Abstract
Objective
Distal hereditary motor neuropathy type V (dHMN-V) and Charcot–Marie–Tooth syndrome (CMT) type 2 presenting with predominant hand involvement, also known as CMT2D and Silver syndrome (SS) are rare phenotypically overlapping diseases which can be caused by mutations in the Berardinelli–Seip Congenital Lipodystrophy 2 (BSCL2) and in the glycyl-tRNA synthetase encoding (GARS) genes. Mutations in the heat-shock proteins HSPB1 and HSPB8 can cause related distal hereditary motor neuropathies (dHMN) and are considered candidates for dHMN-V, CMT2, and SS.
Design
To define the frequency and distribution of mutations in the GARS, BSCL2, HSPB1 and HSPB8 genes we screened 33 unrelated sporadic and familial patients diagnosed as either dHMN-V, CMT2D or SS. Exon 3 of the BSCL2 gene was screened in further 69 individuals with an unclassified dHMN phenotype or diagnosed as hereditary spastic paraplegia (HSP) complicated by pure motor neuropathy.
Results
Four patients diagnosed with dHMN-Vor SS carried known heterozygous BSCL2 mutations (N88S and S90L). In one dHMN-V patient we detected a putative GARS mutation (A57V). No mutations were detected in HSPB1 and HSPB8. The diagnostic yield gained in the series of 33 probands was 12% for BSCL2 mutations and 3% for GARS mutations. In the series of unclassified dHMN and complicated HSP cases no mutations were found.
Conclusions
Our data confirm that most likely only two mutations (N88S, S90L) in exon 3 of BSCL2 may lead to dHMN-V or SS phenotypes. Mutations in GARS, HSPB1 and HSPB8. are not a common cause of dHMN-V, SS and CMT2D. We would therefore suggest that a genetic testing of dHMN-V and SS patients should begin with screening of exon 3 of the BSCL2 gene. Screening of the GARS gene is useful in patients with CMT2 with predominant hand involvement and dHMN-V. The rather low frequencies of BSCL2, GARS, HSPB1 and HSPB8 mutations in dHMN-V, CMT2D and SS patients strongly point to further genetic heterogeneity of these related disorders.
Keywords: Charcot–Marie–Tooth disease type 2D, CMT2, Distal hereditary motor neuropathy, dHMN, BSCL2, GARS, HSPB1, HSPB8
1. Introduction
Autosomal dominant distal hereditary motor neuropathy (dHMN) represents a group of clinically and genetically heterogeneous conditions caused by degeneration of motor neurons leading to distal muscle weakness and wasting. Distal HMN type V (dHMN-V) is characterised by marked and progressive symmetrical or asymmetrical weakness and wasting of the distal muscles of the upper limbs. Foot deformity and peroneal muscle weakness may also be present [1]. Sensory disturbances occur rarely in advanced stages of dHMN-V [1,2]. Recently, patients presenting with axonal hereditary motor and sensory neuropathy (HMSN type 2, i.e. Charcot–Marie–Tooth 2 disease or CMT2 syndrome) and predominant hand muscle involvement have been genetically subclassified as CMT2D [3,4]. The phenotype of CMT2D is thus similar to dHMN-V [4]. Silver syndrome (SS) is a rare motor neuron disease which also frequently resembles a dHMN-V phenotype as patients often show prominent amyotrophy of the small hand muscles [5]. The presence of mild to severe pyramidal tract signs is common in dHMN-V and SS whereas they have rarely been observed in patients with CMT2D [1,2,5,6]. Initially, the presence of spastic paraparesis has led to the subclassification of SS among the group of hereditary spastic paraplegias (HSP) and SS was thus termed as SPG17 [7].
