Summary
This study utilizes a unique data set covering over 19 000 georeferenced records of species presence collected between 1993 and 2008, to explore the distribution and habitat selectivity of an assemblage of 26 carnivore species in the Serengeti–Ngorongoro landscape in northern Tanzania.
Two species, the large-spotted genet and the bushy-tailed mongoose, were documented for the first time within this landscape. Ecological Niche Factor Analysis (ENFA) was used to examine habitat selectivity for 18 of the 26 carnivore species for which there is sufficient data. Eleven ecogeographical variables (EGVs), such as altitude and habitat type, were used for these analyses.
The ENFA demonstrated that species differed in their habitat selectivity, and supported the limited ecological information already available for these species, such as the golden jackals’ preference for grassland and the leopards’ preference for river valleys.
Two aggregate scores, marginality and tolerance, are generated by the ENFA, and describe each species’ habitat selectivity in relation to the suite of EGVs. These scores were used to test the hypothesis that smaller species are expected to be more selective than larger species [Science, 1989, 243, 1145]. Two predictions were tested: Marginality should decrease with body mass; and tolerance should increase with body mass. Our study provided no evidence for either prediction.
Our results not only support previous analyses of carnivore diet breadth, but also represent a novel approach to the investigation of habitat selection across species assemblages. Our method provides a powerful tool to explore similar questions in other systems and for other taxa.
Keywords: Canidae, carnivore biodiversity, coexistence, East Africa, felidae, habitat use, herpestidae, hyaenidae, macroecology, mustelidae
Introduction
Understanding the mechanisms for coexistence in species assemblages is key to understanding how communities are structured, and hence the fundamental drivers of evolutionary processes. Niche partitioning between different species in a community is a key driver of the evolution and structure of species assemblages (Begon, Harper & Townsend 1990; Mayhew 2006). In particular, body size is expected to be a major factor driving niche separation between different species, whereby species with larger body sizes utilize different habitats and diet than species with smaller body sizes (Peters 1983). Energy requirements of mammals scale with 2/3–1 of the power of body mass (Glazier 2010), thus metabolic demand per unit mass is expected to decrease with body mass (Banavar et al. 2002), and animals are expected to become more efficient in energy acquisition as they decrease in size (Brown & Maurer 1989). In addition, smaller species are also expected to be more selective than larger species, because the ability to cover ground and acquire different types of food scales with body mass, allowing larger species access to a broader range of habitats and diet than smaller species (Schoener 1968; Peters 1983). Moreover, in predator guilds, large predators are expected to be able to capture both large and small prey, whilst small predators are usually only able to handle small prey (Barclay & Brigham 1991; Costa 2009). There is some evidence to support this hypothesis, for example, there are positive relationships between geographic range and body size in some fish taxa (Pyron 1999) and between prey range and body size in carnivores (Radloff & Du Toit 2004). However, other studies are more ambiguous. Thus, body size in passerine birds shows no relationship with diet breadth, but beak size has a positive correlation (Brandl, Kristin & Leisler 1994), and an apparent relationship between body size and dietary breadth in insects breaks down when analysed within guilds (Novotny & Basset 1999). Further studies appear to contradict the theory; for example, dietary niche breadth in marine predators (Costa 2009) or lizards (Costa et al. 2008) is not correlated with body size, whilst trophic niche breadth in Poicephalus parrots appears to be inversely related to body size (Boyes & Perrin 2009). Most studies to date have concentrated on dietary breadth as a measure of niche breadth; however, optimal foraging theory suggests that handling time constraints may make small prey unprofitable for larger sized predators (Costa 2009), which might explain the ambiguity of the results. We might therefore expect a relationship between habitat selectivity and body size to be stronger, yet relatively few studies have investigated habitat-based measures of niche breadth.
Carnivores are of particular interest in any exploration of the relationship between niche breadth and body size, as they span an exceptionally wide range of body size (Gittleman & Purvis 1998), and are found across a range of different ecosystems, from polar ice to tropical forest (Macdonald 1989). They are clearly highly adaptable, and able to live in complex species assemblages where over 30 species can be documented in a single ecosystem (Loyola et al. 2009). However, whilst many studies of carnivore biodiversity and distribution have focussed on regional or global patterns, e.g. (Mills, Freitag & van Jaarsveld 2001; Loyola et al. 2009), very few have investigated possible mechanisms underlying multi-species distribution patterns within an ecosystem or landscape (but see Pita et al. 2009). This is unfortunate, as natural selection operates on individuals within ecosystems, rather than across an entire species. Very often clear patterns of distribution at smaller scales can be masked at large scales and vice versa, and hence the scale of investigation can have profound influences on our understanding and interpretation of the factors influencing species distribution (Rahbek & Graves 2001; Shriner, Wilson & Flather 2006; Davies et al. 2007; Anderson et al. 2009).
Recent developments in spatial analysis enable us to parameterize species habitat selectivity based on species occurrence, enabling us for the first time to document the habitat selectivity of entire species communities within a taxon and ecosystem. Ecological Niche Factor Analysis (ENFA) is one such approach, and uses a multifactorial analysis to determine niche selectivity for a species based on its observed presence in relation to a range of ecogeographical variables (EGVs) such as altitude or habitat type (Hirzel et al. 2002). In addition to providing important information on species distribution in relationship to EGVs, ENFA generates two key parameters, marginality and tolerance, which provide aggregated statistics describing two independent measures of habitat selectivity for a particular species (Hirzel et al. 2002; Pettorelli et al. 2010). Broadly, a species with high habitat selectivity is expected to have a high marginality, indicating that it is selecting habitats which differ from the global average, and/or a low tolerance, indicating that it is selecting habitats with a narrow range over the EGVs (see Materials and methods below; Hirzel et al. 2002).
In this study, we aim to explore the ways in which habitat selection influences carnivore species distribution and how this relates to body size within a single landscape. Specifically, we explore the distribution and habitat selection of an assemblage of carnivores, and go on to use the habitat selectivity parameters generated by ENFA, marginality and tolerance, to test the hypothesis that carnivores become less selective as body mass increases through the following predictions:
Marginality should decrease with body mass.
Tolerance should increase with body mass.
We use presence data for 26 species of carnivores in the Serengeti ecosystem and surrounding areas – the Serengeti-Ngorongoro landscape – for this analysis.
Materials and methods
STUDY SITE AND DATA
The study area is located within the Serengeti ecosystem in northern Tanzania, encompassing the Serengeti National Park, Maswa, Grumeti and Ikorongo Game Reserves, Ngorongoro Conservation Area and Loliondo Game Controlled Area (Fig. 1). Habitats range from an extensive grassland plain in the south to thick bush in the north and west, and montane forest in the far south east. The area is irregularly interspersed with kopjes of granite and gneiss, and in the north and west intersected by rivers with remnant riverine forest habitats (Fig. 2; Sinclair & Arcese 1995). The area mainly comprises Serengeti volcanic grasslands and southern Acacia commiphora bush-land and thickets (Fig. 3; Olson et al. 2001).
Fig. 1.
