Abstract
We assessed the relationship between open-source data on net primary production and precipitation for the southern Mongolian Gobi, and related this information to data obtained from a set of 1418 vegetation relevés sampled in the region. Gradients determining plant community diversity and composition were examined, and the relation between α-diversity and key environmental parameters was tested.
The correlation between net primary production and precipitation within our working area was fairly high (r2 = 0.66). The variance of the net primary production was related to the average annual precipitation; at sites with more than ~220 mm/a precipitation the median coefficient of variation in productivity data decreased, indicating a rather gradual shift from a non-equilibrium ecosystem towards an equilibrium ecosystem with increasing moisture. A DCA-ordination showed that the main gradient in plant community composition was closely correlated to environmental variables for altitude, precipitation and net primary production. All three parameters were also significant predictors of the species diversity. The final model, which included an additional quadratic term for longitude, predicted local plant biodiversity at r2 = 0.57.
The results can be directly applied to both resource management and nature conservation within the area. For future studies a closer focus on the characterisation of non-equilibrium rangelands based on modelled productivity layers is suggested.
Keywords: Net primary production, biodiversity, precipitation variability, non-equilibrium system, Gobi desert, GLOPEM, plant community composition
Introduction
The relation between species richness, plant community composition and productivity forms one of the most fundamental topics in ecology, which is still subject of vivid discussions (Mittelbach et al., 2001). Global comparisons reveal complex patterns differing between ecosystems (Waide et al., 1999, Hawkins et al., 2003), and studies from arid environments show varying results. Some authors describe linear relationships, while others found hump-shaped patterns, especially where the assessed productivity gradients were long (Garcia et al., 1993, Gough et al., 1994, Waide et al., 1999, Mittelbach et al., 2001, Alhamad, 2006, Cox et al., 2006, Adler & Levine, 2007).
Large-scale analyses often suffer from a lack of sufficient numbers of comparable samples. Large vegetation databases offer increasingly popular data sources (Tichy, 2005), although assessments of species richness based on traditional vegetation samples may be somewhat biased (Chytry, 2001). Unfortunately, most of the available large datasets cover the comparatively well-studied regions of moist western Eurasia. For central Eurasia, Russian and Mongolian botanists have also collected large numbers of vegetation samples, but no formal statistical evaluation has been conducted as yet. To our knowledge, no publication addresses the relationship between local plant community composition and productivity in the region, which seems surprising because Central Asian drylands are expected to exhibit close climatic controls (Christensen, 2001, Christensen et al., 2004). We aimed at closing this gap by conducting an analysis of a large set of vegetation samples collected in the Gobi of southern Mongolia between 1996 and 2005.
Even where other data on plant communities are available, lack of data on productivity or similar proxies often renders formal correlation analysis difficult. Precipitation is the main driver of productivity within arid environments (Ludwig, 1987), and thus species richness is expected to correlate with precipitation. An open-source climatic extrapolation model has recently become available offering averaged data on a suitable spatial scale (Hijmans et al., 2005). Precipitation data can be used to estimate productivity in drylands (Le Houerou, 2001, Huxman et al., 2004), but there are other, less indirect approaches to productivity assessment. Remote sensing offers suitable methods for ecological assessments of large areas (Nagendra, 2001, Turner et al., 2003). NOAA-AVHRR is an established standard tool in ecology to estimate primary productivity (Campbell, 1996). NDVI transformations (Tucker, 1979) derived from this satellite are closely related to above ground primary production (Paruelo et al., 1997, Pineiro et al., 2006); this relation has been confirmed for Central Asian drylands (Purevdorj et al., 1998, Bai et al., 2004, Yu et al., 2004). Data are thus widely applied in rangeland ecology (e.g. Hobbs, 1995, Schmidt & Karnieli, 2000, Weiss et al., 2001, Al-Bakri & Taylor, 2003, Anyamba & Tucker, 2005); recent studies focused on degradation (Bastin et al., 1995, Weiss et al., 2001, Holm et al., 2003) and other aspects of rangeland dynamics (Oesterheld et al., 1998).
NOAA-based net primary production estimates, which model net CO2 assimilation (total assimilation minus respiration losses, Goetz et al., 1999), have become available on a global scale (Tucker et al., 2005). Thanks to the relatively narrow recording intervals (days), data are easily available as annual means and can be assessed down to the level of weeks. This facilitates insights into the interannual variability of productivity, which is high in dry rangelands and crucial for their ecology (Fernandez-Gimenez & Allen-Diaz, 1999, Vetter, 2005). Annually derived satellite-based productivity estimates thus offer suitable tools (Lim & Kafatos, 2002) to account for the high variability of the rainfall, and thus productivity in drylands including those of southern Mongolia.
Topography is also of fundamental importance to plant community composition (Gough et al., 1994). SRTM-datasets offer an open-source high-resolution digital elevation model (Jarvis et al., 2006).
For the present paper, we combined spatial data for the drylands of southern Mongolia in a GIS with a dataset comprising 1418 vegetation checks from the southern Mongolian Gobi, which included data on plant community composition, species richness and relative abundance. We evaluated relationships among the environmental variables and assessed whether the values are reasonable. In a second step, environmental data was related to our vegetation data. Specifically, we asked the following questions:
Spatial data: How is productivity related to mean annual precipitation? What is the spatial and interannual variability in productivity (Weiss et al. 2001)?