Up to date, 9 different mutations in the glycyl-tRNA synthetase gene (GARS) were identified in families with dHMN-V and CMT2D (www.molgen.ua.ac.be/CMTMutations/), [8]. The phenotypic spectrum of disorders associated with GARS mutations has been summarised in detail [6,9]. Recent functional analyses of several disease-associated GARS mutations suggested tRNA-charging deficits to play a role in disease pathogenesis [10]. In a number of families diagnosed as either dHMN-V or SS two heterozygous mutations (N88S, S90L) located in exon 3 in the Berardinelli–Seip congenital lipodystrophy gene (BSCL2) were identified [11]. Clinical examination of more than 90 patients with the BSCL2 N88S substitution showed that it is most often associated with a dHMN-V phenotype and less frequently, additional prominent spasticity and mild sensory disturbances are found in the lower limbs [2,12–15]. Up to date, the S90L substitution was only described in three families and often resulted in a spastic paraplegia phenotype with marked weakness and wasting in the hands suggesting the clinical diagnosis of SS [11,15,16]. Recently, mutations in the HSPB1 and HSPB8 genes have been reported as a cause of autosomal dominant dHMN and CMT2 in a small number of patients and families [17,18].
The purpose of the present study was to define the mutation spectrum and frequency of mutations in the BSCL2, GARS, HSPB1 and HSPB8 genes in a collection of 33 sporadic and familial cases exhibiting one of the overlapping phenotypes, dHMN-V, CMT2D or SS. Further, a series of 69 individuals with an unclassified dHMN phenotype or with HSP complicated by pure motor neuropathy was screened for mutations in exon 3 of the BSCL2 gene.
2. Methods
2.1. Patients
A cohort of 33 unrelated probands (cohort 1) consisting of 20 sporadic, 1 unknown and 12 familial cases carrying the clinical diagnosis of dHMN-V, CMT2D or SS was analysed for mutations in the BSCL2, GARS, HSPB1 and HSPB8 genes. Clinical classification of patients was based on previous descriptions of the given phenotypes and was made as follows: 14 patients (9 sporadic, 4 familial, 1 unknown) who showed prominent uni- or bilateral wasting of the small hand muscles, which sometimes exclusively affected thenar and dorsalis interosseus I eminences were diagnosed as dHMN-V. Gait disturbances were absent or only mild and spasticity of the lower limbs and sensory abnormalities were not found. Electromyography (EMG) and nerve conduction studies (NCS) confirmed a pure axonal motor neuropathy. Only one of these patients (proband 2) showed a complicated phenotype consisting of typical features of dHMN-V in the hands but also sensory abnormalities and acro-mutilations in the feet with severe lancinating pain attacks. Six probands (1 sporadic, 5 familial) exhibited a CMT2 phenotype characterised by predominant hand muscle involvement, absent pyramidal tract features, and mild to moderate distal sensory loss, which was confirmed by pathological sensory nerve conduction studies (SNCS). Thirteen patients (10 sporadic, 3 familial) represented the classical SS phenotype consisting of mild to severe spasticity in the lower limbs in addition to a pure motor neuropathy in the lower limbs, and symmetrical or asymmetrical hand muscle wasting in most of these patients. Sensory abnormalities were usually absent but rarely occurred with progression of the disease. The clinical features of all probands are presented in Table 1. A further 69 patients (cohort 2) of whom a blood sample had been sent to our laboratory to be screened for dHMN or HSP complicated by pure motor neuropathy were tested for mutations in exon 3 of the BSCL2 gene. No detailed clinical and electrophysiological data were available from these patients. Study participants originated from Austria, Germany, Great Britain, Italy and from Australia and were ascertained at the Medical Universities of Graz (Austria), Friedrich-Baur-Institut at the Ludwig-Maximilians-University Munich and Tübingen (Germany), Department of Pediatrics, Clinical Medical Center Zagreb (Croatia), “C. Besta” Neurological Institute of Milan (Italy), London (Great Britain), and Sydney (Australia). Studies were performed with written informed consent and approved by the local Ethical Committees of the participating centers.
Table 1.