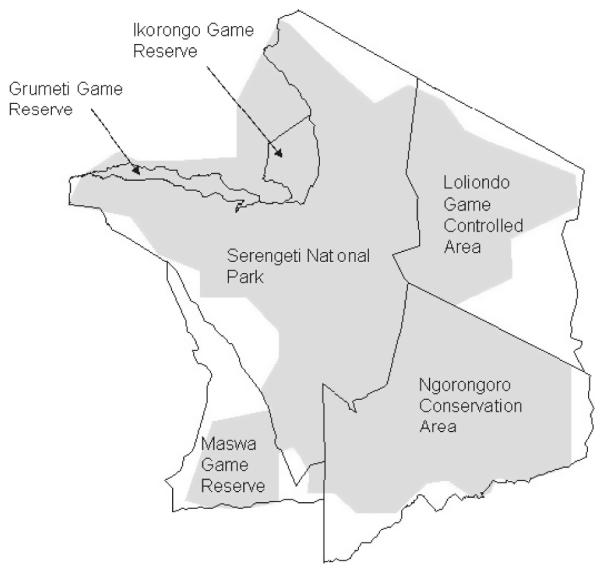
Study area (grey area) used for the background reference set of ecogeographical variables.
Fig. 2.
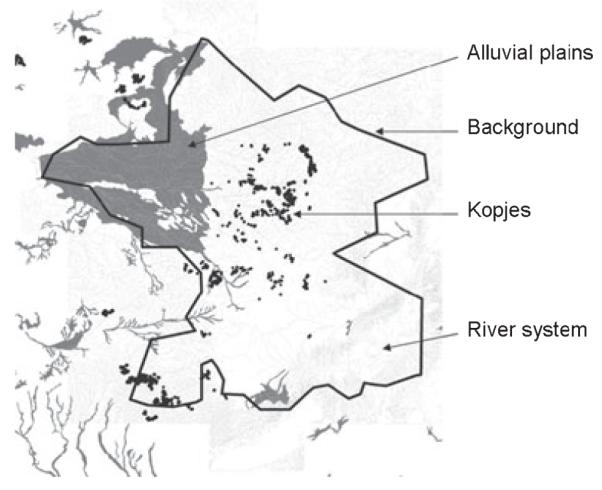
Distribution of rivers, kopjes and alluvial plains in the study area.
Fig. 3.
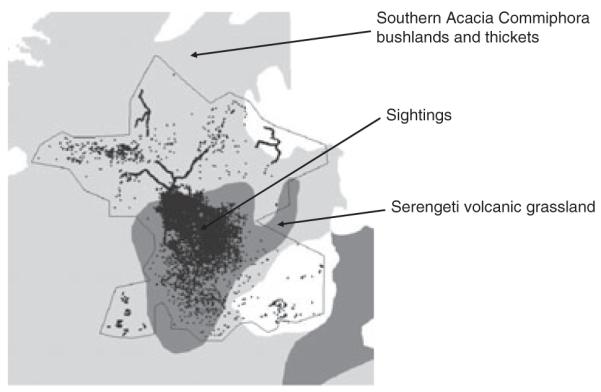
Distribution of the carnivore sightings in the study area (small circles) plotted against the main ecoregions in the study area.
The data used in our analyses originate from six sources of georeferenced sighting data of carnivores gathered by research projects working in the region:
Camera trap data – collected during surveys in the Maswa Game Reserve (79 locations, 1260 camera trap days, June 2008), Ngorongoro highlands (52 locations, 1008 camera trap days, April-August 2005) and Serengeti riverine forest (40 locations, 1219 camera trap days, May-July 2006) by the Tanzania Carnivore Program (TAWIRI) (see Pettorelli et al. 2010 for details of the methodology). No bait was used at camera trap sites.
Night transect data – collected by the Serengeti Viral Transmission Dynamics Team and the Serengeti Biodiversity Project between July 2003 and January 2009. The same 10 transects (four inside the park, six outside the park) were driven on roads monthly (when possible); all carnivores seen with spotlights were recorded with their GPS location.
Opportunistic carnivore sighting data gathered by S.M.D. between 1993 and 2008. All rarely sighted carnivores (i.e. excluding lions Panthera leo; spotted hyaena Crocuta crocuta; golden jackal Canis aureus; black-backed jackal Canis mesomelas; and bat-eared fox Otocyon megalotis) seen whilst searching for cheetah Acinonyx jubatus were recorded alongside the date and GPS location, as well as cheetah.
Opportunistic carnivore sighting data gathered by M.E.C. and the Serengeti Lion Project between April 2004 and May 2008. All carnivores, including lions, seen whilst radio-tracking for lions were recorded alongside date and GPS location.
Geo-referenced observations collected during daytime transect counts conducted quarterly along roads on the plains and in the northern woodlands between 1998 and 2000 (S.M.D., unpublished data) and across the plains in 2002, 2003 and 2005 (S. M. Durant, M.E. Craft, R. Hilborn, S. Bashir & L. Thomas, unpublished data).
Data submitted by volunteer contributors to the Tanzania Carnivore Program database from 2002 to 2008 (http://www.tanzaniacarnivores.org). Only geo-referenced data collected by experienced observers were included in analyses.
Data are biased towards the southern plains, as this is where research projects concentrated their efforts, and towards roads, particularly because of data from road-based transect counts from across the region (Fig. 3). Because of the difficulties in identifying genets to species by inexperienced observers, only verifiable camera trap observations were used in analyses for this taxon.
DATA ANALYSIS – ENFA
We used ENFA (Hirzel et al. 2002) to explore habitat use for species where a sufficient number of observation locations were available. ENFA uses presence-only observation data to define habitat features that promote species presence (Chefaoui, Hortal & Lobo 2005; Santos et al. 2006). The principles and procedures of ENFA have been described in detail elsewhere (Hirzel et al. 2002; Sattler et al. 2007). ENFA uses a factor analysis to aggregate information for each species into two indices, namely (i) ‘marginality’ (equivalent to the first factor) which maximizes the multivariate distance of the EGVs between the cells occupied by the species and the cells within the whole reference area, and (ii) ‘specialization’; an aggregate of the remaining factors, which is defined as the ‘ratio of the ecological variance in mean habitat to that observed for the focal species’ (Hirzel et al. 2002), and denotes to which extent a species’ EGVs distribution is narrow with respect to the overall distribution of the EGVs in the whole reference area. A species’ ‘tolerance’ is measured as the inverse of specialization. Marginality and specialization are uncorrelated factors, with most information contained within the first factors (Hirzel et al. 2002). A global marginality factor close to 1 means that the species lives in a very particular habitat relative to the reference set, whilst a tolerance of < 1 indicates some degree of specialization.
Geographic Information System (GIS) maps were selected for the analyses based on features believed to be possible components of a carnivore’s habitat, such as elevation, ecoregion (southern Acacia commiphora vs. Serengeti volcanic grassland), vegetation type (grassland, shrub or tree) and cover (in %), landform (alluvial plains), kopjes, lakes and rivers. Altogether, 11 EGVs were considered: alluvial plains; elevation; 100% grassland cover; kopjes; rivers; short grassland; 100% shrub cover; 60% shrub cover; southern Acacia commiphora; 100% tree cover; and Serengeti volcanic grassland. Maps were provided by the Tanzanian Wildlife Research Institute and the Serengeti-Mara data initiative (http://www.serengetidata.org). The resolution of the maps used for the analysis was set at 50 m. This resolution represented a trade-off between accuracy and computation time. Before carrying out the analysis, all the EGVs were normalized using the Box-Cox algorithm (Hirzel et al. 2002).
We evaluated the accuracy of the reported patterns by means of k-fold cross-validation (Sattler et al. 2007). k was determined using Huberty’s rule (Fielding & Bell 1997). We computed three presence-only evaluation measures, i.e. Absolute Validation Index (AVI), Contrast Validation Index (CVI; Sattler et al. 2007) and continuous Boyce’s Index (BI; Hirzel et al. 2006). AVI indicates how well the model discriminates high-suitability from low-suitability areas and varies from 0 to 1, while CVI indicates how much the AVI differs from that generated by a random model and varies from 0 to AVI. BI varies from 0 to 1, with 0 indicating a random model. Four classes of habitat suitability were determined in order to estimate AVI and CVI (Sattler et al. 2007). A window width of 20 was used to estimate BI (Hirzel et al. 2006). For all these measures (AVI, CVI and BI), high mean values indicate a high consistency with evaluation data sets. Furthermore, the lower the standard deviation, the more robust the prediction. All the analyses were performed in Biomapper 4.0 (Hirzel, Hausser & Perrin 2004).