Vegetation data: Are there gradients in plant community diversity and composition?
Vegetation data: Are trends related to the available environmental variables (Nagendra & Gadgil, 1999, Oindo & Skidmore, 2002)?
Vegetation data: What are the relationships between α-diversity and productivity data?
Methods
Working area
The southern Mongolian Gobi is an arid ecosystem (Lehmkuhl, 1997). Only a few mountain ranges (Map 1) receive higher precipitation levels and hence support denser mountain steppes (Hilbig, 1995), whereas semi-deserts dominate the largest part of the region. True deserts with, at most, contracted vegetation are found in southern central Mongolia (von Wehrden et al., 2006a). These gain only episodic rainfall due to their being situated between the two principal climatic circulation systems (von Wehrden & Wesche, 2007). The western Gobi still receives rain from disturbances originating over the Mediterranean basin, which is reflected in the unique flora found in the Dzungarian Gobi (Meusel et al., 1965, Jäger et al., 1985, von Wehrden et al., in press). In eastern Mongolia, the climate regime is influenced by the east-Asian monsoon (Weischet & Endlicher, 2000, Wesche et al., 2005a); hence many floristic elements found there show an east-Asian distribution (Wesche et al., 2005b). However, most of the dominant plant species in the Gobi are typical drought-adapted Central Asian elements and occur throughout the entire study region.
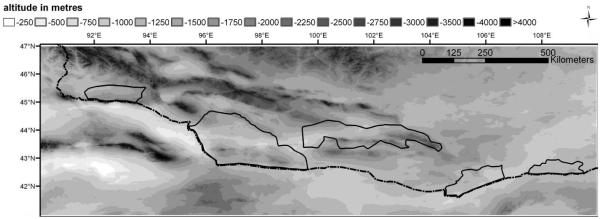
Map 1.
Altitude of the working area, derived from the SRTM-set. The dotted line indicates the southern border of (Outer) Mongolia; the black lined areas indicate the protected areas of the working area
The rangelands of the southern Mongolian Gobi have been grazed by livestock for millennia (Fernandez-Gimenez, 1999); but improvements during the last century in both well digging and veterinarian maintenance (Fernandez-Gimenez, 2006) have led to increased livestock numbers. The political changes in the early 1990′s resulted in additional transformations; in some parts of the Mongolian Gobi the number of goats doubled within the last decade of the 20th century (National Statistical Office of Mongolia, 2003). At the same time, the spatial extent of protected areas increased more than two-fold (Reading et al., 1999, Reading et al., 2006), but population dynamics of the endangered species in the area show worrying (Milner-Gulland & Lhagvasuren, 1998), if not alarming trends (Zevegmid & Dawaa, 1973, Reading et al., 2001, Mix et al., 2002).
Plant community data
In 1996 and from 2001-2005, our working group collected 1418 vegetation samples during a survey of all southern Mongolian nature reserves (von Wehrden & Wesche, 2005). Sampling followed a modified Braun-Blanquet approach (Mueller-Dombois & Ellenberg, 1974, Mucina et al., 2000), with a constant plot size of 10 × 10 m. This seemed appropriate as plots of 100m2 have been shown to capture most of the local plant species diversity (α-diversity) in Central Asian drylands (e.g. He et al., 2006). Plots were deliberately chosen based on printouts of suitably transformed LANDSAT data to ensure that they are representative for a given area and that all relevant vegetation formations were covered; plots were located using a hand-held GPS (see von Wehrden et al., 2006b for details). Detailed vegetation descriptions were compiled for all regions (von Wehrden, 2005, Hilbig & Tungalag, 2006, von Wehrden et al., 2006a, von Wehrden & Wesche, in prep., von Wehrden et al., in press). For the present study, we assigned all relevés to major vegetation units. A finer classification is currently in preparation (von Wehrden & Wesche, in prep.), but for the present paper a classification approximately equivalent to the level of formations, alliances and orders (in phytosociological terminology; Hilbig, 1995, 2000) seemed sufficient (see Tab. 1). Some heterogeneous groups were merged based on their habitat background (namely alpine vegetation and salt adapted communities). The complete dataset was compiled using TABWIN (http://www.uni-olden-burg.de/landeco/21346.html) and then processed with the JUICE-package (Tichy, 2002).
Table 1.
No. of relevés used in the present study, split by principal vegetation units
| Vegetation unit | no. of relevés |
|---|---|
| Alpine vegetation | 128 |
| Mountain steppes | 179 |
| Pediment steppes | 77 |
| Caragana leucophloea-scrub | 185 |
| Anabasis brevifolia-desert steppes | 374 |
| Haloxylon ammodendron-dry scrub | 193 |
| Desert scrub (Reaumuria, Salsola) | 127 |
| Salt-tolerant vegetation | 155 |
Remote sensing and GIS-data
The SRTM-tiles (Jarvis et al., 2006) for the northern Gobi (i.e. southern Mongolia and northern China, cf. Map 1) were merged into one file covering the whole working area with a spatial resolution of 90 metres. Slope and aspect were extracted from this dataset; for the statistical analysis, aspect was recoded into non-circular north-southern and west-eastern values using sine and cosine transformations. Extrapolated climate data were obtained from the model by Hijmans et al. (2005), which is based on a one-kilometre grid. The layers represent modelled mean monthly minimum and maximum temperatures as well as the averaged precipitation from 1950-2000. Since it is an interpolated model, the datasets may contain errors due to the low density of climatic stations in our working area, and due to the pronounced relief of the landscape. Nevertheless, data correspond surprisingly well to short-term measurements obtained by our group in a mountain region of southern Mongolia, and should offer a sufficient accuracy for the present analysis.