Clinical features of all probands
| Proband no. |
Inheritance | Age at onset |
Age at exam |
Selected/ prominent thenar/ FDI atrophy1 |
Bilateral diffuse hand muscle wasting1 |
Peroneal muscle weakness |
PTR | ATR | Foot deformity |
Sensory loss, pathological SNCV2 |
Pyramidal tract signs |
Clinical diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AD | 52 | 54 | +++; R>L | − | ++ | ++ | + | +++ | − | − | dHMN-V |
| 2 | AD | 14 | 66 | ++; L>R | +++ | +++ | ++ | − | +++ | +++3 | − | dHMN-V |
| 3 | S | 3 | 57 | ++; R>L | +++ | +++ | +++ | − | + | ++ | +++ | SS |
| 4 | AD | 16 | 55 | +++; R>L | − | + | +++ | +++ | + | − | − | SS |
| 5 | S | 12 | 40 | − | +++ | − | ++ | ++ | − | − | − | dHMN-V |
| 6 | D | 3rd dec | 42 | − | +++ | − | +++ | + | − | − | − | SS |
| 7 | D | 1st dec | 36 | − | +++ | +++ | − | − | +++ | ++ | − | CMT2D |
| 8 | AD | 15 | 53 | − | +++ | − | ++ | + | + | − | − | dHMN-V |
| 9 | AD | 14 | 42 | +++; Bil | − | + | ++ | + | ++ | + | − | CMT2D |
| 10 | S | 60 | 64 | − | +++ | − | +++ | + | − | − | − | SS |
| 11 | AD | 3rd dec | 43 | − | +++ | +++ | ++ | + | +++ | + | − | CMT2D |
| 12 | S | 42 | 50 | − | +++ | − | ++ | + | ++ | − | − | dHMN-V |
| 13 | S | 46 | 53 | +++; L>R | ++ | − | ++++ | ++++ | − | − | + | SS |
| 14 | S | 25 | 31 | +++; R>L | +++ | + | ++++ | ++++ | − | − | + | SS |
| 15 | AD | 36 | 43 | − | ++ | + | +++ | +++ | + | − | − | SS |
| 16 | U | 27 | 39 | − | +++ | + | ++ | ++ | − | − | − | dHMN-V |
| 17 | S | 9 | 66 | − | ++ | + | +++ | +++ | − | − | + | SS |
| 18 | S | 15 | 18 | − | +++ | − | ++ | ++ | − | − | − | dHMN-V |
| 19 | AD | 2nd dec | 60 | − | +++ | +++ | ++ | − | + | ++ | − | CMT2D |
| 20 | S | 21 | 27 | +++; Bil | − | − | + | + | + | − | − | dHMN-V |
| 21 | S | 15 | 48 | ++ | − | − | ++ | ++ | − | − | − | dHMN-V |
| 22 | S | 62 | 69 | +++; L>R | − | ++ | + | + | − | − | − | dHMN-V |
| 23 | S | 59 | 69 | − | − | + | + | − | ++ | + | + | SS |
| 24 | S | 33 | 63 | − | +++ | +++ | + | − | − | − | − | dHMN-V |
| 25 | S | 36 | 38 | ++ | − | − | ++++ | ++++ | − | − | − | SS |
| 26 | D | 28 | 31 | ++ | − | + | ++ | − | +++ | + | − | CMT2D |
| 27 | S | 49 | 55 | ++ | − | − | ++++ | ++ | − | + | − | SS |
| 28 | S | 17 | 21 | ++ | − | − | ++ | ++ | + | − | − | dHMN-V |
| 29 | S | 60 | 70 | ++; L>R | − | − | + | − | − | + | − | CMT2D |
| 30 | S | 70 | 69 | ++ | − | +++ | ++++ | − | − | − | − | SS |
| 31 | S | ~60 | 60 | +++; Bil | − | + | + | +++ | ++ | − | − | dHMN-V |
| 32 | S | 25 | 35 | − | − | ++ | ++ | − | ++ | − | ++; Bil | SS |
| 33 | AD | ~25 | 42 | +++; Bil | ++ | − | ++ | + | + | − | − | dHMN-V |
(−) absent, normal; (+): mild, (++) moderate, (+++): severe, R = right, L = left; Bil = bilateral; patellar and achilles tendon reflex (PTR, ATR): absent (−), diminished (+), preserved (++), brisk (+++), very brisk, clonus (++++);
at disease onset;
except for pallesthesia,
patient with dHMN-V and ulcero-mutilating neuropathy; S = sporadic cases, AD: autosomal dominant inheritance; D = dominant inheritance; FDI = first dorsalis interosseus I muscle; U = unknown; SNCV = sensory nerve conduction velocity.