BODY SIZE
Body size estimates were gleaned from the literature (Table 1). As body size estimates can vary across the species range, wherever possible we used estimates from within the Serengeti and Ngorongoro landscape. If these were not available, then we used estimates from eastern Africa; however, for a few species estimates were only available from southern Africa (Table 1). Analyses of body size were corrected for phylogeny using independent contrasts and were performed in the statistical package r (http://www.r-project.org).
Table 1.
Body mass of carnivore species used in the analysis. Where possible, mass estimates were derived from within the ecosystem. Scientific names are from Wilson & Reeder (2005)
| Species | Latin name | Weight (kg) | Study location | Reference |
|---|---|---|---|---|
| Common dwarf mongoose | Helogale parvula | 0·35 | Serengeti | Waser et al. (1995) |
| Slender mongoose | Galerella sanguinea | 0·64 | Serengeti | Waser et al. (1995) |
| Banded mongoose | Mungos mungo | 1·66 | Serengeti | Waser et al. (1995) |
| White-tailed mongoose | Ichneumia albicauda | 3·50 | East Africa | Kingdon (1977) |
| Bat-eared fox | Otocyon megalotis | 3·50 | Serengeti | Maas & Macdonald (2004) |
| Wild cat | Felis silvestris | 4·50 | Southern Africa | Nowell & Jackson (1996) |
| Golden jackal | Canis aureus | 6·20 | East Africa | Wayne et al. (1989) |
| Black-backed jackal | Canis mesomelas | 7·30 | Kenya | Wayne et al. (1989) |
| Side-striped jackal | Canis adustus | 7·60 | East Africa | Wayne et al. (1989) |
| Honey badger | Mellivora capensis | 7·80 | South Africa | Begg et al. (2005) |
| Aardwolf | Proteles cristata | 8·70a | South Africa | Williams, Anderson & Richardson (1997) |
| Serval | Leptailurus serval | 11·18 | Africa | Kingdon (1977) |
| Caracal | Caracal caracal | 11·40 | South Africa | Stuart (1981) |
| African civet | Civettictis civetta | 12·00 | East Africa | Kingdon (1977) |
| Leopard | Panthera pardus | 37·78 | Tanzania | Caso (2002) |
| Cheetah | Acinonyx jubatus | 38·65 | Serengeti | Caro (1994) |
| Spotted hyaena | Crocuta crocuta | 52·00 | Serengeti | Kruuk (1972) |
| Lion | Panthera leo | 161·50 | Serengeti | Schaller (1972) |
Average over winter and summer seasons.
Results
CARNIVORE DIVERSITY
Twenty-eight carnivore species have been documented in the Serengeti–Mara region (Mduma & Hopcraft 2008): lion; leopard Panthera pardus; cheetah; serval Leptailurus serval; caracal Caracal caracal; African wild cat Felis silvestris; golden jackal; black-backed jackal; side-striped jackal Canis audustus; African wild dog Lycaon pictus; bat-eared fox; zorilla Ictonyx striatus; African striped weasel Poecilogale albinucha; African honey badger Mellivora capensis; African civet Civettictis civetta; spotted necked otter Hydrictis maculicollis; cape clawless otter Aonyx capensis; African palm civet Nandinia binotata; common genet Genetta genetta; Egyptian mongoose Herpestes ichneumon; slender mongoose Galerella sanguinea; common dwarf mongoose Helogale parvula; marsh mongoose Atilax paludinosus; banded mongoose Mungos mungo; white-tailed mongoose Ichneumia albicauda; aardwolf Proteles cristata; spotted hyaena Crocuta crocuta; and striped hyaena Hyaena hyaena. Data from a total of 26 species were accumulated for this analysis; however, the composition of species differed from the published list. Four species, African palm civet, striped weasel, spotted necked otter and cape clawless otter, were not recorded in this study but are listed as present in the ecosystem (Sinclair & Arcese 1995; Mduma & Hopcraft 2008). The Tanzania Carnivore Program data base included two sightings of striped weasel seen in Loliondo Game Controlled Area (Daudi Peterson, pers. comm.); however, these sightings were not GPS referenced. Two species not previously listed, the large-spotted genet Genetta maculata and the bushy-tailed mongoose Bdeogale crassicauda, were recorded in this study. These two species were identified in a series of camera-trapping surveys (i.e. through verifiable photographs) in the region. The large-spotted genet was recorded at 14 sites around the Mbalageti and Grumeti rivers in the Serengeti National Park, 14 sites in the Maswa Game Reserve and a further nine sites in the Ngorongoro highlands, whilst the bushy-tailed mongoose was recorded at six sites in the Ngorongoro highlands, marking a major range extension for the species (Taylor 1987).
ENFA
There were sufficient data for an ENFA for 18 of the species observed, covering 19 640 locations (Table 2). Species for which there were insufficient data for analysis included the two newly documented species, large-spotted genet and bushy-tailed mongoose, as well as striped hyaena, wild dog, Egyptian mongoose, zorilla, marsh mongoose and common genet.
Table 2.
