Abstract
Among all BH3-only proteins known to date, most information is available on the biological role and function of Bim (Bcl-2 interacting mediator of cell death)/BOD (Bcl-2 related ovarian death agonist), whereas little is still known about its closest relative, Bcl-2 modifying factor (Bmf). Although Bim has been implicated in the regulation of cell death induction in multiple cell types and tissues in response to a large number of stimuli, including growth factor or cytokine deprivation, calcium flux, ligation of antigen receptors on T and B cells, glucocorticoid or loss of adhesion, Bmf seems to play a more restricted role by supporting Bim in some of these cell death processes. This review aims to highlight similarities between Bim and Bmf function in apoptosis signaling and their role in normal development and disease.
Keywords: apoptosis, Bcl2 family, BH3-only proteins, tumorigenesis, drug-response
Searching for Bcl-2 interaction partners—identification of Bim and Bmf
In 1998 O’Connor and colleagues described a BH3-only protein discovered while screening a bacteriophage λ cDNA expression library using recombinant Bcl2 as bait. The protein was termed Bim (Bcl-2 interacting mediator of cell death) and sequence analysis of murine cDNAs revealed the presence of three major isoforms (BimEL-196aa, BimL-140aa and BimS-110aa), produced by alternative splicing (O’Connor et al., 1998). Shortly thereafter, a second group reported the discovery of the same gene in a yeast two-hybrid (Y2H) approach screening an ovarian cDNA library using Mcl1 as bait (Hsu et al., 1998) that they initially termed Bod (Bcl-2 related ovarian death agonist).
The Bim protein sequences did not show any similarity to others, but a short stretch of 9aa displayed homology to the Bcl-2 homology (BH) region 3, found in all known Bcl-2 family members at the time. In addition, the bim sequence encoded for a hydrophobic C-terminal domain required in other Bcl2 family proteins for localization to intra-cellular membranes, and this was also confirmed for Bim in overexpression studies (O’Connor et al., 1998). Further analysis revealed that the pro-apoptotic and Bcl-2 antagonizing potential of Bim/Bod correlated inversely with protein length, for reasons discussed below. Although BimEL and BimL can be found widely expressed in most tissues and cell lines, BimS expression is less abundant, presumably because of its strong pro-apoptotic potential ((O’Reilly et al., 2000) and http://symatlas.gnf.org/SymAtlas). Today, more than a dozen splice variants of bim have been reported in mice and humans in various cell types, but their expression at the protein level, biological relevance and relative contribution to specific cell death signaling events remain largely unknown (Adachi et al., 2005).
Despite the reported low affinity of Bmf (Bcl-2 modifying factor) for interaction with Mcl1 (Chen et al., 2005), it was originally discovered in a Y2H screen of a mouse embryonic cDNA library using Mcl1 as bait (Puthalakath et al., 2001). The truncated cDNA fragment found in the Y2H screen was used to isolate mouse and human full-length bmf transcripts from different T cell-derived expression libraries. Mouse bmf mRNA (~4.7 kb) was found to encode for a 558-bp open reading frame (555 bp in human bmf), giving rise to a 21 kDa protein. Again, the only sequence homology initially observed was the presence of a functional BH3 domain, required and sufficient for binding and neutralization of Bcl-2-like molecules and cell killing. However, as compared with Bim, Bmf only showed limited pro-apoptotic potential in cell death assays for reasons discussed by others in this issue of Oncogene and below.
In lymphocytes, Bmf is found widely expressed, but outside the hematopoietic system expression of Bmf seems more restricted than that of Bim and is still poorly investigated (http://symatlas.gnf.org/SymAtlas/). More recently, novel monoclonal antibodies specific for mouse Bmf have facilitated this analysis and revealed the presence of multiple isoforms in most hematopoietic tissues with the highest levels found in immature T and B cells (Labi et al., 2008a) as well as in the mammary gland (personal observation). Until now, it is unclear how these isoforms arise, but alternative splicing of bmf has been reported to regulate its in vivo function. Two additional splice variants of bmf (termed bmf II and III) were found to be expressed in normal and malignant human B cells, derived from patients with B cell lymphocytic leukemia (Morales et al., 2004). Surprisingly, these novel variants of Bmf lack a functional BH3 domain and Bmf III also contains a different carboxy-terminus. This feature has also been reported to occur frequently in other BH3-only proteins, for example, in Bim and Noxa, but its functional significance remains unclear. Overexpression of Bmf II or Bmf III in Hela cells, however, increased their colony-forming potential, whereas Bmf I, the originally described isoform, acted in a pro-apoptotic manner (Morales et al., 2004). It is noteworthy that the multiple isoforms detected in the mouse lymphocytes most certainly do not resemble Bmf II or III reported in humans, as they are all able to bind to and co-immunoprecipitate Bcl2, and hence possess a functional BH3 domain (A Villunger and V Labi, unpublished results). Therefore, it seems more likely that some of these murine isoforms arise because of post-translational modification and/or alternative start codon usage, in line with the lack of alternatively spliced transcripts found in EST databases.
Mouse bim and bmf are both found on chromosome 2 within a distance of 9 Mb, bmf is located on a syntenic region on chromosome 15 in humans. Although localization of two genes within such a rather large distance may question the idea that they may have evolved from a common ancestor, it is noteworthy that effective gene duplication is actually facilitated by the repositioning of duplicated genes to ectopic sites, reducing the risk of pseudogenization (Rodin et al., 2005). This, and structural similarities that go beyond the BH3 domain allowing similar subcellular localization led to speculations that Bim and Bmf evolved from a common ancestral gene (see below). However, the sequence homology of their zebrafish counterparts seems limited to the BH3 domain (Kratz et al., 2006; Jette et al., 2008), raising the possibility that the additional regulatory domain enabling cytoskeletal sequestration (see below) may have been added to both proteins independently later in evolution.
Molecular basis of apoptosis induced by Bim and Bmf
Although Bim and Puma can bind all pro-survival Bcl2 proteins with high affinity, Bmf or Bad bind only to Bcl2, Bclxl and Bclw at significant rates. In contrast, Noxa selectively binds to Mcl1 and, with lower affinity, Bfl1/A1 (Chen et al., 2005; Kuwana et al., 2005). The molecular basis for this selectivity depends on critical amino acid residues in the amphipathic α-helical BH3 domain contacting key residues in the hydrophobic groove of the anti-apoptotic Bcl2-like pro-survival proteins, formed by parts of the BH1, BH2 and BH3 domains (Liu et al., 2003). As shown by high-resolution structure analyses of Bclxl/Bim complexes, binding of Bim to Bclxl depends on the interaction of hydrophobic pockets, located in the binding groove of Bclxl, with four conserved hydrophobic residues in the BH3 domain of Bim (Liu et al., 2003). Replacement of one or more of these critical residues interferes with the binding specificity and affinity of the entire BH3-only protein. This suggests that the binding property of a BH3 molecule depends only on the nature of the BH3 domain, and hence an exchange of the critical residues leads to an altered binding specificity of the entire molecule. In fact, exchanging the key contact residues of one BH3-only molecule into another transmits the binding characteristic of the donor BH3 molecule. Consistently, when the shortest isoform of BimS was modified to contain the Noxa-BH3 domain, it lost its cell death-inducing potential. This correlated with the binding capacity restricted to Mcl1. Consistently, BimS chimeras containing the BH3 domain of Bad no longer bound to Mcl1, but both mutants of BimS complemented each other in promoting apoptosis (Chen et al., 2005). These experiments support the hypothesis that the nature of BH3 domains defines binding specificity and affinity of the entire BH3-only molecule and that mitochondrial cell death ensues when all pro-survival molecules present in a given cell type are neutralized by BH3-only proteins. However, most recently, evidence has been provided that Bim-BH3 carbon-stapled helices can trigger Bax oligomerization and cytochrome c release through an earlier unrecognized interaction surface on Bax. This interaction site, defined by helices α1 and α6, together with the inter-helical junction and proximal hydrophilic and charged amino acids, is distinct from the classical binding pocket required for interaction with anti-apoptotic Bcl2-like molecules (Gavathiotis et al., 2008). Taken together, this leaves us with the possibility that Bim can trigger apoptosis by at least two different mechanisms, that is, by the interaction and neutralization of Bcl2-like molecules and/or direct activation of Bax (see also below), and that the decision on which mode of action ensues perhaps depends on the nature of the incoming apoptosis signal.