History and family history were obtained and a full neurological examination was carried out by experienced neurologists (M. A-G, H. L., B.S-W, M.M.R., D.P., G.N., L.S.) in each proband.
2.2. Clinical neurophysiology
NCS were performed in the upper and lower limbs to detect or exclude an underlying polyneuropathy. NCS followed standard techniques using a portable EMG Myohandy (Micromed Neurodata, Mogliano Veneto, Italy), and the electromyograph Keypoint (Dantec Medical, Skovlunde, Denmark). Responses for motor NCS (MNCS) were recorded from distal muscles using surface electrodes. SNCS were performed ortho- or antidromically with the use of surface or ring electrodes. EMG was performed with the use of concentric needle electrodes in the majority of probands to confirm a neuropathic origin of the disease and to exclude myopathic changes.
2.3. Genetic sequencing
After informed consent genomic DNA was extracted from peripheral blood samples by standard techniques. Mutation analysis of the coding regions and all splice sites of BSCL2, GARS, HSPB1 and HSPB8 was performed by sequencing all probands of cohort 1. In addition, all coding exons and flanking splice sites of the HSN2, SPTLC1 and RAB7 genes were sequenced in proband 2. Primer sequences are available on request. Reactions were separated using an ABI 3130-xL Genetic Analyzer (Applied Biosystems, Foster City, CA). Sequencing data were collected using the ABI DNA Sequence Analysis Software, version 5.2 (Applied Biosystems, Foster City, CA). Sequencing analysis was carried out using the SeqScape Software 2.5 (Applied Biosystems, Foster City, CA). BSCL2 exon 3 was sequenced in DNA samples from probands of cohort 2.
3. Results
Four of 33 probands in cohort 1 (three familial and one isolated case) were found to carry a known mutation in the BSCL2 gene and in one patient a novel, putative GARS mutation was detected. The phenotypes of these patients are summarised here.
3.1. Proband 1
This Austrian patient first noticed muscle wasting of thenar and dorsalis interosseus I muscles on the right side and mild unsafeness of gait at age 52. Subsequently, wasting in the left hand and weakness of the other small hand muscles developed. High arches were known from early childhood. On neurological examination there was prominent weakness and wasting predominantly in the thenar and dorsalis interosseus I muscles on both sides with the right side more severely affected than the left. In the lower limbs there was mild to moderate symmetric weakness and wasting of toe and foot extensors and severe pes cavus foot deformity. No sensory abnormalities could be detected. Tendon reflexes were reduced except for the patellar reflexes which were well preserved on both sides. Babinski sign was absent. NCS revealed an axonal motor neuropathy in upper and lower limbs. Five further family members were known to be affected and the pedigree of the family indicated autosomal dominant inheritance.