Coefficients of the ecogeographical variables, marginality and tolerance for 18 species of Serengeti carnivores. N indicates the number of locations were the species was recorded
| Species | Alluvial plains (freq.) |
Elevation | Grassland 100% cover (freq.) |
Kopjes (dist.) |
Rivers (dist.) |
Short grass (freq.) |
Shrub 100% cover (freq.) |
Shrub 60% cover (freq.) |
Southern Acacia (freq.) |
Trees 100% cover (dist.) |
Volcanic grassland (freq.) |
Marginality | Tolerance | N | % explained by marginality |
% explained by first axis of specialization |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aardwolf | −0·174 | −0·009 | 0·025 | −0·602 | 0·249 | 0·459 | 0·088 | −0·159 | −0·212 | 0·375 | 0·341 | 0·861 | 0·547 | 107 | 17·6 | 26·9 |
| African civet | 0·260 | −0·482 | −0·115 | −0·400 | −0·195 | −0·236 | 0·181 | −0·141 | 0·510 | −0·022 | −0·347 | 0·794 | 0·577 | 58 | 19·2 | 24·9 |
| Banded mongoose |
−0·144 | −0·028 | −0·074 | −0·510 | 0·192 | 0·493 | 0·045 | −0·110 | −0·295 | 0·223 | 0·531 | 1·278 | 0·517 | 274 | 29·2 | 29·5 |
| Black-backed jackal |
−0·116 | −0·066 | −0·167 | −0·564 | 0·124 | 0·441 | 0·097 | −0·244 | −0·259 | 0·191 | 0·503 | 1·013 | 0·687 | 1858 | 11·7 | 32·1 |
| Bat·eared fox | −0·139 | 0·046 | −0·110 | −0·510 | 0·263 | 0·481 | 0·157 | −0·108 | −0·338 | 0·328 | 0·384 | 0·815 | 0·723 | 1298 | 15·1 | 25·4 |
| Caracal | −0·099 | 0·031 | −0·039 | −0·357 | 0·139 | 0·599 | 0·154 | −0·231 | −0·371 | 0·220 | 0·468 | 0·952 | 0·573 | 77 | 12·2 | 32·2 |
| Cheetah | −0·087 | 0·024 | 0·019 | −0·369 | 0·270 | 0·599 | −0·002 | −0·050 | −0·370 | 0·192 | 0·497 | 1·308 | 0·564 | 1273 | 18·4 | 24·7 |
| Common dwarf mongoose |
0·033 | −0·175 | −0·232 | −0·615 | −0·039 | 0·180 | 0·299 | −0·455 | −0·085 | −0·043 | 0·444 | 1·101 | 0·348 | 57 | 38·0 | 23·3 |
| Golden jackal | −0·150 | 0·104 | 0·133 | −0·333 | 0·402 | 0·547 | −0·040 | 0·029 | −0·348 | 0·238 | 0·443 | 1·604 | 0·413 | 1001 | 17·5 | 24·8 |
| Honey badger | −0·127 | 0·045 | −0·041 | −0·269 | 0·324 | 0·625 | 0·075 | 0·010 | −0·376 | 0·127 | 0·499 | 1·140 | 0·625 | 147 | 7·5 | 26·0 |
| Leopard | −0·037 | −0·187 | −0·163 | −0·505 | −0·139 | 0·598 | −0·045 | −0·212 | −0·240 | 0·135 | 0·428 | 0·677 | 0·603 | 248 | 21·9 | 30·7 |
| Lion | −0·079 | −0·019 | −0·040 | −0·471 | 0·079 | 0·565 | 0·017 | −0·074 | −0·352 | 0·248 | 0·504 | 1·315 | 0·509 | 6699 | 35·2 | 21·7 |
| Serval | 0·126 | −0·261 | −0·145 | −0·653 | 0·118 | 0·489 | 0·138 | −0·203 | −0·113 | 0·203 | 0·318 | 0·600 | 0·660 | 365 | 17·4 | 22·5 |
| Slender mongoose |
−0·318 | 0·144 | −0·354 | −0·419 | −0·104 | 0·055 | 0·334 | −0·451 | −0·363 | −0·156 | 0·304 | 0·703 | 0·699 | 86 | 9·7 | 22·1 |
| Spotted hyena | −0·108 | −0·002 | −0·032 | −0·449 | 0·258 | 0·579 | 0·023 | −0·025 | −0·330 | 0·227 | 0·472 | 1·203 | 0·622 | 5066 | 15·2 | 24·1 |
| Side-striped jackal |
−0·100 | −0·032 | −0·086 | −0·412 | 0·228 | 0·600 | −0·043 | −0·093 | −0·340 | 0·215 | 0·477 | 1·268 | 0·440 | 126 | 35·2 | 19·8 |
| White-tailed mongoose |
−0·182 | 0·125 | −0·380 | −0·009 | −0·014 | −0·342 | 0·308 | −0·318 | 0·145 | 0·491 | −0·481 | 0·522 | 0·523 | 503 | 23·1 | 32·3 |
| Wild cat | 0·105 | −0·321 | −0·087 | −0·770 | 0·198 | 0·230 | 0·365 | −0·200 | 0·121 | 0·050 | 0·063 | 0·571 | 0·639 | 397 | 17·9 | 20·2 |
The results of the ENFA are presented in Table 2. The number of locations available for analysis ranged from 57 for the dwarf mongoose, up to 6699 for lions. The high number of sightings for lions and cheetah reflect the fact that data from two long-term research projects on these species were included in this analysis. There was no relationship between marginality or tolerance with the number of locations at which a species was recorded (marginality F1,16 = 2·65, NS; tolerance F1,16 = 0·02, NS). For EGV that are a measure of distance, a positive correlation means that the species is more likely to be found as distance increases, and hence the overall association with that EGV is negative. All 18 species were thus positively associated with kopjes; and positively associated with the volcanic grasslands and short grasslands, except for the civet and white-tailed mongoose.
For the other EGVs, the pattern was less straightforward (Table 2). Golden jackal, white-tailed mongoose and slender mongoose were the species most positively associated with higher elevations, while civet, wild cat and serval were species most associated with lower elevations. Golden jackal was the only species showing a strong positive association with 100% grass cover, while black-backed jackal, leopard, slender mongoose, white-tailed mongoose and common dwarf mongoose, all thought to be dependent on some degree of bush or woodland, were most negatively associated with this habitat type. Rather surprisingly, most species were negatively associated with rivers, leopards, civet and slender mongoose being the only species showing a strong positive association. In terms of shrub cover, most species seemed to prefer thick 100% cover, except golden jackal, side-striped jackal and leopard, while all except golden jackal and honey badger avoided 60% cover. Rather interestingly, preferences for shrub cover largely did not extend to an association with southern Acacia commifera woodlands, which all species, except for civet, white-tailed mongoose and wild cat appeared to avoid. Neither were species likely to be associated with 100% tree cover, only slender mongoose showing a strong association with this habitat. The serval, a species thought to be associated with wetland habitat (Bowland 1990), showed a strong association with alluvial plains areas, together with the African civet and wild cat, two species whose habitat preferences have not been previously documented.
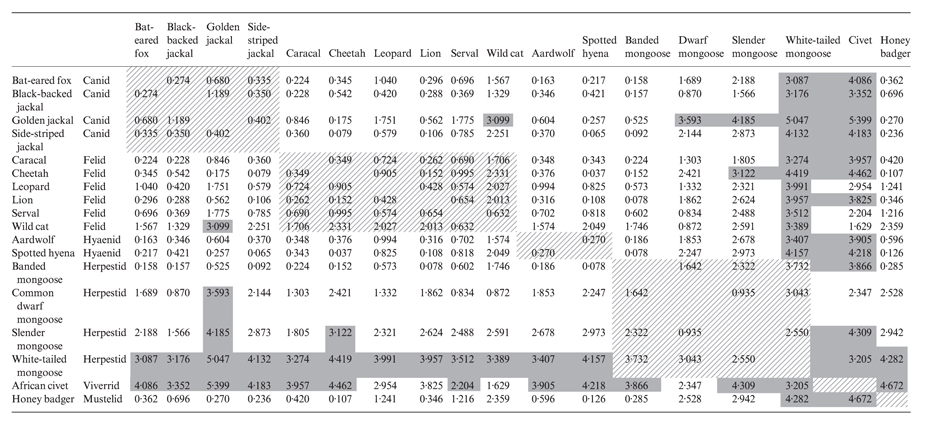
Overall, the analysis shows habitat partitioning variation between species. Table 3 shows the average mean squared differences between the EGV coefficients (corrected to ensure equal weighting between EGVs), demonstrating that within the canids: golden jackal; black-backed jackal; side-striped jackal, all of which have some overlap in their diets, occur in relatively similar habitats. The golden jackal shows the highest differences from other canids, showing use of grassland and non-bushy habitats relative to the other species, as has been documented elsewhere (Sillero-Zubiri, Hoffmann & Macdonald 2004). Within the felids, the wild cat, the smallest felid in this analysis, is least similar to the other felids in habitat use, a reflection of its preference to dense shrub and acacia habitats compared to the other species. Within the herpestids, a family whose habitat preferences are largely undocumented in any great detail, the white-tailed mongoose is the least similar to the other mongooses in terms of habitat preferences. Within the guild of large carnivores, which in this analysis comprises leopard, lion, cheetah and spotted hyaena, there is broad overlap. Only the leopard differs to any great extent in habitat use under the parameters explored in this analysis, reflecting a relative avoidance of higher elevations and grasslands and selection of rivers and bushy habitats. Interestingly, two species, the African civet (a viverrid) and the white-tailed mongoose, appeared to have the most marked differences in habitat use compared with the other carnivores examined here.