The binding affinities of Bmf seem to resemble that of Bad, but it is still unclear whether transplantation of the Bmf BH3 domain into the Bim backbone would have the same consequence as the transplantation of the Bad BH3 domain, that is, loss of Mcl1 binding capacity. Although this behavior may be predicted, as based on BIACORE analysis using Bmf–BH3 peptides (Chen et al., 2005), Bmf was originally discovered in a Y2H interaction screen using Mcl1 as bait (Puthalakath et al., 2001), and in the overexpression analyses Bmf efficiently co-immunoprecipitates Mcl1, but not Bfl1/A1 (A Villunger, unpublished results). This suggests that amino acid residues outside the core BH3 domain may contribute to defining the binding specificities of Bmf in vivo.
The interaction of Bcl2-like molecules with BH3-only proteins can lead to two possible outcomes: stabilization, causing functional inactivation of their pro-survival function, or, as in the case of Mcl1 or A1, proteasomal degradation. It is interesting to note that degradation of Mcl1 is not always a prerequisite for apoptosis to occur, as interaction with Bim, for example, in response to glucocorticoid treatment of T-ALL cells (R Kofler; personal communication), Puma overexpression (Mei et al., 2005) or arsenic trioxide treatment of myeloma cells (Morales et al., 2008) actually leads to its stabilization, whereas interaction with Noxa targets it for proteasomal degradation. It is interesting to note that whereas the Noxa BH3 domain in the context of Bim protein caused Mcl1 degradation, it failed to do so in the context of Bad (and presumably Bmf), suggesting that the residues outside the BH3 domain, functionally conserved in Noxa and Bim, contribute to this process (Czabotar et al., 2007). Although protein stability of the pro-survival Bcl2 homolog, Bfl1/A1, also seems to be regulated by the proteasome, its stability can be increased by Bim binding, suggesting similar mechanisms to be responsible for Mcl1 as well as A1 degradation and stabilization (Herold et al., 2006).
Early biochemical analyses suggested that both proteins associate with components of the cytoskeleton, that is, microtubules for Bim and actin filaments for Bmf. On certain cellular stresses, such as those caused by UV radiation or loss of adhesion, both proteins were found to change their subcellular localization pattern and could then be co-immunoprecipitated with Bcl2 in the mitochondria (Puthalakath et al., 1999, 2001). This has led to the assumption that Bim and/or Bmf are important mediators of apoptosis caused by UV radiation or drugs interfering with cytoskeletal dynamics such as taxol or cytochalasin D, but also for the loss of signals provided by the extracellular matrix, leading to a special form of apoptosis called anoikis (Puthalakath and Strasser, 2002).
However, cell biological evidence for such subcellular localization at the endogenous level is currently still lacking for either protein, and at least in primary lymphocytes (Zhu et al., 2004) endogenous Bim, or when overexpressed, both proteins reside almost exclusively in the mitochondria (O’Connor et al., 1998 and personal observations). However, this could be a secondary consequence of their interaction with pro-survival proteins in this organelle and may not necessarily reflect its localization in healthy cells. Notably, at least for Bim, it has been shown that its C-terminus harbors a mitochondrial import sequence that allows insertion into the outer mitochondrial membrane with the help of the TOM complex (translocase of the mitochondrial outer membrane), thereby targeting Bim to the mitochondria, independent of its ability to interact with Bcl2 (Weber et al., 2007). A mutant of BimS that still retains the ability to bind to Bcl2, but is no longer targeted to the mitochondria because of a C-terminal truncation, loses the ability to initiate apoptosis upon induced expression. Conversely, a mutant of Bim carrying the mitochondrial-targeting domain of TOM5 promotes cell death, but only in the presence of a functional BH3 domain. However, a mutant of BimS (D69A), which no longer binds to Bcl2, but is still targeted correctly, triggers apoptosis, indicating that neutralization of Bcl2 by BimS is not a prerequisite for killing (Weber et al., 2007), whereas yet another mutant (R66E) was found to localize to the mitochondria and to bind to Bcl2, but failed to trigger apoptosis. Several conclusions can be drawn. First, this proves beyond doubt that localization of Bim to the mitochondria is separable from Bcl2 binding and, second, this mitochondrial localization is a prerequisite for apoptosis induction, which also requires a functional BH3 domain in BimS. Third, mitochondrial targeting seems more important than Bcl2 binding for killing, leaving the possibility that BimS, which upon induced expression also triggers translocation of Bax to the mitochondria, may facilitate cell killing through the activation of Bax. Consistently, expression of BimS, albeit non-toxic to yeast per se, enhanced Bax-induced killing in this organism (Weber et al., 2007). This activity of BimS does not necessarily depend on direct binding of BimS to Bax, as mutants of Bim that have lost this feature still potently induce apoptosis in mammalian cells (Willis et al., 2007). Although pure speculation, this Bax-activating effect of Bim may be achieved by morphological changes imposed upon the outer mitochondrial membrane after the insertion of supraphysiological amounts of BimS.
On the basis of the overexpression analysis in fibroblasts lacking Bax or Bak, as well as BH3-peptide profiling, Bim and Bmf proteins can engage both multi-domain pro-apoptotic Bcl2 family members for apoptosis induction (Figure 1), triggering cytochrome-c release and activation of the classical caspase activation cascades (Chen et al., 2005; Kuwana et al., 2005; Certo et al., 2006).
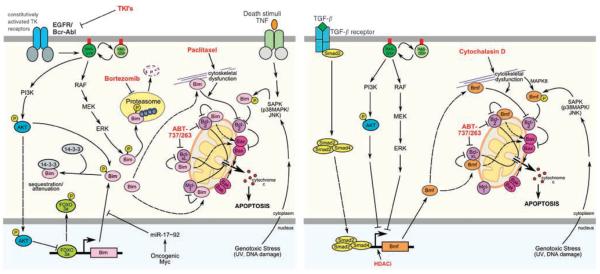
Figure 1.
The BH3-only proteins Bim and Bmf are subjected to multiple layers of regulation. Gene transcription of Bim can be induced by inhibition of the AKT survival pathway leading to FOXO3a nuclear translocation and transcriptional activation of bim. Both BH3-only protein genes can be activated by TGF-β-mediated signals, engaging Smad4 and/or RUNX1/3 transcription factors. Translation of Bim can be blocked by binding of c-Myc-induced micro RNAs to the 3′UTR of bim mRNA. At the post-translational level, Bim and Bmf can be rendered inactive by sequestration along microtubules or actin filaments through binding to dynein light chain molecules, DLC1 or DLC2, respectively. Activation of the Ras/Raf/ERK-signaling cascade leads to phosphorylation and to proteasomal degradation of Bim and interferes with induction of Bmf during anoikis by a so far unknown mechanism. Alternatively, phosphorylation of Bim was also reported to prompt cytoplasmic sequestration by 14-3-3 proteins, whereas JNK-mediated phosphorylation of Bim and Bmf is suggested to render the proteins more active. Once activated, Bim and Bmf can neutralize Bcl-2 pro-survival proteins at mitochondria, leading to Bax/Bak activation and Bim may possibly also trigger Bax oligomerization by direct protein–protein interaction. BH, Bcl-2 homology; Bmf, Bcl-2 modifying factor.