3.2. Proband 2
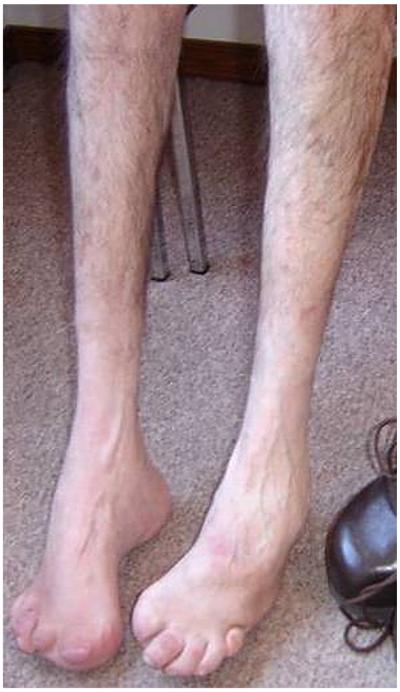
This 66-year-old patient from Austria exhibited a typical dHMN-V phenotype with disease onset at 14 years. However, at age 50, he started to suffer from shooting and lancinating pain in the lower limbs. Subsequently, he developed severe sensory loss affecting all modalities in the distal parts of the lower limbs which was complicated by foot ulcerations and infections and necessitated amputations of several toes (Fig. 1). Circulatory disturbances in the lower limbs and all frequent causes of neuropathies were excluded. NCS revealed a severe axonal sensory motor neuropathy of the upper and lower limbs. A family history was present and the origin of this patient could be traced back to the large Austrian family with a BSCL2 mutation, which has been reported elsewhere [2].
Fig. 1.
Prominent distal muscle wasting of lower limbs, foot deformity and amputations of toes in proband 2.
3.3. Proband 3
This now 57-year-old German patient developed spastic paraparesis and symmetric muscle wasting and weakness in the distal parts of the lower limbs at the age of 3 years. Family history was negative for neuromuscular disorders. Over the following decades the disease was slowly progressive. Later on moderate sensory deficits such as a reduction of pain and vibration sensitivity were detected. Atrophy of the right thenar muscle was first noticed at the age of 35, bilateral atrophy was found at the age of 54. On examination muscle tone in the lower limbs was increased and patellar reflexes were brisk. The patient walked with a spastic gait and exhibited severe muscular atrophy in distal parts of the upper and lower limbs.
3.4. Proband 4
In this 55-year-old English woman the disease started at age 16 with weakness in the right hand. Her left hand became similarly involved a few years later but has always been less affected than the right hand. Subsequently she also developed high arches and distal muscle weakness in the legs. Moreover, she complained troublesome shooting pains in both legs and cramps in both feet. On examination, all reflexes were present with brisk knees and ankle jerks. Plantars were flexor and full sensory examination including temperature testing was normal.
Autosomal dominant inheritance was suggested with her mother and two of 4 children being similarly affected.
3.5. Proband 5
This 40-year-old man who lives in the UK but was originally from Ghana noticed difficulty in writing as his right index finger was weak at age 12. He also noticed wasting in the right thenar at about the same time. His symptoms worsened when he was badly beaten up at age 34 in Ghana. We only have limited details of the injuries but he was unconscious and woke up to find both arms in plaster but never knew why. When the plaster was removed 3 weeks later he noticed that both hands were more wasted than previously and since that time has described wasting and weakness of both hands but feels it has progressed only slightly since then. On examination he had wasting and weakness of both triceps, both distal forearms and the small muscles of both hands. Upper limb reflexes were absent except for the right biceps which was present with reinforcement. The lower limbs were completely normal with normal tone, power and reflexes. Plantars were flexor. Sensation was normal throughout. In addition this patient also had bilateral ptosis and chronic denervation of the right orbicularis oculi was confirmed by EMG. All his family including 6 siblings and 6 children lives in Ghana and has not been examined. One of his sisters is reported having ptosis.