Table 3.
Mean square differences in ecogeographical variables (EGVs) between species. EGVs are scaled to represent equal weighting between EGVs and then the square of the differences for each species averaged across all EGVs. Hatched cells denote different families: canids, felids, hyaenids, and herpestids whilst solid grey cells denote high mean square differences of 3 or more
Tolerance scores ranged from 0·35 to 0·72 (Table 2). In descending order, bat-eared fox, slender mongoose, black-backed jackal, serval, wild cat, honey badger, spotted hyaena and leopard showed the highest tolerance (tolerance of ≥ 0·6), whilst dwarf mongoose, golden jackal and side-striped jackal showed the lowest tolerance (tolerance of < 0·5). Marginality ranged from 0·52 to 1·60. Half the species had marginalities higher than 1, indicating that the mean EGVs where they are found were very different from the average EGVs across the ecosystem, and hence that they are likely to be fairly specialized. Species with the highest marginality (> 1·3) in descending order were golden jackal, lion and cheetah while species with lowest marginality (<< 0·6) in ascending order were white-tailed mongoose, wild cat and serval.
The percentage of variance in the ENFA explained by marginality ranged from 8% for honey badger up to 38% for common dwarf mongoose. Marginality explained < 10% of the variance for only one other species: slender mongoose. Overall, a higher percentage of variance was explained by the first axis of specialization, ranging from 20% for side-striped jackal and wild cat up to 32% for white-tailed mongoose, caracal and black-backed jackal. The BI (Table 4) is a correlative measure of the predicted outputs generated by the ENFA analysis compared to observed results and hence provides a validation of the model for each species (Sattler et al. 2007). The index ranges from 0 to 1 and a value closest to 1 suggests a model with better predictive power. In our data, this index is high for black-backed jackal, white-tailed mongoose, spotted hyaena and golden jackal. However for several species (aardwolf, banded mongoose, bat-eared fox, caracal, dwarf mongoose, serval, lion and slender mongoose), it is not significantly different from zero, suggesting that for these species the model may be no better than a random model. Two other measures of model validation, the AVI and the CVI provide more confidence. The AVI is robust for all species, whilst the CVI is robust for all except for the aardwolf and caracal.
Table 4.
Validation test for the ENFAs performed. Boyce Index (BI) was determined using a window size of 20.
| Species | AVI | CVI | BI | Nb factorsa | Nb partitionsb |
|---|---|---|---|---|---|
| Aardwolf | 0·52 ± 0·09 | 0·07 ± 0·11 | 0·22 ± 0·30 | 3 | 4 |
| Banded mongoose | 0·52 ± 0·23 | 0·41 ± 0·22 | 0·40 ± 0·54 | 3 | 5 |
| Black-backed jackal | 0·45 ± 0·07 | 0·32 ± 0·06 | 0·86 ± 0·09 | 3 | 4 |
| Bat-eared fox | 0·45 ± 0·12 | 0·19 ± 0·08 | 0·14 ± 0·19 | 3 | 4 |
| Goldenjackal | 0·50 ± 0·16 | 0·43 ± 0·15 | 0·68 ± 0·29 | 5 | 4 |
| Caracal | 0·36 ± 0·27 | 0·24 ± 0·24 | 0·31 ± 0·48 | 4 | 5 |
| Cheetah | 0·48 ± 0·03 | 0·36 ± 0·03 | 0·28 ± 0·19 | 5 | 4 |
| Civet | 0·48 ± 0·17 | 0·31 ± 0·16 | 0·38 ± 0·26 | 5 | 4 |
| Dwarf mongoose | 0·54 ± 0·12 | 0·50 ± 0·12 | 0·38 ± 0·38 | 4 | 5 |
| Honey badger | 0·47 ± 0·12 | 0·32 ± 0·11 | 0·41 ± 0·21 | 4 | 4 |
| Leopard | 0·47 ± 0·05 | 0·16 ± 0·01 | 0·35 ± 0·01 | 2 | 2 |
| Lion | 0·36 ± 0·22 | 0·29 ± 0·22 | 0·23 ± 0·59 | 5 | 2 |
| Serval | 0·46 ± 0·13 | 0·18 ± 0·13 | 0·33 ± 0·40 | 5 | 10 |
| Slender mongoose | 0·49 ± 0·33 | 0·30 ± 0·28 | 0·31 ± 0·52 | 5 | 4 |
| Spotted hyaena | 0·43 ± 0·07 | 0·32 ± 0·08 | 0·77 ± 0·18 | 5 | 4 |
| Side-striped jackal | 0·50 ± 0·21 | 0·40 ± 0·20 | 0·56 ± 0·14 | 5 | 5 |
| White-tailed mongoose | 0·50 ± 0·21 | 0·36 ± 0·17 | 0·83 ± 0·15 | 3 | 4 |
| Wild cat | 0·43 ± 0·16 | 0·27 ± 0·12 | 0·54 ± 0·30 | 4 | 3 |
AVI, Absolute Validation Index; CVI, Contrast Validation Index.
Determined by the broken-stick heuristics (Jackson 1993).
Determined by Huberty’s rule of thumb (Fielding & Bell 1997).
HABITAT SPECIALIZATION AND BODY SIZE
A linear regression was used to explore the relationship between marginality and tolerance, and body size. The logarithm of body mass was used to transform this measure to a normal distribution for the analysis. Neither marginality nor tolerance showed a relationship with body mass (marginality on log body mass R2 = 0·058, NS; tolerance on log body mass R2 = 0·007, NS, Fig. 4), and the relationship between marginality and body size was in the opposite direction to that predicted, although it was non-significant. These relationships remained non-significant when correcting for phylogeny (marginality t16 = 1·22, NS; tolerance t16 = −0·29, NS).
Fig. 4.
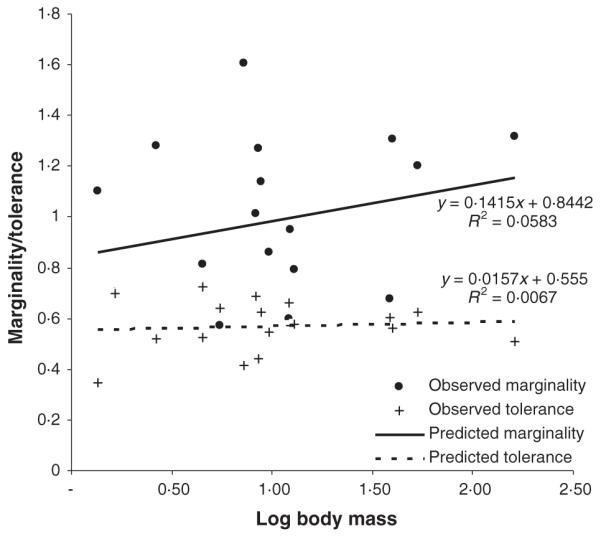
Relationship between axes of specialization, marginality and tolerance, and carnivore body mass (log transformed).
Discussion
Our results represent the first synthesis of the carnivore community in the Serengeti and its environs. We were able to document 26 species in the area, including two species for the first time: the large-spotted genet in the Serengeti–Mara ecosystem, and the bushy-tailed mongoose in the Ngorongoro highlands. We were able to use ENFA across 18 species, providing information on habitat selectivity for hitherto little known species, and explore the distribution of better known species across a broader landscape than has been previously possible. This analysis demonstrated clear evidence of differences in habitat selectivity between different species, which, where information was available, supported what was known about these species. We could find little evidence that habitat selectivity amongst similar species was strongly partitioned, either within a taxon, or within the large carnivore guild (Table 3). Furthermore, the aggregate statistics generated by our analysis provided no support for the hypothesis that species should become less selective as body size increases (Brown & Maurer 1989). There was no evidence for either of our predictions: (i) marginality did not decrease with body mass; and (ii) tolerance did not increase with body mass. In fact, marginality increased with body mass, against a predicted decline, albeit non-significantly. Our analysis suggests that there is no trend for carnivore species to become less selective as body size increases across the range of EGVs used in this analysis.
