruzzo PM, Hogstrand K, Grandien A, Zhivotovsky B. DISC-mediated activation of caspase-2 in DNA damage-induced apoptosis. Oncogene. 2009;28:1949–1959. doi: 10.1038/onc.2009.36. [DOI] [PubMed] [Google Scholar]
- Samraj AK, Sohn D, Schulze-Osthoff K, Schmitz I. Loss of caspase-9 reveals its essential role for caspase-2 activation and mitochondrial membrane depolarization. Mol Biol Cell. 2007;18:84–93. doi: 10.1091/mbc.E06-04-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CL, Schuler M, Marsden VS, Egle A, Pellegrini M, Nesic D, et al. Apaf-1 and caspase-9 do not act as tumor suppressors in myc-induced lymphomagenesis or mouse embryo fibroblast transformation. J Cell Biol. 2004;164:89–96. doi: 10.1083/jcb.200310041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Vivian CJ, Lee KJ, Ge C, Morotomi-Yano K, Manzl C, et al. DNA-PKcs-PIDDosome: a nuclear caspase-2-activating complex with role in G2/M checkpoint maintenance. Cell. 2009;136:508–520. doi: 10.1016/j.cell.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R, et al. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell. 2008;133:864–877. doi: 10.1016/j.cell.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- Yi CH, Yuan J. The Jekyll and Hyde functions of caspases. Dev Cell. 2009;16:21–34. doi: 10.1016/j.devcel.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Padalecki SS, Chaudhuri AR, De Waal E, Goins BA, Grubbs B, et al. Caspase-2 deficiency enhances aging-related traits in mice. Mech Ageing Dev. 2007;128:213–221. doi: 10.1016/j.mad.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivotovsky B, Orrenius S. Carcinogenesis and apoptosis: paradigms and paradoxes. Carcinogenesis. 2006;27:1939–1945. doi: 10.1093/carcin/bgl035. [DOI] [PubMed] [Google Scholar]