3.6. Gene mutation analysis
In our series of 33 probands we screened the 10 coding exons of BSCL2, the 17 coding exons of GARS, and each 3 coding exons of HSPB1 and HSPB8 for mutations. Probands 1, 2 and 4 were heterozygous for the known BSCL2 p.N88S mutation in exon 3. In proband 3 we detected a heterozygous C to T transition changing serine to leucine at codon 90 (c.269 C > T, p.S90L) in exon 3 of the BSCL2 gene. Both mutations have been described previously [11–13,15,16]. In proband 5 we detected a heterozygous C to T substitution changing alanine to valine at codon 57 (c.688 C > T, p.A57V) in the GARS gene which is novel. There were no family members or ethnically matched controls available to screen for this variation. This variant has not been described previously and was not found in any other patient screened in this study. Proband 2 was also screened for a mutation in the SPTLC1, RAB7 and HSN2 genes due to the additional ulcero-mutilating complications. In neither of these genes we could find any pathogenic mutations or polymorphisms.
Neither of the 69 probands with an unclassified dHMN phenotype or complicated HSP in our second series harboured a mutation in exon 3 of BSCL2. Location and frequencies of known and novel polymorphisms detected in the BSCL2 and GARS genes are summarised in Table 2.
Table 2.
Polymorphisms found in the BSCL2 and the GARS gene
| Polymorphism | Literature | Number | |
|---|---|---|---|
| BSCL2 | |||
| Exon 3: c.294+ 11 G>T |
None | 8 heterozygous, 2 homozygous |
|
| Exon 4: c.295-34 A>T |
None | 2 heterozygous | |
| Exon 6: c.574-49 T>C |
rs2850597 | 10 heterozygous, 8 homozygous |
|
| Exon 8: c.814-50 T>G |
None | 2 heterozygous | |
| Exon 9: c.945 A>G, p.E315E |
None | 11 heterozygous, 2 homozygous |
|
| GARS | |||
| Exon 1: c.60 + 5 C>T |
rs2072236 | 2 heterozygous | |
| Exon 6: c.177-43 C>A |
rs1558064 | 6 heterozygous, 10 homozygous |
|
| Exon 9: c.870-23 A>T |
rs2527878 | 2 heterozygous | |
| Exon 12: c.1451+ 9 T>C |
None | 2 heterozygous | |
| Exon 17: c.1933-6 C>T |
rs2240401 | 7 heterozygous, 10 homozygous |
4. Conclusions
The present study provides an overview about the frequency of mutations in the BSCL2, GARS, HSPB1 and HSPB8 genes in 33 patients diagnosed as dHMN-V, CMT2D or SS. The diagnostic yield gained in this series of probands with a well defined phenotype was 12% for BSCL2 mutations and 3% for GARS mutations. Moreover, we screened another series of 69 probands with an undefined dHMN phenotype or HSP complicated by pure motor neuropathy for mutations in exon 3 of BSCL2 but did not find any pathogenic mutations. These results strongly point to further, non-allelic heterogeneity in this group of disorders. This study further indicates that mutations in the HSPB1 and HSPB8 genes might not produce complicated phenotypes such as dHMN-V, SS and CMT2D but certainly more patients have to be screened before drawing any firm conclusions.
So far, only the two heterozygous mutations N88S and S90L in exon 3 of the BSCL2 gene have been reported in patients with dHMN [11–16]. Our study highlights these two mutations as a cause of dHMN-V. Notably, the fourth patient carrying the S90L mutation exhibited prominent spastic paraparesis of the lower limbs in addition to axonal polyneuropathy and was originally diagnosed as HSP. Our findings confirm the marked phenotypic variation between the N88S and the S90L mutations as described earlier [2,15] which both affect glycosylation of seipin, the protein encoded by BSCL2 [11]. Based on the previous reports and on the results presented in this study it seems most likely that the N88S and S90L substitutions are indeed the only two mutations in the BSCL2 gene that may cause dHMN-V or SS. We therefore suggest that an analysis of BSCL2 of patients with dHMN-V and SS may be restricted to exon 3. On the other hand, in non-classifiable dHMN and HSP cases this algorithm has no rationale.