Our results should be regarded as a first approach to reveal habitat use patterns for a carnivore assemblage in a single landscape: altogether, the ability of our models to describe the observed distribution was relatively low, compared with those reported using similar approaches elsewhere (e.g. on pipistrelle bats Pipistrellus spp., see Sattler et al. 2007; on smooth snakes Coronella austriaca, see Santos et al. 2009). Such results may be partly due to the fact that carnivores are a relatively generalist taxon, hence their ecological niche might be expected to be relatively broad, and less habitat specific than other taxa. As far as we are aware, and aside from Pettorelli et al. (2009, 2010) which are discussed in detail below, indices of model fit (CVI, AVI and BI) have only previously been published for two other species of carnivore, the giant panda Ailuropoda melanoleuca (Xuezhi et al. 2008) and the Eurasian otter Lutra lutra (Cianfrani et al. 2010). These species are unusual amongst carnivores in that they are dependent on particular habitat types, bamboo forests and rivers, hence it is perhaps not surprising that the fit of the ENFA was much better than the species examined here. However, it is also possible that the heterogeneity in the EGVs used was insufficient to detect clear habitat specializations in the carnivore assemblage examined here. It is therefore important to repeat these analyses as more sighting data and more sophisticated GIS layers become available.
While there were an impressive number of locations for a number of the species in this study, for some species the number of locations where the presence of the species was confirmed may have sometimes been too low for high accuracy, as estimated by indices such as BI. For these species, habitat suitability models were not particularly robust. Three species were observed in fewer than 100 locations: caracal; common dwarf mongoose; and slender mongoose, and all except African civet had a BI that was not significantly different from zero. A fourth species, aardwolf, was recorded at only 107 sites and also had a BI that was not significantly different from zero. Another two species with a non-significant BI were banded mongoose and serval, both of which were recorded between 200 and 400 times. However, under alternative and less conservative measures of model validation, the AVI and CVI, all models were robust using the AVI, while only models of aardwolf and caracal were not significantly different from random using the CVI. More data on these species and on additional EGVs would help to refine the analysis. However, many of these species are rarely seen and hence increasing sample sizes is likely to be arduous and time consuming.
Several other species, with a high number of records, had a low correlation with the EGVs. With these species, the model was unable to reach high values of BI, even though confidence intervals could be relatively tight. Three species fell into this category: lion, cheetah and bat-eared fox. The cheetah and lion are wide-ranging species that are likely to move across the EGVs used in this analysis. Cheetah movement, in particular, are driven by avoidance of other larger carnivores in the ecosystem (Durant 1998, 2000), and these may thus play a greater role on the distribution of cheetah than direct measures of habitat. The remaining species, the bat-eared fox, is territorial and does not range particularly widely (Maas & Macdonald 2004). However, it is possible that the EGVs measured here do not correlate well with the termites on which it depends (Maas & Macdonald 2004), and which are likely to have the primary influence on its distribution.
The data used were from many sources, and included data from visual sightings, camera traps and transects. While some records, such as from camera traps and radiotracking, are likely to be unbiased in relationship to habitat, other records, particularly visual observation, are likely to be influenced by habitat, as species are more likely to be seen in open habitats than in closed habitats. All species recorded have been seen through a combination of visual observation (such as through transect counts) and through unbiased records, such as radiotracking and camera traps – and no species has only been recorded through these latter methods. This means that caution is needed when interpreting different EGV scores directly, and scores should always be interpreted as relative measures between species, rather than as absolute scores. In our analyses, we have been careful to interpret EGV scores in this way, and the aggregate statistics, tolerance and marginality, are intrinsically relative measures of EGVs. Any interactions between visibility and habitat between species would further confound the results.
A previous ENFA using only presence data collected in camera traps across a much broader region (Pettorelli et al. 2010) included five species analysed here: leopard, spotted hyaena, serval, slender mongoose and white-tailed mongoose, but used mostly different EGVs, which were more appropriate for the larger scale of this analysis. This earlier analysis largely concentrated on ecoregions, many of which were not found in the Serengeti landscape. However, it demonstrated a preference of spotted hyaena for acacia communities, and white-tailed mongoose and serval for open grassland. All species were attracted to rivers, unlike the results presented here, which demonstrated avoidance by most species. Interestingly, whilst the tolerance scores were very similar between the two studies (R2 = 0·440), marginality was negatively correlated (R2 = 0·155). However, sample sizes (n = 5) were below that necessary for sufficient power to detect statistically significant trends. Another study, on a single species, the cheetah, used a smaller area – around 10% of the area explored here – and a wider range of EGVs, but still recorded similar measures of marginality and tolerance to those found here (Pettorelli et al. 2009).
There are three possible explanations for the differences between our results and those of previous studies. First, this study used sighting data, as well as camera trap and radiotracking data, and sighting data are likely to be biased towards more open habitats. Whilst the comparative approach used here should account for much of this bias, some biases may remain if there is a complex relationship between species detectability and habitat openness. Second, scale is known to impact ecological patterns (Levin 1992), and changing the spatial resolution and/or the spatial extent of the background could affect our results. For example, on a country-wide scale species may appear to be attracted to rivers, however at a finer scale, species may move towards watersheds yet avoid the immediate vicinity of the river itself, which would be seen as avoidance. Finally, the broader Tanzania study used data from 11 different protected areas. These areas varied greatly in size, degree of protection and in consumptive use. Areas where carnivore species have been impacted by humans, may be subject to meso-predator release and hence a relaxation in competition (see Vance-Chalcraft et al. 2007; Ritchie & Johnson 2009), whereby smaller or intermediate-sized carnivore species, which may have avoided certain habitats due to competition, are less likely to be so constrained, and hence might use a wider range of habitats than would be apparent in ecosystems with a full complement of species. If this hypothesis is correct, and is responsible for the differences observed, then we should see an increase in habitat specialization as competition increases. Given how little is known about many of these species in this analysis, our results present a first approach to determining species habitat use in relation to other species, that should be tested with more detailed data, across different scales, should it become available.
The lack of a significant relationship between body size and habitat selectivity, for both marginality and tolerance scores, suggests that niche breadth does not correlate with body size in this landscape. This finding contradicts the hypothesis that species should become less selective as body mass increases (Brown & Maurer 1989). Furthermore, we found no evidence that habitat partitioning increased within taxon groups such as felids and canids or within the large carnivore guild (Table 3). Instead, two medium-sized species, African civet and white-tailed mongoose, seemed to be most dissimilar to the other species examined here. However, our study is in agreement with findings elsewhere based on measures of diet breadth, which show no variation in prey size range and body size within the large carnivore guild (Radloff & Du Toit 2004; Owen-Smith & Mills 2008); matching findings in other taxa (Costa et al. 2008; Costa 2009). We are aware of only one study which tested the hypothesis on niche-based parameters that are not diet based; Pyron (1999) used geographic range of fish species as an indicator of habitat breadth and demonstrated a relationship with body size for both sunfish and suckers. However, our study is the first, to our knowledge, to use an ENFA-based approach to investigate habitat niche breadth in carnivores. Our approach could be used to investigate similar questions for other taxa and ecosystems, and represents a potentially powerful tool to increase our understanding of the distribution of species within community assemblages.