Regulation of Bim and Bmf protein expression and effector function
At the epigenetic level, bim and bmf gene expression is regulated by the presence of methylation-sensitive CpG islands in their promoter regions that, at least in the case of Bim, account for its silencing in Burkitt lymphoma. Along that line, recent reports describe that Bim and Bmf protein expression can be induced in melanoma and colorectal adenocarcinoma as well as in oral and esophageal squamous cell carcinoma cell lines, respectively, by the addition of novel histone deacetylase inhibitors (HDACi) (Zhang et al., 2006a).
Bim gene transcription can be induced by E2F1 on induced overexpression of this transcription factor (Hershko and Ginsberg, 2004) by members of the forkhead family such as FOXO3a on cytokine deprivation in lymphocytes (Dijkers et al., 2000; Stahl et al., 2002), by CHOP-C/EBPalpha on endoplasmic reticulum stress (Puthalakath et al., 2007), by the Nf-κB family member RelA in primary cortical neurons on ischemia (Inta et al., 2006) or by the AP1 family member c-Jun on nerve growth factor deprivation (Biswas et al., 2007), as well as by direct binding of the glucocorticoid receptor on ligand treatment of primary lymphocytes and leukemia cells (Ploner et al., 2008). Both bim and bmf are targeted by TGF-β-mediated signals in mammary epithelial cells (Ramjaun et al., 2007), possibly through activation of the Runx3, as described for bim in normal and malignant gastric epithelial cells (Yamamura et al., 2006).
At the post-transcriptional level, bim mRNA stability is regulated by heat shock cognate protein 70 that binds to AU-rich elements in the 3′UTR and enhances its stability on cytokine deprivation. Binding efficiency of heat shock cognate protein 70 is fine-tuned by co-chaperones such as Bag-4 and HIP, which by themselves are regulated by cytokine-activated Ras signaling, leading to destabilization of bim mRNA (Matsui et al., 2007). Micro RNAs (miR) of the miR-17~92 cluster have been reported not only to repress the tumor suppressor phosphatase and tensin homologue (PTEN), but also Bim. Transgenic expression of this cluster in mice led to lymphoproliferative disease with autoimmune pathology and premature death of these animals (Xiao et al., 2008), resembling features observed in bim−/− mice (Bouillet et al., 1999). Consistently, deletion of the miR-17~92 cluster led to increased Bim expression and defects in early B-cell development (Ventura et al., 2008). It is interesting to note that this cluster is also upregulated by c-Myc (O’Donnell et al., 2005), and may dampen c-Myc-mediated signals aiming to drive apoptosis through Bim, ultimately facilitating tumor outgrowth. As no direct interaction of c-Myc with the Bim promoter has been shown today, it will be interesting do define the signaling intermediates in this process (Figure 1).
Yeast two-hybrid interaction screens revealed that Bim and Bmf can bind to dynein light chain 1 and 2, respectively, through a highly conserved motif (K/RXTQT) near their N-termini targeting Bmf to the actin cytoskeleton and Bim to microtubules, respectively (Puthalakath et al., 1999, 2001). This binding domain is also found conserved in the Xenopus ortholog of Bmf. Bim and Bmf were hypothesized to be released from the cytoskeleton in response to the loss of adhesion and/or integrin signals preceding a distinct form of cell death, called anoikis, which is observed in fibroblasts, epithelial or endothelial cells and prevents detached cells from colonizing elsewhere (Puthalakath et al., 1999, 2001). The signals triggering the release of Bim or Bmf are still ill-defined, but phosphorylation of Bim or Bmf within the DLC interaction motif has been proposed as a putative mechanism.
Members of the MAP kinase family, in particular JNK and ERK, were found to exert opposing effects on Bim protein and function upon phosphorylation. ERK-mediated serine (Ser-59/69/77) phosphorylation in human BimEL (Ser-55/65/73 in mouse BimEL), for example, on growth factor-mediated activation of the Ras/RAF/ERK-signaling cascade, causes destabilization of the protein, facilitating ubiquitination and subsequent proteasomal degradation, thus promoting cell survival (Ley et al., 2005). In contrast, phosphorylation of threonine 112 in Bim by JNK was discussed to increase its proapoptotic potential, presumably by triggering release from the cytoskeleton, as this phospho-site resides within the DLC-1-binding domain and its phosphorylation may compromise protein–protein interaction (Lei and Davis, 2003). This phosphorylation site is conserved in Bmf and is phosphorylated by JNK, at least in vitro (Lei and Davis, 2003). In addition, p38-MAPK or JNK-mediated phosphorylation of Bim at Ser-65 upon trophic factor deprivation (Putcha et al., 2003) or sodium arsenite treatment of neuronal cells (Cai et al., 2006) was also discussed to enhance its proapoptotic potential, although the mechanism behind it remained elusive.
The importance of the individual modes of post-translational modification on Bim for cell death signaling and initiation are still largely unclear. The generation of animal models carrying three serine-to-alanine knock-in mutations (Bim3SA) or a threonine-to-alanine mutation (BimT112A), thus impairing phosphorylation of Bim by MAPK family members, has shed some light on the role and relevance of this type of post-translational modification (Hubner et al., 2008). First of all, all mice carrying such knock-in mutations are viable and express protein levels of Bim comparable to those found in wt mice, suggesting that the steady-state levels and turnover of Bim do not depend on any of these phospho-sites.
Further analysis revealed that phosphorylation of Ser-55/65/73 is mediated by ERK on re-stimulation of serum-starved fibroblasts, whereas Thr-112 is targeted by JNK on UV radiation. It is interesting to note that Thr-112 can also be targeted by ERK on serum stimulation, but seems dispensable for the destabilization of Bim mediated by ERK under these conditions. Consistently, Bim3SA-expressing MEF were most susceptible to apoptosis triggered by serum deprivation and Bim3SA efficiently bound to Bcl2, Bclxl and Mcl1 (Hubner et al., 2008). In contrast, BimT112A mutant cells were equally susceptible as wt MEF to serum withdrawal, but thymocytes expressing this mutant version of Bim were less susceptible to the systemic effects of glucocorticoids in vivo. As Bim is also a primary response gene of the glucocorticoid receptor (Ploner et al., 2008), at least two different glucocorticoid-triggered signals seem to impinge here on Bim function. Similarly, BimT112A mice also displayed defects in the negative selection of thymocytes, simulated either by the injection of anti-CD3 antibody or by the presentation of the male-specific HY-self-antigen in a TCR transgenic mouse model (Hubner et al., 2008). Taken together these observations support an important role for JNK-mediated Thr112 phosphorylation of Bim in developmental and glucocorticoid-triggered lymphocyte apoptosis in vivo. This observation was explained by the fact that BimT112A could no longer bind efficiently to Bcl2, but still bound Bclx or Mcl-1, suggesting that this phosphorylation can facilitate apoptosis by enabling efficient neutralization of Bcl2 and not, as suggested earlier, by triggering the release of Bim from the cytoskeleton, although these effects are likely not mutually exclusive (Lei and Davis, 2003). However, CD4+8+ immature thymocytes do not express significant amounts of Bcl2 (Veis et al., 1993), and hence inefficient neutralization of Bcl2 by BimT112A cannot account for the reduced thymocyte-mediated killing. Alternatively, one may speculate that this phospho-site may inhibit the postulated interaction of Bim with Bax at the recently defined novel interaction site (Gavathiotis et al., 2008).