Proband 2 is an offspring of a large Austrian dHMN-V/Silver syndrome family reported in 2005 [2]. The phenotype of this patient, who shows the typical dHMN-V features in the hands and carries the N88S mutation, is highly unusual with regard to the severe ulcero-mutilating complications in the lower limbs and the lancinating pain attacks. This phenotype is difficult to explain on the basis of the present mutation only. Because other common causes of acquired and inherited sensory neuropathies (such as mutations in the HSN2, SPTLC1 and RAB7 genes) have been excluded, it is possible that this patient is afflicted with a second rare genetic or acquired disease.
The GARS variant A57V identified in this study is novel, but its pathogenicity has to be confirmed in further CMT2D and dHMN-V patients because we did not have access to ethnic matched controls and there were no other family members available to show segregation with the phenotype. However, the initial phenotype in this case is typical for the phenotype described with GARS mutations and is the main evidence for considering A57V pathogenic. The finding of ptosis is unusual but since it is present in another family member who does not reportedly have other symptoms suggesting distal HMN or CMT2, it may be co-incidental and not related to the GARS phenotype.
The high phenotypic variation between and within families with BSCL2 and GARS mutations has already been documented very well [6]. An overlapping of features consisting of uni- or bilateral selected hand muscle wasting and a variable degree of weakness in the lower limbs, which is often accompanied by pure motor neuropathy, has been demonstrated in both instances. Mild to severe pyramidal tract features are common in patients with BSCL2 mutations whereas they have only been observed in five patients with GARS mutations to a mild degree [6]. On the other hand, sensory abnormalities are more frequent in patients with GARS mutations making the boundaries between hereditary motor and sensory neuropathies and distal HMN less clear. Having seen both patients with BSCL2 and GARS mutations we may state here that it is often impossible to distinguish the matching phenotypes on a clinical and electrophysiological basis particularly in the absence of pyramidal tract features. The latter strongly favour the diagnosis of SS and indicate that exon 3 of BSCL2 should be screened first. To avoid confusion of classification between these related diseases it might be important to use the term SS (which is also subcategorized as SPG17 among the group of hereditary spastic paraplegias) for cases with hand muscle involvement and additional spastic paraparesis only.
It still remains poorly understood how mutations in the BSCL2 and GARS genes lead to this broad spectrum of phenotypically overlapping disorders. It will be a challenge for future cellular and functional studies to detect the underlying mechanisms and maybe also to define common pathways between these two genes.
Acknowledgements
We gratefully acknowledge the cooperation of the probands and their participation in this study. This project was supported by the Austrian Science Fund (FWF, P17494-B14 and P19455-B05), the Telethon-Italy grant GUP02169, and by the German Bundesministerium für Bildung und Forschung (Grant 01GM0304-GeNeMove).
References
- [1].Auer-Grumbach M, Loscher WN, Wagner K, et al. Phenotypic and genotypic heterogeneity in hereditary motor neuronopathy type V: a clinical, electrophysiological and genetic study. Brain. 2000;123(Pt8):1612–23. doi: 10.1093/brain/123.8.1612. [DOI] [PubMed] [Google Scholar]
- [2].Auer-Grumbach M, Schlotter-Weigel B, Lochmuller H, et al. Phenotypes of the N88S Berardinelli–Seip congenital lipodystrophy 2 mutation. Ann Neurol. 2005;57:415–24. doi: 10.1002/ana.20410. [DOI] [PubMed] [Google Scholar]