Acknowledgements
We gratefully acknowledge Tanzania National Parks (TANAPA), Ngorongoro Conservation Area Authority (NCAA), Wildlife Division, the Tanzania Wildlife Research Institute (TAWIRI) and the Tanzania Commission for Science and Technology (CosTech) for providing permission to conduct field work in the Serengeti. We would like to also thank the Tanzania Carnivore Centre team for the camera trap survey data; the Serengeti Cheetah Project, volunteers on the Serengeti Carnivore Survey, contributors to the Tanzania Carnivore Atlas for sighting data; the Serengeti Viral Transmission Dynamics Project (especially B. Chunde, C. Mentzel and T. Lembo) and the Serengeti Biodiversity Project (A.R.E. Sinclair, J. Fryxell, S. Mduma and J. Masoy) for the night transect data; Kris Metzger for the short grassland GIS layer; and M.E.C. and the Serengeti Lion Project (C. Packer, I. Taylor, K. Skinner, H. Brink, I. Jansson, P. Jigsved) for daytime tracklog data. The Tanzania Carnivore Program, Serengeti Cheetah Project and the Serengeti Carnivore Census were supported by the Darwin Initiative, the Wildlife Conservation Society, St Louis Zoo WildCare Institute and Frankfurt Zoological Society. The night transects were supported by NSF grant EF-0225453, Lincoln Park Zoo and Canadian NSERC funding. The daytime tracklog sightings were supported by NSF grants awarded to Craig Packer (DEB-0343960, DEB-0308486) with support to M.E.C. by NSF grants (DEB-0710070 & OISE-0804186). K.H. was supported by NSF grant DEB-0513994, Pew Charitable Trusts Award 2000-002558, the Leverhulme Trust, the Heinz Foundation and the Wellcome Trust. Finally, we would like to thank Tim Blackburn and two anonymous referees for their comments on previous drafts of this manuscript.
References
- Anderson BJ, Armsworth PR, Eigenbrod F, Thomas CD, Gillings S, Heinemeyer A, Roy DB, Gaston KJ. Spatial covariance between biodiversity and other ecosystem service priorities. Journal of Applied Ecology. 2009;46:888–896. [Google Scholar]
- Banavar JR, Damuth J, Maritan A, Rinaldo A. Supply-demand balance and metabolic scaling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10506–10509. doi: 10.1073/pnas.162216899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay RMR, Brigham RM. Prey detection, dietary niche breadth, and body size in bats – why are aerial insectivorous bats so small? The American Naturalist. 1991;137:693–703. [Google Scholar]
- Begg CM, Begg KS, Du Toit JT, Mills MGL. Life-history variables of an atypical mustelid, the honey badger Mellivora capensis. Journal of Zoology. 2005;265:17–22. [Google Scholar]
- Begon M, Harper JL, Townsend CR. Ecology: Individuals, Populations and Communities. Blackwell Scientific Publications; Oxford: 1990. [Google Scholar]
- Bowland JM. Diet, Home Range and Movement Patterns of Serval on Farmland in Natal. Department of Zoology and Entomology; University of Natal, Pietermaritzburg: 1990. pp. 1–96. [Google Scholar]
- Boyes RS, Perrin MR. Generalists, specialists and opportunists: niche metrics of Poicephalus parrots in southern Africa. Ostrich. 2009;80:93–97. [Google Scholar]
- Brandl R, Kristin A, Leisler B. Dietary niche breadth in a local-community of passerine birds, an analysis using phylogenetic contrasts. Oecologia. 1994;98:109–116. doi: 10.1007/BF00326096. [DOI] [PubMed] [Google Scholar]
- Brown JH, Maurer BA. Macroecology – the division of food and space among species on continents. Science. 1989;243:1145–1150. doi: 10.1126/science.243.4895.1145. [DOI] [PubMed] [Google Scholar]
- Caro TM. Cheetahs of the Serengeti Plains: Group Living in an Asocial Species. University of Chicago Press; Chicago: 1994. [Google Scholar]
- Caso MSA. Leopard Pilot Population Study at Rungwa/Piti Ecosystem, Report. A report to the Tunzanian Wildlife Division, Dar es Salaam, Tanzania; Tanzania, East Africa: 2002. [Google Scholar]
- Chefaoui RM, Hortal J, Lobo JM. Potential distribution modelling, niche characterization and conservation status assessment using GIS tools: a case study of Iberian Copris species. Biological Conservation. 2005;122:327–338. [Google Scholar]
- Cianfrani C, Le Lay G, Hirzel AH, Loy A. Do habitat suitability models reliably predict the recovery areas of threatened species? Journal of Applied Ecology. 2010;47:421–430. [Google Scholar]
- Costa GC. Predator size, prey size, and dietary niche breadth relationships in marine predators. Ecology. 2009;90:2014–2019. doi: 10.1890/08-1150.1. [DOI] [PubMed] [Google Scholar]
- Costa GC, Vitt LJ, Pianka ER, Mesquita DO, Colli GR. Optimal foraging constrains macroecological patterns: body size and dietary niche breadth in lizards. Global Ecology and Biogeography. 2008;17:670–677. [Google Scholar]
- Davies RG, Orme CDL, Storch D, Olson VA, Thomas GH, Ross SG, Ding TS, Rasmussen PC, Bennett PM, Owens IPF, Blackburn TM, Gaston KJ. Topography, energy and the global distribution of bird species richness. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2007;274:1189–1197. doi: 10.1098/rspb.2006.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant SM. Competition refuges and coexistence: an example from Serengeti carnivores. Journal of Animal Ecology. 1998;67:370–386. [Google Scholar]
- Durant SM. Living with the enemy: avoidance of hyenas and lions by cheetahs in the Serengeti. Behavioral Ecology. 2000;11:624–632. [Google Scholar]
- Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation. 1997;24:38–49. [Google Scholar]
- Gittleman JL, Purvis A. Body size and species-richness in carnivores and primates. Proceedings of the Royal Society of London, Series B: Biological Sciences. 1998;265:113–119. doi: 10.1098/rspb.1998.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier DS. A unifying explanation for diverse metabolic scaling in animals and plants. Biological Reviews. 2010;85:111–138. doi: 10.1111/j.1469-185X.2009.00095.x. [DOI] [PubMed] [Google Scholar]
- Hirzel AH, Hausser J, Perrin N. Biomapper 3.1. Division of Conservation Biology. University of Bern; 2004. Available at: http://www.unil.ch/biomapper. [Google Scholar]
- Hirzel AH, Hausser J, Chessel D, Perrin N. Ecological-niche factor analysis: how to compute habitat-suitability maps without absence data? Ecology. 2002;83:2027–2036. [Google Scholar]
- Hirzel AH, Le Lay G, Helfer V, Randin C, Guisan A. Evaluating the ability of habitat suitability models to predict species presences. Ecological Modelling. 2006;199:142–152. [Google Scholar]
- Jackson DA. Stopping rules in principal components analysis: a comparison of heuristic and statistical approaches. Ecology. 1993;74:2204–2214. [Google Scholar]
- Kingdon J. East African Mammals: An Altas of Evolution in Africa, Volume IIIA Carnivores. Academic Press; London: 1977. [Google Scholar]
- Kruuk H. The Spotted Hyaena: A Study of Predation and Social Behavior. University of Chicago; Chicago: 1972. [Google Scholar]
- Levin SA. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. [Google Scholar]