Another form of post-translational modification reported to modulate Bim function involves cleavage by active caspase-3 that has been reported to generate an N-terminally truncated version of the protein by proteolysis at position D17, termed BimELΔN13. This truncated version of Bim was reported to possess increased killing potential when compared with BimEL on overexpression, presumably because of increased affinity for Bcl2 (Chen and Zhou, 2004). This feature resembles the processing of Bid into tBid during death receptor-mediated apoptosis by caspase-8. As Bim has also recently been discussed to contribute to death receptor-induced hepatocyte apoptosis (Corazza et al., 2006), it will be interesting to see whether Bim can be cleaved under these conditions by caspases other than caspase-3, for example, by caspase-8 or -10 to connect the mitochondrial and the death receptor pathway of apoptosis. However, thus far the physiological relevance of caspase-mediated Bim cleavage remains unclear.
Physiological roles for Bim and Bmf
Much of what we know about the physiological roles of Bim and Bmf has come from gene-targeting experiments in mice. To date the targeted disruption of all known BH3-only genes has been achieved (Youle and Strasser, 2008) and deletion of any one of these genes by themselves has resulted in viable and fertile animals, indicating that they are largely dispensable for, and most likely have redundant functions in, embryonic development. The exception is bim, whose deletion results in >40% lethality before embryonic day 10 (Bouillet et al., 1999), and must therefore play an important, albeit thus far incompletely defined, role in embryogenesis, which may be related to its role in trophoblast stem cell survival downstream of FGF4 (Ralston and Rossant, 2006).
Bim−/− mice that survive to birth go on to accumulate excess lymphoid and myeloid cells (Bouillet et al., 1999). In the hematopoietic compartment, two major roles can be ascribed to Bim. First, Bim is an important effector of cell death induced by a variety of signals including growth factor withdrawal, deregulated Ca2+ + flux or glucocorticoid treatment (Bouillet et al., 1999), which reflects in part the second role of Bim, which is its involvement in a number of checkpoints of the developing immune system. Deletion of Bim strongly interferes with the deletion of autoreactive T (Bouillet et al., 2002) and B cells (Enders et al., 2003) or with their maintenance in an anergic state (Oliver et al., 2006; Barron et al., 2008). Bim is also required for the developmentally programmed death of germinal center-derived memory B cells and antibody-forming cells (Fischer et al., 2007), and for controlling the homeostasis of naive and memory T cells (Wojciechowski et al., 2007). Finally, the apoptosis of activated T and B cells, and the proper termination of an immune response, is largely dependent on Bim (Strasser, 2005). For example, Bim-deficient mice were not able to terminate CD8-driven immune responses after clearing a viral challenge (Pellegrini et al., 2003) and super-antigen-induced T cell death was greatly impaired in bim−/− mice (Hildeman et al., 2002), whereas both functions did not require death receptor signaling through CD95.
In contrast to the abundance of information that has accumulated for Bim, comparatively little is known about the physiological role of Bmf. Recently, however, Bmf-deficient mice were generated and an extensive characterization of the hematopoietic compartment of these mice in direct comparison with bim−/− mice was carried out to define the physiological function of Bmf and the extent to which its role may overlap with that of Bim (Labi et al., 2008a). Bmf is dispensable for normal embryonic development and organogenesis. In hematopoietic cells Bmf, like Bim, is highly expressed in CD4+8+ immature thymocytes, and in B cell development from the pre-B cell stage onward. At first glance, the increases in the total cellularity of the various lymphoid organs of bmf−/− mice seemed modest, when compared with Bim-deficient mice (Labi et al., 2008a). However, an in-depth comparison with the different cell subsets within these organs revealed that the loss of bmf very specifically perturbs B cell homeostasis, without affecting T cell and myeloid cell development. In particular, the number of pre-B and transitional B cells was significantly elevated in bmf−/− mice, suggesting that the checkpoints between pre-B to naive B cell and T1 to T2 or mature B cells are partially regulated by Bmf (Labi et al., 2008a). Taking the data from these two individual mouse models together, it seems likely that apoptosis of progenitor B cells in the bone marrow, because of the loss of cytokine support, as well as the developmental regulation of splenic B cells are co-regulated by Bim and Bmf. Consistent with this hypothesis the numbers of progenitor and splenic B cells in vav-bcl-2 tg mice far exceeded those observed in bim−/− mice, and this extent of hyperplasia could not be achieved by the additional deletion of puma (Erlacher et al., 2006), blk (Coultas et al., 2005) or bad (A Strasser personal communication). It will be interesting to see whether the combined deletion of bim and bmf entirely recapitulates the B cell hyperplasia observed in vav-bcl-2 tg mice.
The signaling cascades leading to the accumulation of pre-B and mature B cells in the absence of Bmf are still undefined. Although Bim is a critical target of IL-7R (Pellegrini et al., 2004) and BCR-emanating signals (Enders et al., 2003), it is less clear whether or not the same signaling cascades also regulate Bmf. BCR ligation-induced apoptosis was normal in Bmf-deficient mice, and the application of IL-7 or BAFF potently extended the survival of pre-B and mature B cells, respectively, in the absence of Bmf (Labi et al., 2008a). It is therefore possible that Bmf functions at developmental checkpoints that ascertain the proper expression of a pre-BCR or BCR. Deletion of Bmf in Rag1-deficient or severe combined immunodeficient (SCID) mice will help to address this hypothesis.
As mentioned before, Bim and Bmf have both been implicated in the anoikis of epithelial cells (Puthalakath and Strasser, 2002). It is noteworthy that their individual loss did not interfere with anoikis induced in gastrointestinal epithelial cells, suggesting that both proteins can compensate for each other, or may not be important for the process, at least in this tissue (Labi et al., 2008a). In the breast tissue, however, epithelial cell death during lumen formation in the terminal end bud during puberty, considered to occur by anoikis, clearly depended on the presence of Bim (Mailleux et al., 2007) and Bmf mRNA, and was observed to be induced during acinar morphogenesis (Schmelzle et al., 2007). Consistently, apoptosis was delayed in acini-like 3D structures, in which Bmf expression was knocked down by shRNA, confirming the involvement of both proteins in luminal cell death and mammary gland morphogenesis (Schmelzle et al., 2007). Taken together, this suggests that Bim and Bmf are relevant mediators of anoikis in vivo, but their role in this process may be a tissue-restricted one and can be compensated for by alternative death mechanisms in their absence (Mailleux et al., 2007).
Outside the hematopoietic system, Bim has also been shown to exert important developmental functions. Loss of Bim corrected the developmental defects in kidney formation and melanocyte maintenance that were apparent in Bcl2-deficient mice (Bouillet et al., 2001), suggesting an important role for Bim in the maintenance of these lineages and derivatives.
Bim and Bmf in autoimmune pathology
In mice, the combined immunological defects attributed to the loss of Bim lead to a relevant increase in the amount of circulating autoantibodies and the accumulation of Ig-secreting plasma cells, resulting in hypergammaglobulinemia, splenomegaly, and, depending on genetic background, progressive lymphoadenopathy and fatal systemic autoimmunity (Bouillet et al., 1999). It is interesting to note that mice with enforced pan-hematopoietic expression of pro-survival Bcl2 developed similar symptoms that are akin to human systemic lupus erythematosus (SLE), and fits with the role of Bim as a major physiological antagonist of Bcl2 (Bouillet et al., 2001; Egle et al., 2004a). In humans, gene expression profiling revealed an increase in the pro-survival members of both the intrinsic and extrinsic apoptosis pathways in mononuclear cells from patients with SLE, suggesting that a dysregulation of both pathways contributes to the pathogenesis of this disease (Hutcheson et al., 2008). Indeed, Fas mutant lpr mice also exhibit some aspects of SLE-like disease (Cohen and Eisenberg, 1991; Watanabe-Fukunaga et al., 1992), and the onset of full-blown SLE could be shortened to just 16 weeks in mice that simultaneously lacked Bim and carried the lpr mutation in the Fas gene (Hughes et al., 2008; Hutcheson et al., 2008; Weant et al., 2008).