GLOPEM-data was obtained from the Global Landcover Facility (see Tab. 2). This product is largely based on NOAA-AVHRR satellite data and offers a robust estimate of combined net above- and below-ground carbon assimilation (Prince & Goward, 1995), which is a better indicator of productivity than raw NDVI-values (Goetz et al., 1999). The climate datasets were extracted using DIVA-GIS (ver. 5.2), and then combined with all other layers within ArcMap (ver. 8.2). A grid-based shapefile matching the resolution of the NOAA-AVHRR-based productivity data (8 × 8 km) was derived for the whole working area, and all layers were collated into a common database file. All raster data were sub-sampled to the GLOPEM-resolution by averaging the values (e.g. the SRTM-set). The GIS was amended by vector layers available from the Global Landcover Facility on boundaries of those reserves recognized by the IUCN (WDPA Consortium, 2006).
Table 2.
Spatially explicit data used for the present study
For analyses with the vegetation relevés all spatial datasets were extracted based on the highest spatial resolution and compiled into a complete data matrix.
Statistical analysis
We analysed the environmental data with correlation and regression analysis and refrained from performing formal spatial analysis. Minimum, maximum, mean and median values were derived for the complete set of net primary production layers. Since standard deviation strongly depends on the absolute net primary production of any given pixel, the coefficient of variance was calculated in order to normalise the variance over the mean values (Weiss et al., 2001). All spatially comprehensive data were analysed for covariance and redundant information using Principal Components Analysis (data centred and standardised) and further regressions. Species-area curves were computed for all vegetation units using the EstimateS-software (http://viceroy.eeb.uconn.edu/EstimateS). Relevé composition and its environmental background was analysed using Detrended Correspondence Analysis (Hill & Gauch, 1980), which is a robust ordination technique for datasets with large gradients. For ordination analysis, data were transformed into presence/absence; rare species were down-weighted, extra-zonal vegetation was excluded. Further steps of analysis again involved simple correlations and regressions. Multiple regressions were calculated based on knowledge obtained from the ordinations.
Ordinations were performed using CANOCO software (ter Braak & Smilauer, 2002). For other calculations the R-package was used (R Development Core Team, 2005). The results of the linear models were exported as Excel files and linked to the GIS, where some raster data were visualized (e.g. annual precipitation).
Results
Both results and discussion are divided according to the two principal types of datasets processed within this study. The first parts deal with the spatial data derived from both remotely sensed and GIS layers, which are related to the vegetation data in the second parts.
Environmental datasets
The PCA ordination of the spatial data indicates that the predictors can be divided into three groups, which contain largely redundant information (analysis not shown). All temperature variables and the slope vector correlated highly and positively with the first axis, which also captured the closely, yet inversely related elevation gradient. Mean precipitation was also negatively correlated with the first axis, as was the net primary production. The vectors for monthly precipitation point in slightly different directions; the winter precipitation loads also on the second axis. The coefficient of variance shows no mentionable correlation with any other parameter.
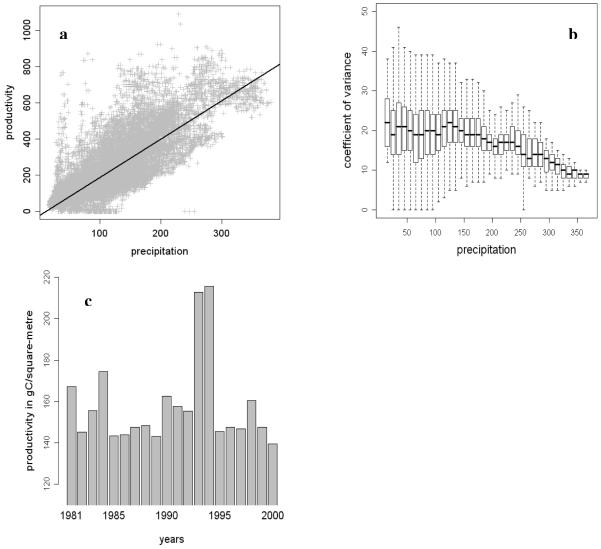
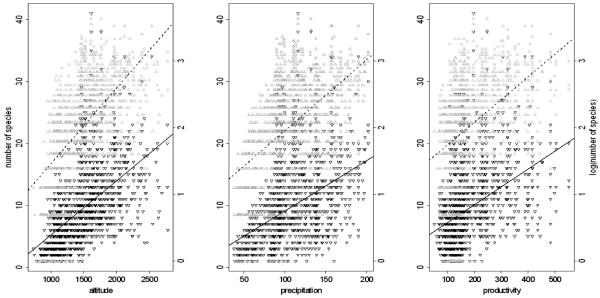
A map of the area shows that mean net primary production is indeed related to mean annual precipitation (Map 2) and this is confirmed by correlation analysis (r2 = 0.66, see Fig. 1a). The slope of the respective linear regression is b = 2.21. The coefficients of variance in net primary production were generally high at around 20% (Fig. 1b), and decreased only above a mean annual precipitation of 220 mm (equivalent to an estimated productivity of some 500 kgC/ha). The high interannual variability was also apparent when data were averaged over the entire study region and expressed on an annual basis (Fig. 1c).