- [3].Ionasescu V, Searby C, Sheffield VC, Roklina T, Nishimura D, Ionasescu R. Autosomal dominant Charcot–Marie–Tooth axonal neuropathy mapped on chromosome 7p (CMT2D) Hum Mol Genet. 1996;5:1373–5. doi: 10.1093/hmg/5.9.1373. [DOI] [PubMed] [Google Scholar]
- [4].Sambuughin N, Sivakumar K, Selenge B, et al. Autosomal dominant distal spinal muscular atrophy type V (dSMA-V) and Charcot–Marie–Tooth disease type 2D (CMT2D) segregate within a single large kindred and map to a refined region on chromosome 7p15. J Neurol Sci. 1998;161:23–8. doi: 10.1016/s0022-510x(98)00264-0. [DOI] [PubMed] [Google Scholar]
- [5].Silver JR. Familial spastic paraplegia with amyotrophy of the hands. Ann Hum Genet. 1966;30:69–75. doi: 10.1111/j.1469-1809.1966.tb00007.x. [DOI] [PubMed] [Google Scholar]
- [6].Sivakumar K, Kyriakides T, Puls I, et al. Phenotypic spectrum of disorders associated with glycyl-tRNA synthetase mutations. Brain. 2005;128:2304–14. doi: 10.1093/brain/awh590. [DOI] [PubMed] [Google Scholar]
- [7].Patel H, Hart PE, Warner TT, et al. The Silver syndrome variant of hereditary spastic paraplegia maps to chromosome 11q12–q14, with evidence for genetic heterogeneity within this subtype. Am J Hum Genet. 2001;69(1):209–15. doi: 10.1086/321267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Antonellis A, Ellsworth RE, Sambuughin N, et al. Glycyl tRNA synthetase mutations in Charcot–Marie–Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet. 2003;72:1293–9. doi: 10.1086/375039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].James PA, Cader MZ, Muntoni F, et al. Severe childhood SMA and axonal CMT due to anticodon binding domain mutations in the GARS gene. Neurology. 2006;67(9):1710–2. doi: 10.1212/01.wnl.0000242619.52335.bc. [DOI] [PubMed] [Google Scholar]
- [10].Antonellis A, Lee-Lin SQ, Wasterlain A, et al. Functional analyses of glycyl-tRNA synthetase mutations suggest a key role for tRNA-charging enzymes in peripheral axons. J Neurosci. 2006;26(41):10397–406. doi: 10.1523/JNEUROSCI.1671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Windpassinger C, Auer-Grumbach M, Irobi J, et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nat Genet. 2004;36:271–6. doi: 10.1038/ng1313. [DOI] [PubMed] [Google Scholar]
- [12].Van de Warrenburg BP, Scheffer H, van Eijk JJ, et al. BSCL2 mutations in two Dutch families with overlapping Silver syndrome–distal hereditary motor neuropathy. Neuromuscul Disord. 2006;16:122–5. doi: 10.1016/j.nmd.2005.11.003. [DOI] [PubMed] [Google Scholar]
- [13].Bienfait HM, Baas F, Koelman JH, et al. Phenotype of Charcot–Marie–Tooth disease Type 2. Neurology. 2007;68:1658–67. doi: 10.1212/01.wnl.0000263479.97552.94. [DOI] [PubMed] [Google Scholar]
- [14].Irobi J, De Jonghe P, Timmerman V. Molecular genetics of distal hereditary motor neuropathies. Hum Mol Genet. 2004;13:R195–202. doi: 10.1093/hmg/ddh226. Spec No 2. [DOI] [PubMed] [Google Scholar]
- [15].Irobi J, Van den Bergh P, Merlini L, et al. The phenotype of motor neuropathies associated with BSCL2 mutations is broader than Silver syndrome and distal HMN type V. Brain. 2004;127:2124–30. doi: 10.1093/brain/awh232. [DOI] [PubMed] [Google Scholar]
- [16].Cho HJ, Sung DH, Ki CS. Identification of de novo BSCL2 Ser90Leu mutation in a Korean family with Silver syndrome and distal hereditary motor neuropathy. Muscle Nerve. 2007 May 7; doi: 10.1002/mus.20792. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [17].Irobi J, Van Impe K, Seeman P, et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet. 2004;36:597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- [18].Evgrafov OV, Mersiyanova I, Irobi J, et al. Mutant small heat-shock protein 27 causes axonal Charcot–Marie–Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–6. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]