The role of Bim in the preclusion of autoimmunity has also been illustrated in specific cases of autoimmune lymphoproliferative syndrome (ALPS), in which Fas-mediated apoptosis is unaffected. Although ALPS is classified according to genotype as type Ia, Ib and II, corresponding to germline mutations in CD95, CD95 ligand and caspase-8 or 10, respectively, in some ALPS individuals no such mutations could be found (type III). In one specific case, an activating N-RAS mutation, resulting in enhanced ERK signaling and a marked reduction in Bim, was identified as the underlying molecular mechanism leading to the elevated numbers of TCRαβ CD4−8− T cells, chronic lymphoid accumulation, and a predisposition to the development of hematological tumors—symptoms that are hallmarks of ALPS (Oliveira et al., 2007).
Besides a B-cell restricted splenomegaly, none of the above-mentioned pathologies have been observed in Bmf-deficient mice. However, preliminary analysis of mice lacking both Bim and Bmf suggests that loss of bmf over bim can exacerbate the pathologies caused by loss of bim, even on the C57BL/6 genetic background, leading to premature death of bim−/−bmf−/− mice. Together, this suggests that although Bmf may not be rate-limiting for the deletion of autoreactive lymphocytes it may well contribute to this process, together with Bim, in vivo (A Villunger and V Labi, unpublished results).
Bim and Bmf in malignant disease
The tumor suppressor function of Bim was first reported under conditions of oncogenic expression of c-Myc. Oncogenic Myc promotes both the induction of Bim and the downregulation of Bcl2 and Bclxl, as part of an oncogenic stress program and results in a disturbed balance between pro- and anti-apoptotic members of the Bcl-2 family that is heavily biased toward death (Eischen et al., 2001). Tipping the scales in favor of survival by the deletion of bim dramatically enhances the rate of Myc-induced tumorigenesis. For example, whereas Eμ-Myc transgenic mice develop B-cell lymphomas with a latency of 23 weeks, the additional loss of bim in this setting decreased tumor latency to just 10 weeks (Egle et al., 2004b). Loss of just one allele of bim was nearly as effective as loss of both, and in none of the primary tumors from bim+/− Eμ-Myc mice was loss of the second bim allele observed. This suggests that there is a critical threshold below which Bim is unable to serve as a tumor suppressor in B-cell lymphopoiesis. It is important that, although a significant number of tumors arising from the sole deregulation of c-Myc require additional defects along the p53 axis either by the loss of the function of p53 or p19ARF, all of the bim−/− Eμ-Myc tumors retained normal p53 function, showing that the loss of bim relieves the selection pressure for the inactivation of the p19ARF/mdm2/p53 pathway. Thus, Bim can clearly act as a suppressor in Myc-induced tumorigenesis.
Consistent with its role as a tumor suppressor in Myc-induced oncogenesis, Bim was found to be frequently silenced in human Burkitt’s lymphoma. Analyses of the methylation status of the Bim promoter in a panel of Burkitt’s lymphoma cell lines and primary patient tumor biopsies revealed that 86% of the cell lines and 50% of patient biopsies presented a hypermethylation of the Bim promoter (Mestre-Escorihuela et al., 2007). The functional silencing of Bim has also been shown for other tumor entities. Using array-based comparative genomic hybridization, it was shown that regions of chromosome 2q13 spanning certain exons of Bim presented with a recurrent biallelic loss in mantle cell lymphoma (Tagawa et al., 2005). This finding was subsequently verified in another study, in which array-based comparative genomic hybridization and gene expression microarrays were combined to analyse various B-cell non-Hodgkin’s lymphomas and showed the homozygous loss of bim in 42% of mantle cell lymphoma cell lines and in one Burkitt’s lymphoma cell line (Mestre-Escorihuela et al., 2007). Thus, blocking Bim expression by gene deletion or epigenetic silencing may play a role in the pathogenesis of these tumors, although inactivating mutations of these pro-apoptotic proteins are very rare in human cancer (Labi et al., 2006).
In addition to its prominent role within the lymphoid compartment, Bim makes relevant contributions to the normal homeostasis of a number of tissues such as the kidney or skin. In line with these observations the loss of Bim mRNA and protein expression has been observed in melanoma and renal cell carcinoma (Labi et al., 2006). The mechanism suppressing Bim in these cancer types has not been clarified yet. However, as discussed above, Bim function is regulated by transcriptional, post-transcriptional and post-translational mechanisms that are frequently deregulated in cancer. Regardless of the mode of repression, restoration of the Bim function usually correlates well with increased drug responsiveness and tumor cell apoptosis (Zantl et al., 2007).
Although these observations provide mainly circumstantial evidence of the tumor suppressive role of Bim in certain cancer types, a prognostic value of Bim and Puma has recently been shown in human colon carcinoma (Sinicrope et al., 2008). The expression levels of Bim, Puma and Noxa in surgically resected primary colon carcinomas from stages II and III patients receiving 5-fluorouracil-based adjuvant therapy trials were evaluated and correlated with clinicopathological variables and disease-free survival (DFS) and overall survival (OS) rates. Neither Bim nor Puma expression correlated with clinicopathological features, nor did their expression levels depend on tumor stage. However, patients with an elevated expression of Bim had significantly improved DFS and OS, compared with patients that had lost Bim expression, and increased Puma expression also correlated with improved OS. The combined variable of Bim and Puma was a strong independent prognostic marker for both DFS and OS in univariate and multivariate analyses, showing for the first time how these two BH3-only proteins significantly impact treatment outcome and patient survival (Sinicrope et al., 2008).
In comparison with the well-established role of Bim in counteracting oncogenesis, the tumor suppressive potential of Bmf is just emerging. Loss of bmf has been shown to accelerate the development of thymic lymphoma in a γ-irradiation carcinogenesis protocol in mice (Labi et al., 2008b). However, in the human system, bmf has only indirectly been implicated in carcinogenesis. Loss of 15q14/15, which includes the bmf gene, has been reported in lung and breast cancer (Wick et al., 1996; Schmutte et al., 1999). The observation in breast cancer is intriguing, as Bmf has been shown to be involved in mammary morphogenesis and to be repressed by oncogenic Ras (Schmelzle et al., 2007).