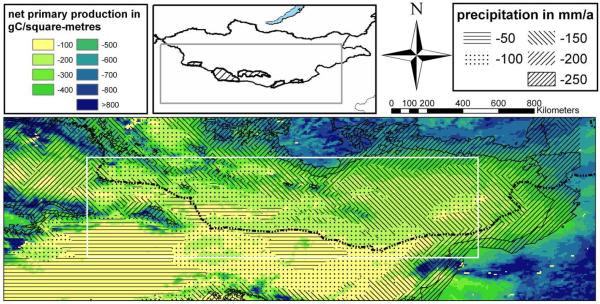
Map 2.
Productivity (colour codes) and precipitation (shadings) in Central Asia. For data sources see Tab. 2. The black dotted line indicates the southern border of Outer Mongolia. The white box demarcates the area, which was analysed for this work
Fig. 1. Trends in productivity data for the northern Gobi.
1a: Regression plot between the modelled mean annual precipitation and the estimated mean productivity (in gC/square metre, computed over the years from 1981-2000). The black line represents the linear model (r2 =0.66)
1b: Coefficients of variance (of the net primary production, computed for 1981-2000) plotted against the averaged precipitation, which is pooled within 10 mm/a intervals
1c: Changes in mean annual mean net primary production from 1981 to 2000
Vegetation data
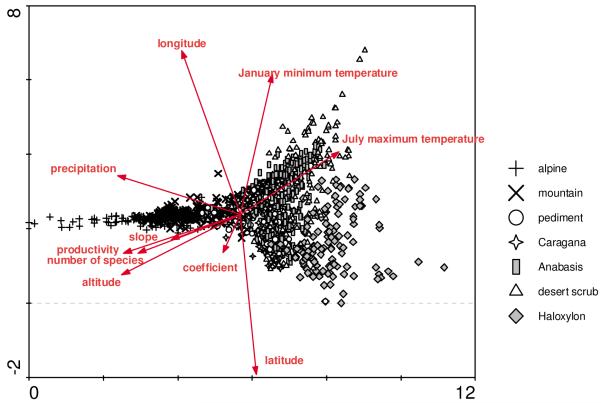
The species-area curves derived for the different formations show initially steep and then more smoothly rising curves for all drier formations (Fig. 2); only the alpine vegetation and, to some extent, the extrazonal vegetation of saline sites showed a different pattern indicating a higher ß-diversity. All other formations showed a quite similar slope, although the overall number of species varied somewhat. The ordination of the zonal vegetation (Fig. 3) clearly indicated a pronounced floristic gradient along axis 1 (length 11.2 multivariate standard deviations) and a shorter but similarly pronounced gradient along axis 2 (length 6.8 multivariate standard deviations). The vegetation units formed only partly overlapping clusters. The first axis reflected the gradient from the alpine and mountainous groups to the driest semi-desert and true desert stands. The second axis indicated the differentiation within the semi-desert and desert stands (see Fig. 3).
Fig. 2.
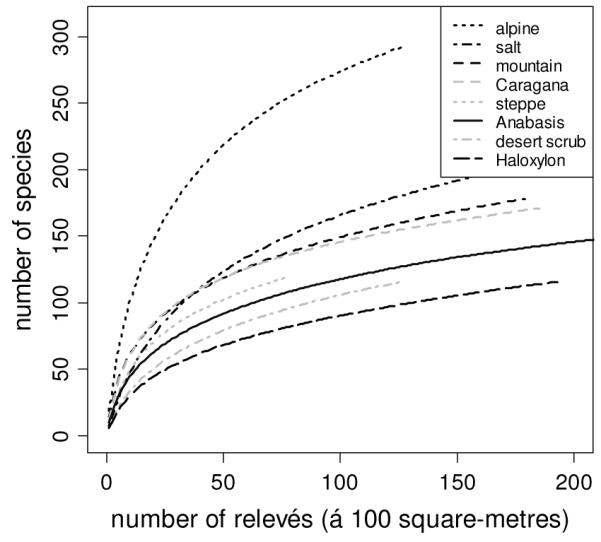
Species-area curves for the 1418 relevés. The classes are described in Table 1
Fig. 3.
Ordination of the zonal vegetation set, salt-adapted vegetation was excluded (presence/absence data, eigenvalue axis 1 = 11.2, axis 2 = 6.8). Environmental vectors were implemented by post hoc correlations with ordination axes; for the vegetation units refer to Table 1
The overlaid vector for species richness was closely related to the alpine vegetation, and vectors for altitude and net primary production pointed in the same direction. Precipitation shows a somewhat different behaviour with some relation to the second axis, which mainly reflects differences in January minimum temperatures and in the variables capturing the geographical coordinates.
The relationship between local plant species richness and the variables of altitude, precipitation and productivity was again confirmed by correlation analysis of raw and log-transformed richness data (Fig. 4). In bivariate analyses, altitude showed the closest correlation with species richness, followed by mean precipitation and mean productivity (Tab. 3). A multiple regression (forward selection) indicated that four environmental variables contributed significantly to the model, which explained more than 50% of the total variance in the diversity data (log-transformed, Tab. 3).