- Loyola RD, Oliveira-Santos LGR, Almeida-Neto M, Nogueira DM, Kubota U, Diniz-Filho JAF, Lewinsohn TM. Integrating economic costs and biological traits into global conservation priorities for carnivores. PLoS ONE. 2009;4:e6807. doi: 10.1371/journal.pone.0006807. doi:10.1371/journal.pone.0006807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas B, Macdonald DW. In: Bat-eared foxes. Biology and Conservation of Wild Canids. Macdonald DW, Sillero-Zuberi C, editors. Oxford University Press; Oxford: 2004. pp. 227–242. [Google Scholar]
- Macdonald DW. The Encyclopedia of Mammals. Unwin Hyman Limited; London: 1989. [Google Scholar]
- Mayhew P. Discovering Evolutionary Ecology. Oxford University Press; Oxford: 2006. [Google Scholar]
- Mduma SAR, Hopcraft JGC. The main herbivorous mammals and crocodiles in the greater Serengeti ecosystem. In: Sinclair ARE, Packer C, Mduma SAR, Fryxell JM, editors. Serengeti III: Human Impacts on Ecosystem Dynamics. University of Chicago; Chicago: 2008. pp. 497–505. [Google Scholar]
- Mills MGL, Freitag S, van Jaarsveld AS. Geographic priorities for carnivore conservation in Africa. In: Gittleman JL, Funk SM, Macdonald DW, Wayne RK, editors. Carnivore Conservation. Cambridge University Press; Cambridge: 2001. pp. 467–483. [Google Scholar]
- Novotny V, Basset Y. Body size and host plant specialization: a relationship from a community of herbivorous insects on Ficus from Papua New Guinea. Journal of Tropical Ecology. 1999;15:315–328. [Google Scholar]
- Nowell K, Jackson P. Wild Cats: Status Survey and Conservation Action Plan. IUCN/SSC Cat Specialist Group, Gland; Switzerland: 1996. [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’Amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Ricketts TH, Kura Y, Lamoreux JF, Wettengel WW, Hedao P, Kassem KR. Terrestrial ecoregions of the worlds: a new map of life on Earth. BioScience. 2001;51:933–938. [Google Scholar]
- Owen-Smith N, Mills MGL. Predator-prey size relationships in an African large-mammal food web. Journal of Animal Ecology. 2008;77:173–183. doi: 10.1111/j.1365-2656.2007.01314.x. [DOI] [PubMed] [Google Scholar]
- Peters RH. The Ecological Implications of Body Size. Cambridge University Press; Cambridge: 1983. [Google Scholar]
- Pettorelli N, Hilborn A, Broekhuis F, Durant SM. Exploring habitat use by cheetahs using ecological niche factor analysis. Journal of Zoology. 2009;277:141–148. [Google Scholar]
- Pettorelli N, Lobora AL, Msuha MJ, Foley C, Durant SM. Carnivore biodiversity in Tanzania: revealing the distribution patterns of secretive mammals using camera traps. Animal Conservation. 2010;13:131–139. [Google Scholar]
- Pita R, Mira A, Moreira F, Morgado R, Beja P. Influence of landscape characteristics on carnivore diversity and abundance in Mediterranean farmland. Agriculture, Ecosystems & Environment. 2009;132:57–65. [Google Scholar]
- Pyron M. Relationships between geographical range size, body size, local abundance, and habitat breadth in North American suckers and sunfishes. Journal of Biogeography. 1999;26:549–558. [Google Scholar]
- Radloff FGT, Du Toit JT. Large predators and their prey in a southern African savanna: a predator’s size determines its prey size range. Journal of Animal Ecology. 2004;73:410–423. [Google Scholar]
- Rahbek C, Graves GR. Multiscale assessment of patterns of avian species richness. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4534–4539. doi: 10.1073/pnas.071034898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie EG, Johnson CN. Predator interactions, mesopredator release and biodiversity conservation. Ecology Letters. 2009;12:982–998. doi: 10.1111/j.1461-0248.2009.01347.x. [DOI] [PubMed] [Google Scholar]
- Santos X, Brito JC, Sillero N, Pleguezuelos JM, Llorente GA, Fahd S, Parellada X. Inferring habitat-suitability areas with ecological modelling techniques and GIS: a contribution to assess the conservation status of Vipera latastei. Biological Conservation. 2006;130:416–425. [Google Scholar]
- Santos X, Brito JC, Caro J, Abril AJ, Lorenzo M, Sillero N, Pleguezuelos JM. Habitat suitability, threats and conservation of isolated populations of the smooth snake (Coronella austriaca) in the southern Iberian Peninsula. Biological Conservation. 2009;142:344–352. [Google Scholar]
- Sattler T, Bontadina F, Hirzel AH, Arlettaz R. Ecological niche modelling of two cryptic bat species calls for a reassessment of their conservation status. Journal of Applied Ecology. 2007;44:1188–1199. [Google Scholar]
- Schaller GB. The Serengeti Lion: A Study of Predator-Prey Relations. University of Chicago Press; Chicago: 1972. [Google Scholar]
- Schoener TW. Sizes of feeding territories among birds. Ecology. 1968;49:123. [Google Scholar]
- Shriner SA, Wilson KR, Flather CH. Reserve networks based on richness hotspots and representation vary with scale. Ecological Applications. 2006;16:1660–1673. doi: 10.1890/1051-0761(2006)016[1660:rnborh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sillero-Zubiri C, Hoffmann M, Macdonald DW. Canids: Foxes, Wolves, Jackals and Dogs, Status Survey and Conservation Action Plan. IUCN/SSC Canid Specialist Group, Gland; Switzerland and Cambridge, UK: 2004. [Google Scholar]
- Sinclair ARE, Arcese P. Serengeti II: Dynamics, Management and Conservation of an Ecosystem. University of Chicago Press; Chicago: 1995. [Google Scholar]
- Stuart CT. Notes on the mammalian carnivores of the Cape Province, South Africa. Bontebok. 1981;1:1–58. [Google Scholar]
- Taylor ME. Bdeogale crassicauda. Mammalian Species. 1987;294:1–4. [Google Scholar]
- Vance-Chalcraft HD, Rosenheim JA, Vonesh JR, Osenberg CW, Sih A. The influence of intraguild predation on prey suppression and prey release: a meta-analysis. Ecology. 2007;88:2689–2696. doi: 10.1890/06-1869.1. [DOI] [PubMed] [Google Scholar]
- Waser PM, Elliot LF, Creel NM, Creel SR. Habitat variation and mongoose demography. In: Sinclair ARE, Arcese P, editors. Serengeti II: Dynamics, Management, and Conservation of an Ecosystem. University of Chicago Press; Chicago: 1995. pp. 421–447. [Google Scholar]
- Wayne RK, Van Valkenburgh B, Kat PW, Fuller TK, Johnson WE, O’Brien SJ. Genetic and morphological divergence among sympatric canids. Journal of Heredity. 1989;80:447–454. doi: 10.1093/oxfordjournals.jhered.a110896. [DOI] [PubMed] [Google Scholar]
- Williams JB, Anderson MD, Richardson PRK. Seasonal differences in field metabolism, water requirements, and foraging behavior of free-living aardwolves. Ecology. 1997;78:2588–2602. [Google Scholar]
- Wilson DE, Reeder DM. Mammal Species of the World. A Taxonomic and Geographic Reference. Johns Hopkins University Press; Baltimore, Maryland: 2005. [Google Scholar]
- Xuezhi W, Weihua X, Zhiyun O, Jianguo L, Yi X, Youping C, Lianjun Z, Junzhong H. Application of ecological-niche factor analysis in habitat assessment of giant pandas. Acta Ecologica Sinica. 2008;28:821–828. [Google Scholar]