Implications for anti-tumor therapy
As mentioned above, a number of potentially oncogenic upstream signals have been shown to regulate Bim and Bmf expression or half-life. The aim of modern therapies that target these specific pathways is the selective induction of apoptosis in tumor cells. As a potent initiator of cell death, Bim has been implicated in a number of such therapies as the principal proapoptotic initiator on one hand, and as part of relevant resistance mechanisms, when lost, on the other hand. Increased expression of Bim and Bmf has been documented for some tumor types, in which high levels of pro-survival Bcl2 proteins are also observed. For example, in chronic lymphocytic leukemia (CLL), which has long been characterized by an overexpression of Bcl2, paradoxically high levels of Bim, Bmf and Noxa have also been observed (Mackus et al., 2005; Del Gaizo Moore et al., 2007). In particular, Bim could already be found in mitochondria associated with Bcl2 (Del Gaizo Moore et al., 2007). Thus, the normal response to constant oncogenic stress, that is, the induction of proapoptotic BH3-only proteins, seems intact, but the cells survive, nonetheless, because they are able to overexpress Bcl2. This phenomenon has come to be known as ‘Bcl2 addiction’, and such a cancer cell is thought to be ‘primed’ for apoptosis, requiring only a nudge to jumpstart the cell death cascade. Regardless of which model of BH3-only protein modus operandi one supports (see review by DH & PB this issue of Oncogene), it is clear that these proteins are fundamental to the initiation of apoptosis by virtue of their ability to bind some or all anti-apoptotic Bcl2 family members. Building on this concept, a number of BH3 mimetics have been developed to specifically neutralize the anti-apoptotic Bcl2 family members that are frequently overexpressed in cancers and trigger the release of the sequestered proapoptotic protein, whether it might be the effector proteins Bak or Bax, or activator proteins Bim and Bid (for a detailed review on BH3 mimetics, please also see (Labi et al., 2008b)).
The most studied and most promising small-molecule BH3 mimetic to date is ABT-737, along with its second-generation orally bioavailable derivative, ABT-263 (Tse et al., 2008). Functionally similar to the BH3-only proteins Bad and Bmf, both compounds exhibit single-agent efficacy in tumor types that express high levels of Bcl2, including follicular lymphoma, CLL or multiple myeloma. Given the pivotal role of Bim in lymphoid homeostasis, it is not surprising that in CLL, follicular lymphoma and some samples of multiple myeloma, ABT-737 is discussed to induce apoptosis by freeing Bim from sequestration by Bcl2 and subsequently triggering oligomerization of Bax and Bak (Del Gaizo Moore et al., 2007), although other molecular mechanisms may also explain its mode of action (van Delft et al., 2006). However, because of their specificity for Bcl2, Bclw and BclxL, BH3 mimetics in general have limited efficacy as single-agent compounds on tumor types that rely on high Mcl1 activity such as non-small-cell lung cancer (NSCLC). Consistently, knocking down Mcl1 in combination with ABT-737 significantly increases the efficacy of these compounds (van Delft et al., 2006).
Recently, the biochemical characterization and anti-leukemic activity of a novel Bim-derived BH3 mimetic, named 072RB, has been reported (Ponassi et al., 2008). Addition of an internalizing sequence and replacement of specific residues with natural and non-natural amino acids resulted in an improved stability in the serum, and an increased ability to penetrate membranes. This peptide was efficacious in inducing apoptosis in leukemia-derived cell lines and ex vivo leukemia cells from AML patients in vitro, while exhibiting little effect on PBMCs and bone marrow cells taken from healthy donors. It is important to note that administration of 072RB could significantly delay the growth of AML in xenograft NOD (non-obese diabetic)/SCID mouse models (Ponassi et al., 2008). Although selected on the basis of their increased affinity for Bclxl, the important amino acids determined by molecular modeling to be required for interaction with the other pro-survival proteins were preserved, and 072RB could efficiently kill tumor cell lines with high expression levels of Bcl2 and Mcl1 as well, although biochemical confirmation of these interactions remains to be shown.
Although BH3 mimetics generally lack impressive single-agent efficacy, they show a high synergism with some of the currently used therapeutic compounds such as the tyrosine kinase inhibitors (TKIs), for example, imatinib and erlotinib/gefitinib (Figure 1). These TKIs have proven successful as single agents in the treatment of chronic myelogenous leukemia and NSCLC, respectively. However, resistant clones persist and eventually grow out, leading to relapse and the concomitant need for improved treatment strategies. Imatinib (GLEEVEC, Novartis, East Hanover, NJ, USA) targets the Bcr–Abl fusion protein, the oncogenic product of a reciprocal t(9,22)(q34;q22) chromosomal translocation (Phildelphia chromosome, Ph), which is essential for leukemogenesis in chronic myelogenous leukemia and Ph-positive acute lymphoblastic leukemia. Erlotinib and gefitinib are inhibitors of a hyperactive kinase mutant of the epidermal growth factor receptor that is involved in the pathogenesis of a broad spectrum of tumors including carcinomas of the lung, breast and colon. It is interesting to note that these TKIs have been shown to induce apoptosis largely through the action of Bim, which is transcriptionally activated and dephosphorylated (hypophosphorylated Bim is more stable) because of the loss of ERK activity. In refractory cell lines of chronic myelogenous leukemia or NSCLC, resistance could be correlated with either an overexpression of Bcl2 pro-survival proteins or an inability to rapidly induce Bim. Under such conditions, the use of a BH3 mimetic to neutralize Bcl2/Bclxl and increase the functional concentration of BH3-only proteins makes sense. Indeed, combined treatment with the BH3 mimetic, ABT-737, increased the efficacy of these TKIs in inducing apoptosis in both sensitive and refractory cell lines in chronic myelogenous leukemia and NSCLC (Kuroda et al., 2006; Cragg et al., 2007; Deng et al., 2007). In addition, inhibition of MEK in cell lines carrying activating B-Raf mutations led to a Bim-dependent apoptosis response, which could elegantly be augmented by combination with ABT-737 (Cragg et al., 2008). Moreover, this effect also translated to a murine xenograft model, in which the combination treatment allowed for long-term tumor regression. These studies not only illustrate the elegance of such rationally designed therapies, but also underscore the advantages of combined regimens in the treatment of refractory disease, and even possibly as first-line therapies to immediately eradicate all neoplastic clones.
The recognition that some cancer types down-modulate Bim protein levels by enhancing its proteasomal degradation also provides a therapeutic window for intervention. Proteosome inhibitors, including bortezomib (also known as Velcade) and carfilzomib, have been shown to have clinical efficacy against various hematologic malignancies, including multiple myeloma, follicular lymphoma and mantle cell lymphoma, all of which are known to have a modified Bcl2 family system, some even with known alterations in Bim level (Labi et al., 2006). Their effects are manifold because of the diverse array of proteins that are stabilized in their presence, including Bim and Noxa (for a detailed review on proteasome inhibitors see contribution by M Soengas, this issue of Oncogene).
At least from the biochemical evidence the binding of the Bim and Bmf to microtubules or actin, respectively, suggests that they may act as sensors for cytoskeletal perturbations. Indeed, in response to drugs that interefere with cytoskeletal dynamics, such as taxanes, Bim can be rapidly activated (Sunters et al., 2003). The taxanes, paclitaxel and docetaxel, are currently used in the clinics for the treatment of lung, ovarian, prostrate, breast cancer, head and neck cancers, and advanced forms of Kaposi’s sarcoma. Paclitaxel is particularly effective in aggressive cancer cells that have a rapid rate of cell division, which requires the constant re-structuring of the cytoskeleton. A significant variation in susceptibility to paclitaxel-mediated apoptosis has been observed among panels of cell lines derived from NSCLC, breast and prostrate cancer (Sunters et al., 2003; Li et al., 2005). The differences in paclitaxel susceptibility could be ascribed to the differences in Bim expression levels, as well as the expression levels of its transcriptional regulator, FOXO3a. In contrast, the sensitivity to paclitaxel treatment could not be correlated with the expression levels of the pro-apoptotic proteins Bmf, Bax and Bak, or the pro-survival proteins Bcl2 and Bclxl. In addition, both transient and stable knockdown of Bim or FOXO3a resulted in decreased sensitivity to paclitaxel-mediated killing (Sunters et al., 2003). These results, taken together, establish the PKB/Akt-FOXO3a-Bim cascade as a critical link between microtubule dysfunction and apoptosis. It is important to note that paclitaxel treatment also induced the transcription of BimS, the most potent form of Bim, while expression of BimEL was decreased (Sunters et al., 2003). Whether or not paclitaxel influences the alternative splicing of Bim remains to be clarified.