Fig. 4.
Regression plots of the number of species (indicated by black triangles and lines) and log-scaled number of species (indicated by grey triangles and hatched lines). The r2− values of the given predictors are shown in Table 3
Table 3.
Environmental parameters implemented in the linear models. Models were calculated for all samples save those from extrazonal saline sites
| Model | response | predictor | r2 |
|---|---|---|---|
| 1 | no. of species | altitude | 0.29 |
| 2 | no. of species | precipitation | 0.22 |
| 3 | no. of species | productivity | 0.19 |
| 4 | no. of species | altitude*precipitation*productivity | 0.38 |
| 5 | no. of species | altitude*precipitation*productivity*(longitude)2 | 0.45 |
| 6 | log (no. of species) | altitude | 0.35 |
| 7 | log (no. of species) | precipitation | 0.27 |
| 8 | log (no. of species) | productivity | 0.19 |
| 9 | log (no. of species) | altitude*precipitation*productivity | 0.51 |
| 10 | log (no. of species) | altitude*precipitation*productivity*(longitude)2 | 0.57 |
Discussion
Spatial dataset
The altitudinal gradients shown in Map 1 clearly determine both precipitation and net primary production. The mountain regions gain the highest rainfall and hence show the highest C-assimilation. Moreover, the low temperatures reduce evapotranspiration and contribute to the moisture surplus in the mountains. Map 2 highlights the close relationship between precipitation and net primary production, and both parameters show similar spatial patterns including an altitudinal increase. Apart from the vertical gradient, there are also horizontal gradients related to large-scale circulation systems. The extent of the two main precipitation regimes is indicated by the relatively high values of productivity in the east and in the west of the study area, while the net primary production is low in the region at around 100° eastern latitude (see Map 1 & Map 2). This area is known as a biogeographical border determining the distribution of certain Gobi endemics (Wesche et al., 2005b) and also forms the easternmost distribution limit for many western species or the westernmost limit for eastern species (Meusel et al., 1965, Hilbig, 1995, Jäger, 2005). In southern Mongolia, this longitude corresponds to the floristic sub-region of the Transaltay Gobi (Grubov, 2000ff, 2001), which lacks the mountain steppes that are found in other mountainous parts of the Gobi (von Wehrden et al., 2006a). It is the poorest region in Mongolia with respect to both precipitation (von Wehrden & Wesche, 2007) and net primary production, and low levels of land use may be the main reason why it has become a retreat zone for Mongolia’s wildlife (Zevegmid & Dawaa, 1973). Comparably dry spots are found in the Chinese Alashan Gobi (Kürschner, 2004) as well as in the Taklamakan in northern China (Map 2).
The close correlation between precipitation and net primary production (see Fig. 1a) is typical of arid environments (Le Houerou, 1984), and data from Inner Mongolia suggest a roughly comparable relationship (Bai et al., 2004). The estimates of net primary production given for several particular ecosystems by Prince & Goward (1995) are well in line with the values we obtained for the Gobi. Our estimates for rainfall efficiency are not directly comparable to those reported for most other studies because these mainly measured aboveground biomass, which represents only a small proportion of the net primary production (Gill et al., 2002). Direct in situ measurements of above- and below-ground standing crop by Titlyanova et al. (1999) suggest that between 80-90% of standing crop are found belowground in dry steppes, data from northern Chinese drylands point in the same direction (Ni, 2004). A study by Huxman et al. (2004) gives an RUE/regression slope of b = 5.67 (mm rain vs. kg dry biomass/ha*year) over the precipitation range described here, which is intermediate among values reported by Le Houerou (1984, 4.6 - 7.6). An analysis of data from northern China indicated a slope of b = 6.61 (Ni, 2004). We obtained an RUE of b = 2.12 when expressed as gC/m2, or b = 21.2 for kgC/ha respectively. If we assume that 90% of productivity occurs below ground (Titlyanova et al., 1999), and that C-content of dry biomass is around 40%, we arrive at a slope of b = 5.3 (kg dry biomass/ha*year), which is reasonably close to previously published values. A certain bias may have been introduced from the precipitation models where data are extrapolated from a limited number of stations. However, an RUE of around 5.3 corresponds reasonably well to in situ measurements of precipitation and aboveground standing crop obtained by our group in the Gobi Altay (Wesche et al., submitted).
A generally decreasing aridity and thus productivity from the 1980′s compared to the 1990′s as reported by Lioubimtseva et al. (2005) is not supported by our data. The early 1990′s were instead blessed with relatively favourable conditions. This facilitated the widely reported increase in livestock numbers in the Mongolian part of the Gobi (National Statistical Office of Mongolia, 2003), but drier conditions from 2000 onwards brought herds back to pre-transformation numbers (Reading et al., 2006).
Relevé dataset
Our species-area curves showed that patterns of richness differed among vegetation units (Fig. 2). The alpine ecosystems include mountain steppes, some shrubby formations (Wesche et al., 2005c), and even a forest community; and mountains clearly host the highest biodiversity within the Mongolian Gobi (Jäger, 2005).