A rationale for combining paclitaxel with other treatment regimens, such as the proteasome inhibitor bortezomib, was elegantly illustrated in tumors in which the H-ras/MAPK pathway is activated. Mutations that activate the ras gene, leading to the constitutive activation of the RAF/MAPK pathway and consequently ERK1/2, are commonly found in tumors. Bim is a target for ERK1/2 phosphorylation at Ser-69, and this phosphorylation leads to ubiquitinylation of Bim and its targeting for proteasomal degradation. Tan et al. showed this to be the molecular basis for paclitaxel resistance in tumors with activating ras mutations, and reasoned that blocking the proteasomal degradation of Bim could restore the sensitivity of these tumors to paclitaxel treatment (Tan et al., 2005). Indeed, baby mouse kidney cells treated with paclitaxel rapidly accumulated Bim and this could be blocked by the enforced expression of H-ras, which conferred resistance to these cells through the proteasomal degradation of Bim. Significantly, the application of bortezomib restored Bim accumulation and paclitaxel sensitivity in these H-ras tumor cells. The therapeutic benefits of this combinatorial treatment strategy could also be shown in transplanted tumor models in mice (Tan et al., 2005).
The role of Bmf in apoptosis induction in response to current clinical therapies is much less defined. Bmf was also shown to be upregulated in response to TKIs; however, its role was not significant, as knockdown of Bmf in this setting did not affect the apoptosis rates achieved. Consistent with the idea that it plays a supporting role to Bim, both Bmf and Bim have been shown to be involved in apoptosis induction in response to treatment with glucocorticoids in ALL (Ploner et al., 2008), arsenic trioxide in multiple myeloma (Morales et al., 2008), and in TGF-β-induced cell death in a number of tissue types (Ramjaun et al., 2007; Romano et al., 2008). Bmf seems to be preferentially upregulated after the application of HDACi in a broad range of cancer cells (Zhang et al., 2006a). Thus, histone hyperacetylation seems to cause the transcription of bmf; however, the relevance of this induced expression is not completely clear. For example, the upregulation of Bmf in response to HDACi does not seem to translate into apoptosis in CLL cells, as RNAi of Bmf did not abrogate apoptosis. Instead, Bim and Noxa, both upregulated after HDACi treatment in CLL cells, mediated apoptosis in response to HDACi (Inoue et al., 2007). In squamous cell carcinoma, however, pretreatment with low nanomolar amounts of HDACi could increase Bmf expression, and thus sensitize these cells also to radiation-induced death (Zhang et al., 2006b). It is important to note that HDACis represent a novel class of promising anti-cancer agents, and understanding their molecular mechanism of action will greatly improve the design of effective therapeutic combinations for the treatment of different cancer types.
Taken together the increasing knowledge of the pathophysiological role of both Bim and Bmf will most certainly allow us to increase the specific armentarium available for the treatment of malignant disease and will, equally importantly, help to properly target these strategies.
Acknowledgements
The work in our laboratories is supported by fellowships and grants from the Austrian Science Fund (FWF): Y212-B13 START, P19481-B12, L488-B13, the Doctoral College MCBO, the SFB021, the Association for International Cancer Research (AICR), EU-FP7 (ApopTrain), the Austrian National Bank (OeNB) # 109900 and the Tyrolean Science Fund (TWF). We apologize to the many scientists in this field whose excellent research was not cited, but was only referred to indirectly through reviews.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- Adachi M, Zhao X, Imai K. Nomenclature of dynein light chain-linked BH3-only protein Bim isoforms. Cell Death Differ. 2005;12:192–193. doi: 10.1038/sj.cdd.4401529. [DOI] [PubMed] [Google Scholar]
- Barron L, Knoechel B, Lohr J, Abbas AK. Cutting edge: contributions of apoptosis and anergy to systemic T cell tolerance. J Immunol. 2008;180:2762–2766. doi: 10.4049/jimmunol.180.5.2762. [DOI] [PubMed] [Google Scholar]
- Biswas SC, Shi Y, Sproul A, Greene LA. Pro-apoptotic Bim induction in response to nerve growth factor deprivation requires simultaneous activation of three different death signaling pathways. J Biol Chem. 2007;282:29368–29374. doi: 10.1074/jbc.M702634200. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev Cell. 2001;1:645–653. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Cai B, Chang SH, Becker EB, Bonni A, Xia Z. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J Biol Chem. 2006;281:25215–25222. doi: 10.1074/jbc.M512627200. [DOI] [PubMed] [Google Scholar]
- Certo M, del G Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhou Q. Caspase cleavage of BimEL triggers a positive feedback amplification of apoptotic signaling. Proc Natl Acad Sci USA. 2004;101:1235–1240. doi: 10.1073/pnas.0308050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Corazza N, Jakob S, Schaer C, Frese S, Keogh A, Stroka D, et al. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493–2499. doi: 10.1172/JCI27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Loveland KL, Meachem S, Perlman H, Adams JM, et al. Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis. EMBO J. 2005;24:3963–3973. doi: 10.1038/sj.emboj.7600857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Jansen ES, Cook M, Harris C, Strasser A, Scott CL. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest. 2008;118:3651–3659. doi: 10.1172/JCI35437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg MS, Kuroda J, Puthalakath H, Huang DC, Strasser A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 2007;4:1681–1689. doi: 10.1371/journal.pmed.0040316. discussion 1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DC, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci USA. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Shimamura T, Perera S, Carlson NE, Cai D, Shapiro GI, et al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867–11875. doi: 10.1158/0008-5472.CAN-07-1961. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bath ML, O’Reilly L, Cory S. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood. 2004a;103:2276–2283. doi: 10.1182/blood-2003-07-2469. [DOI] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA. 2004b;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen CM, Woo D, Roussel MF, Cleveland JL. Apoptosis triggered by myc-induced suppression of Bcl-XL or Bcl-2 Is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SF, Bouillet P, O’Donnell K, Light A, Tarlinton DM, Strasser A. Proapoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody-forming cells. Blood. 2007;110:3978–3984. doi: 10.1182/blood-2007-05-091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold MJ, Zeitz J, Pelzer C, Kraus C, Peters A, Wohlleben G, et al. The stability and anti-apoptotic function of A1 are controlled by its C terminus. J Biol Chem. 2006;281:13663–13671. doi: 10.1074/jbc.M600266200. [DOI] [PubMed] [Google Scholar]
- Hershko T, Ginsberg D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem. 2004;279:8627–8634. doi: 10.1074/jbc.M312866200. [DOI] [PubMed] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, et al. Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Lin P, Hsueh AJW. BOD (Bcl-2-related ovarian death gene) is an ovarian BH3 domain-containing proapoptotic Bcl-2 protein capable of dimerization with diverse antiapoptotic Bcl-2 members. Mol Endocrinol. 1998;12:1432–1440. doi: 10.1210/mend.12.9.0166. [DOI] [PubMed] [Google Scholar]
- Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell. 2008;30:415–425. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, III, Wu T, Li QZ, et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Inoue S, Riley J, Gant TW, Dyer MJ, Cohen GM. Apoptosis induced by histone deacetylase inhibitors in leukemic cells is mediated by Bim and Noxa. Leukemia. 2007;21:1773–1782. doi: 10.1038/sj.leu.2404760. [DOI] [PubMed] [Google Scholar]