The slightly different shape of the curve for salt-tolerant vegetation is related to the fact that the most extreme sites are covered by very species-poor communities including mono-dominant stands (e.g. species of Salicornia, Crypsis, Suaeda). Stands are clearly not representative for the zonal vegetation and were thus excluded from further ordination and correlation analysis. Mountain steppes and Caragana scrub are somewhat richer in species, while the drier Haloxylon (Saxaul) stands and other semi-desert scrub are less diverse (Wesche et al., 2005a, von Wehrden et al., 2006a, von Wehrden et al., in press). Still, curves for zonal vegetation were largely similar to each other (Fig. 2). All curves indicate that the collection of additional samples would have yielded additional species, which may be a consequence of the sheer size of the working area where supra-regional gradients come into effect. Our plots describe the local richness within a large macro-climatic gradient; thus the lack of a plateau in the species accumulation curves may reflect the gradients in the Central Asian biogeography (sensu Gaston, 2000), indicating trends in γ- rather than in β-diversity.
The overall species pool in southern Mongolia comprises around 800 species (data from Gubanov, 1996). The overall biodiversity is comparable to values from the old world desert belt (Ayyad et al., 2000, Le Houerou, 2001), with the exception of the Namib, which is exceptionally rich in species (Cowling et al., 1998, Breckle, 2006). The Map and data given by Kier et al. (2005) reflect the diversity of the Gobi’s lowlands quite well, while the much higher diversity in the mountains is not indicated; although these often host large fractions of the diversity in desert regions (Breckle, 2006). Thus, even for the rather simple Gobi system, data with higher spatial resolution are urgently needed (Balmford & Gaston, 1999).
The ordination of the relevés illustrates the principal gradients in plant community composition. These are closely correlated to vectors for numbers of species, altitude and productivity. Mean precipitation has some relation with axis 2, which reflects an east - west gradient.
Compared to the major vegetation gradient from montane to desert vegetation along axis 1 of the DCA, the semi-desert vegetation units share a more uniform habitat and species composition. These are differentiated along the second, less important axis. While the January minimum temperature and longitude are positively correlated to the second axis, latitude correlates negatively to the second axis. Winter temperatures are influenced by the Siberian anti-cyclone (Weischet & Endlicher, 2000) and are lower in the west than in the east (see Map 2), which lacks high mountains (Map 1).
Figure 4 gives regressions for the main predictors based on normal scaled and log-scaled species numbers. The optimal multiple regression for species richness included a quadratic term for the longitude (Tab. 3). This is related to the two precipitation regimes mentioned above, with low precipitation in the central Transaltay region. A stepwise regression model yielded an r2 of 0.45 for the raw data, while a logarithmic transformation of species number raised the coefficient of determination to r2 = 0.57 (see Tab. 3). Compared to global models (Kier et al., 2005) this reveals a reasonably high model performance, which is also comparable to available sub-continental models of species richness (Waide et al., 1999, Evans et al., 2005). A potential explanation for the unexplained variance in our species-richness environment models might lie in the influence of grazing, although the (few) available data do not suggest that plant community composition is strongly affected by grazers in our study area nor does it change much over consecutive years (Wesche et al., submitted).
Implications for land use
Though it is not the primary focus of our paper, the productivity data may hold important lessons for land use. The ongoing debate regarding non-equilibrium conditions in dry rangelands (Vetter, 2005) led to the need to quantify relations between livestock density and productivity (Oesterheld et al., 1998). The coefficient of variance in precipitation data (as a proxy for productivity, see Weiss et al., 2001) is seen as an important variable for understanding livestock dynamics. In the more direct analysis presented above, covering 19 years of productivity data, coefficients of variation in net primary production have a median of 18-22% in areas below some 180 mm mean precipitation (see Fig. 1b). They remain between 16 and 20% at precipitation levels up to 220 mm, and decrease only at higher average moisture availability (Fig. 1b & Fig. 1c). Even the relatively moist montane sites, which receive up to 200 mm and have the highest densities of livestock, regularly experience droughts, which induce high animal mortality and force herders to leave the affected region in drought years.
This supports the idea that from 180 to 220 mm precipitation, land use conditions in southern Mongolia gradually change from highly variable non-equilibrium conditions to rather stable equilibrium conditions. This pattern differs from the description by Reading et al. (2006), who regard almost all parts of Outer Mongolia as a non-equilibrium ecosystem.
Large parts within the Outer Mongolian Gobi contain protected areas, which were established within the last decades in order to protect some large herbivores. Wild ass and camel, two gazelle species and the Przewalski horse once had a much larger distribution (Zevegmid & Dawaa, 1973), but are nowadays restricted to the southern drylands with their non-equilibrium conditions. Land-use conflicts with Mongolian herders continue to threaten these species (Fernandez-Gimenez, 2000, Retzer et al., 2006). Spatially explicit knowledge about the pastures of the Gobi might facilitate nature conservation within the area, as protected area boundaries could be reshaped to minimise conflicts between humans and wildlife (Rodrigues & Gaston, 2001, Woinarski & Fisher, 2003).
Further steps may include the incorporation of the spatial model of species diversity into a biodiversity Map of the region to enhance protection schemes of plant biodiversity (Rodrigues & Gaston, 2002, Gaston & Rodrigues, 2003). In order to improve our understanding of the inter-annual and intra-annual variability of this ecosystem, remotely sensed NDVI-based products (e.g. Tucker et al., 2005) as well as GLOPEM-time series should be correlated with Meteosat-based annual precipitation estimates in future studies.