- Inta I, Paxian S, Maegele I, Zhang W, Pizzi M, Spano P, et al. Bim and Noxa are candidates to mediate the deleterious effect of the NF-kappa B subunit RelA in cerebral ischemia. J Neurosci. 2006;26:12896–12903. doi: 10.1523/JNEUROSCI.3670-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette CA, Flanagan AM, Ryan J, Pyati UJ, Carbonneau S, Stewart RA, et al. BIM and other BCL-2 family proteins exhibit cross-species conservation of function between zebrafish and mammals. Cell Death Differ. 2008;15:1063–1072. doi: 10.1038/cdd.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz E, Eimon PM, Mukhyala K, Stern H, Zha J, Strasser A, et al. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 2006;13:1631–1640. doi: 10.1038/sj.cdd.4402016. [DOI] [PubMed] [Google Scholar]
- Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA. 2006;103:14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L, et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med. 2008a;205:641–655. doi: 10.1084/jem.20071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Villunger A. BH3-only proteins in cell death initiation, malignant disease and anticancer therapy. Cell Death Differ. 2006;13:1325–1338. doi: 10.1038/sj.cdd.4401940. [DOI] [PubMed] [Google Scholar]
- Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a break-through in anticancer therapy? Cell Death Differ. 2008b;15:977–987. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–1014. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- Li R, Moudgil T, Ross HJ, Hu HM. Apoptosis of non-small-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ. 2005;12:292–303. doi: 10.1038/sj.cdd.4401554. [DOI] [PubMed] [Google Scholar]
- Liu X, Dai S, Zhu Y, Marrack P, Kappler JW. The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity. 2003;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- Mackus WJ, Kater AP, Grummels A, Evers LM, Hooijbrink B, Kramer MH, et al. Chronic lymphocytic leukemia cells display p53-dependent drug-induced Puma upregulation. Leukemia. 2005;19:427–434. doi: 10.1038/sj.leu.2403623. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Asou H, Inaba T. Cytokines direct the regulation of Bim mRNA stability by heat-shock cognate protein 70. Mol Cell. 2007;25:99–112. doi: 10.1016/j.molcel.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Mei Y, Du W, Yang Y, Wu M. Puma(*)Mcl-1 interaction is not sufficient to prevent rapid degradation of Mcl-1. Oncogene. 2005;24:7224–7237. doi: 10.1038/sj.onc.1208873. [DOI] [PubMed] [Google Scholar]
- Mestre-Escorihuela C, Rubio-Moscardo F, Richter JA, Siebert R, Climent J, Fresquet V, et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood. 2007;109:271–280. doi: 10.1182/blood-2006-06-026500. [DOI] [PubMed] [Google Scholar]
- Morales AA, Gutman D, Lee KP, Boise LH. BH3-only proteins Noxa, Bmf, and Bim are necessary for arsenic trioxide-induced cell death in myeloma. Blood. 2008;111:5152–5162. doi: 10.1182/blood-2007-10-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AA, Olsson A, Celsing F, Osterborg A, Jondal M, Osorio LM. Expression and transcriptional regulation of functionally distinct Bmf isoforms in B-chronic lymphocytic leukemia cells. Leukemia. 2004;18:41–47. doi: 10.1038/sj.leu.2403183. [DOI] [PubMed] [Google Scholar]
- O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- O’Reilly LA, Cullen L, Visvader J, Lindeman G, Print C, Bath ML, et al. The pro-apoptotic BH3-only protein Bim is expressed in hemopoietic, epithelial, neuronal and germ cells. Am J Pathol. 2000;157:449–461. doi: 10.1016/S0002-9440(10)64557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JB, Bidere N, Niemela JE, Zheng L, Sakai K, Nix CP, et al. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci USA. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver PM, Vass T, Kappler J, Marrack P. Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med. 2006;203:731–741. doi: 10.1084/jem.20051407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci USA. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S, et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia. 2008;22:370–377. doi: 10.1038/sj.leu.2405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponassi R, Biasotti B, Tomati V, Bruno S, Poggi A, Malacarne D, et al. A novel Bim-BH3-derived Bcl-XL inhibitor: biochemical characterization, in vitro, in vivo and ex-vivo anti-leukemic activity. Cell Cycle. 2008;7:3211–3224. doi: 10.4161/cc.7.20.6830. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Huang DCS, O’Reilly LA, King SM, Strasser A. The pro-apoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, et al. Bmf: a pro-apoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- Ralston A, Rossant J. How signaling promotes stem cell survival: trophoblast stem cells and Shp2. Dev Cell. 2006;10:275–276. doi: 10.1016/j.devcel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J. Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene. 2007;26:970–981. doi: 10.1038/sj.onc.1209852. [DOI] [PubMed] [Google Scholar]
- Rodin SN, Parkhomchuk DV, Rodin AS, Holmquist GP, Riggs AD. Repositioning-dependent fate of duplicate genes. DNA Cell Biol. 2005;24:529–542. doi: 10.1089/dna.2005.24.529. [DOI] [PubMed] [Google Scholar]
- Romano S, Mallardo M, Chiurazzi F, Bisogni R, D’Angelillo A, Liuzzi R, et al. The effect of FK506 on transforming growth factor beta signaling and apoptosis in chronic lymphocytic leukemia B cells. Haematologica. 2008;93:1039–1048. doi: 10.3324/haematol.12402. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Mailleux AA, Overholtzer M, Carroll JS, Solimini NL, Lightcap ES, et al. Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. Proc Natl Acad Sci USA. 2007;104:3787–3792. doi: 10.1073/pnas.0700115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutte C, Tombline G, Rhiem K, Sadoff MM, Schmutzler R, von Deimling A, et al. Characterization of the human Rad51 genomic locus and examination of tumors with 15q14-15 loss of heterozygosity (LOH) Cancer Res. 1999;59:4564–4569. [PubMed] [Google Scholar]
- Sinicrope FA, Rego RL, Okumura K, Foster NR, O’Connell MJ, Sargent DJ, et al. Prognostic impact of bim, puma, and noxa expression in human colon carcinomas. Clin Cancer Res. 2008;14:5810–5818. doi: 10.1158/1078-0432.CCR-07-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, et al. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A, et al. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 2005;24:1348–1358. doi: 10.1038/sj.onc.1208300. [DOI] [PubMed] [Google Scholar]
- Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, et al. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Weber A, Paschen SA, Heger K, Wilfling F, Frankenberg T, Bauerschmitt H, et al. BimS-induced apoptosis requires mitochondrial localization but not interaction with anti-apoptotic Bcl-2 proteins. J Cell Biol. 2007;177:625–636. doi: 10.1083/jcb.200610148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W, Petersen I, Schmutzler RK, Wolfarth B, Lenartz D, Bierhoff E, et al. Evidence for a novel tumor suppressor gene on chromosome 15 associated with progression to a metastatic stage in breast cancer. Oncogene. 1996;12:973–978. [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem. 2006;281:5267–5276. doi: 10.1074/jbc.M512151200. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zantl N, Weirich G, Zall H, Seiffert BM, Fischer SF, Kirschnek S, et al. Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene. 2007;26:7038–7048. doi: 10.1038/sj.onc.1210510. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Adachi M, Kawamura R, Imai K. Bmf is a possible mediator in histone deacetylase inhibitors FK228 and CBHA-induced apoptosis. Cell Death Differ. 2006a;13:129–140. doi: 10.1038/sj.cdd.4401686. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Adachi M, Kawamura R, Zou HC, Imai K, Hareyama M, et al. Bmf contributes to histone deacetylase inhibitor-mediated enhancing effects on apoptosis after ionizing radiation. Apoptosis. 2006b;11:1349–1357. doi: 10.1007/s10495-006-8266-1. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Swanson BJ, Wang M, Hildeman DA, Schaefer BC, Liu X, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci USA. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]