Acknowledgements
G. & S. Miehe made early steps in data collection for this project. Several people, namely K. Appel, M. Beckmann, A. Hilbig, E. J. Jäger, F. Rütrich, A. Tsolmon and D. Walter aided in fieldwork. W. Hilbig also helped with the data analysis. The UNDP project “Conservation of the Great Gobi and its umbrella species” and the Protected Area bureau in Ulaanbataar provided logistical support, and our partners at the National University of Ulaanbataar assisted in organizing the fieldwork. The data source of the Global land cover facility is crucial to our work. J. Hanspach’s help was essential for the statistical analysis and plotting of results, as were comments by K. Ronnenberg on how realistic the data are. H. Zimmermann and D. McCluskey proofread earlier versions of this manuscript and polished our English. Financial support was initially granted by the gtz and the DFG. The Takhi-project (funded by the FWF project P14992) supported work in the Great Gobi B strictly protected area. H. v. Wehrden was supported by the German Academic Exchange Service. Ongoing studies are financed by the FWF (project P18624), in close cooperation with P. Kaczensky and C. Walzer.
References
- Adler PB, Levine JM. Contrasting relationships between precipitation and species richness in space and time. Oikos. 2007;116:221–232. [Google Scholar]
- Al-Bakri JT, Taylor JC. Application of NOAA AVHRR for monitoring vegetation conditions and biomass in Jordan. J. Arid Environ. 2003;54:579–593. [Google Scholar]
- Alhamad MN. Ecological and species diversity of arid Mediterranean grazing land vegetation. J. Arid Environ. 2006;66:698–715. [Google Scholar]
- Anyamba A, Tucker CJ. Analysis of Sahelian vegetation dynamics using NOAA-AVHRR NDVI data from 1981-2003. J. Arid Environ. 2005;63:596–614. [Google Scholar]
- Ayyad MA, Fakhry AM, Moustafa ARA. Plant biodiversity in the Saint Catherine area of the Sinai Peninsula, Egypt. Biodivers. Conserv. 2000;9:265–281. [Google Scholar]
- Bai YF, Han XG, Wu JG, Chen ZZ, Li LH. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature. 2004;431:181–184. doi: 10.1038/nature02850. [DOI] [PubMed] [Google Scholar]
- Balmford A, Gaston KJ. Why biodiversity surveys are good value. Nature. 1999;398:204–205. [Google Scholar]
- Bastin GN, Pickup G, Pearce G. Utility of AVHRR data for land degradation assessment - a case-study. Int. J. Remote Sens. 1995;16:651–672. [Google Scholar]
- Breckle SW. Deserts and biodiversity - is it area- or resource related? J. Arid Land Stud. 2006;16:61–74. [Google Scholar]
- Campbell JB. Introduction to Remote Sensing. Guilford Publications; New York: 1996. [Google Scholar]
- Christensen L. Simulation of vegetation change due to grazing and climate change on the typical steppe. In: Chuluun T, Ojima D, editors. Change and sustainability of pastoral land use systems in temperate and central Asia. Ulanbataar, Mongolia: 2001. [Google Scholar]
- Christensen L, Coughenour MB, Ellis JE, Zuo ZC. Vulnerability of the Asian typical steppe to grazing and climatic change. Climatic Change. 2004;63:351–368. [Google Scholar]
- Chytry M. Phytosociological data give biased estimates of species richness. J. Veg. Sci. 2001;12:439–444. [Google Scholar]
- Cowling RM, Rundel PW, Desmet PG, Esler KJ. Extraordinary high regional-scale plant diversity in southern african arid lands: Subcontinental and global comparisions. Divers. Distrib. 1998;4:27–36. [Google Scholar]
- Cox SB, Bloch CP, Stevens RD, Huenneke LF. Productivity and species richness in an arid ecosystem: a long-term perspective. Plant Ecol. 2006;186:1–12. [Google Scholar]
- Evans KL, Greenwood JJD, Gaston KJ. Dissecting the species-energy relationship. Proc. Roy. Soc. B-Biol. Sci. 2005;272:2155–2163. doi: 10.1098/rspb.2005.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gimenez ME. Sustaining the steppes: a geographical history of pastoral land use in Mongolia. Geogr. Rev. 1999;89:315–342. doi: 10.1111/j.1931-0846.1999.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gimenez ME. The role of Mongolian nomadic pastoralists: Ecological knowledge in rangeland management. Ecol. Appl. 2000;10:1310–1326. [Google Scholar]
- Fernandez-Gimenez ME. Land use and land tenure in Mongolia: A brief history and current issues. In: Bedunah DJ, McArthur ED, Fernandez-Gimenez ME, editors. Rangelands of Central Asia: Proceedings of the conference on transformations, issues, and future challenges. Salt Lake City: 2006. pp. 30–37. [Google Scholar]
- Fernandez-Gimenez ME, Allen-Diaz B. Testing a non-equilibrium model of rangeland vegetation dynamics in Mongolia. J. Appl. Ecol. 1999;36:871–885. [Google Scholar]
- Garcia LV, Maranon T, Moreno A, Clemente L. Aboveground biomass and species richness in a Mediterranean salt-marsh. J. Veg. Sci. 1993;4:417–424. [Google Scholar]
- Gaston KJ. Global patterns in biodiversity. Nature. 2000;405:220–227. doi: 10.1038/35012228. [DOI] [PubMed] [Google Scholar]
- Gaston KJ, Rodrigues ASL. Reserve selection in regions with poor biological data. Cons. Biol. 2003;17:188–195. [Google Scholar]
- Gill RA, Kelly RH, Parton WJ, Day KA, Jackson RB, Morgan JA, Scurlock JMO, Tieszen LL, Castle JV, Ojima DS, Zhang XS. Using simple environmental variables to estimate below-ground productivity in grasslands. Global Ecol. Biogeogr. 2002;11:79–86. [Google Scholar]
- Goetz SJ, Prince SD, Goward SN, Thawley MM, Small J. Satellite remote sensing of primary production: an improved production efficiency modeling approach. Ecol. Model. 1999;122:239–255. [Google Scholar]
- Gough L, Grace JB, Taylor KL. The relationship between species richness and community biomass - the importance of environmental variables. Oikos. 1994;70:271–279. [Google Scholar]
- Grubov VI. Plants of Central Asia. Vol. I-VIII. Science Publishers; Enfield: 2000ff. [Google Scholar]
- Grubov VI. Key to the vascular plants of Mongolia. Volume I & II. Science Publishers; Plymouth: 2001. [Google Scholar]
- Gubanov IA. Conspectus of the Flora of Outer Mongolia (Vascular Plants) Valang Publishers; Moscow: 1996. [Google Scholar]
- Hawkins BA, Field R, Cornell HV, Currie DJ, Guegan JF, Kaufman DM, Kerr JT, Mittelbach GG, Oberdorff T, O’Brien EM, Porter EE, Turner JRG. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- He Z, Zhao W, Chang X, Chang X, Fang J. Scale dependence in desert plant diversity. Biodivers. Conserv. 2006;15:3055–3064. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. [Google Scholar]
- Hilbig W. The vegetation of Mongolia. SPB Academic Publishing; Amsterdam: 1995. [Google Scholar]
- Hilbig W. Kommentierte Übersicht über die Pflanzengesellschaften und ihre höheren Syntaxa in der Mongolei. Feddes Repert. 2000;111:75–120. [Google Scholar]
- Hilbig W, Tungalag R. Vegetationskundliche Untersuchungen in der Borzongijn und Galbyn-Gobi (Ömnögov Aimak, Mongolei) Feddes Repert. 2006;117:399–429. [Google Scholar]
- Hill MO, Gauch HG. Detrended Correspondence-Analysis - an Improved Ordination Technique. Vegetatio. 1980;42:47–58. [Google Scholar]
- Hobbs TJ. The Use of NOAA-AVHRR NDVI data to assess herbage production in the arid rangelands of central Australia. Int. J. Remote Sens. 1995;16:1289–1302. [Google Scholar]
- Holm AM, Cridland SW, Roderick ML. The use of time-integrated NOAA NDVI data and rainfall to assess landscape degradation in the arid shrubland of Western Australia. Remote Sens. Environ. 2003;85:145–158. [Google Scholar]
- Huxman TE, Smith MD, Fay PA, Knapp AK, Shaw MR, Loik ME, Smith SD, Tissue DT, Zak JC, Weltzin JF, Pockman WT, Sala OE, Haddad BM, Harte J, Koch GW, Schwinning S, Small EE, Williams DG. Convergence across biomes to a common rain-use efficiency. Nature. 2004;429:651–654. doi: 10.1038/nature02561. [DOI] [PubMed] [Google Scholar]
- Jäger EJ. The occurrence of forest plants in the desert mountains of Mongolia and their bearing on the history of the climate. Erforsch. Biol. Ress. Mongolei. 2005;9:237–245. [Google Scholar]
- Jäger EJ, Hanelt P, Davazamc C. Contribution to the knowledge of the flora of the Dsungarian Gobi (Mongolian-Peoples-Republic) Flora. 1985;177:45–89. [Google Scholar]
- Jarvis A, Reuter HI, Nelson A, Guevara E. Hole-filled SRTM for the globe. (Version 3) 2006 http://srtm.csi.cgiar.org/PDF/Jarvis4.pdf.
- Kier G, Mutke J, Dinerstein E, Ricketts TH, Kuper W, Kreft H, Barthlott W. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 2005;32:1107–1116. [Google Scholar]
- Kürschner H. Phytosociological studies in the Alashan Gobi - a contribution to the flora and vegetation of Inner Mongolia (NW China) Phytocoenologia. 2004;34:169–224. [Google Scholar]
- Le Houerou HN. Rain Use Efficiency - a Unifying Concept in Arid-Land Ecology. J. Arid Environ. 1984;7:213–247. [Google Scholar]
- Le Houerou HN. Biogeography of the arid steppeland north of the Sahara. J. Arid Environ. 2001;48:103–128. [Google Scholar]
- Lehmkuhl F. Der Naturraum Zentral- und Hochasiens. Geogr. Rundschau. 1997;49:300–306. [Google Scholar]
- Lim C, Kafatos M. Frequency analysis of natural vegetation distribution using NDVI/AVHRR data from 1981 to 2000 for North America: correlations with SOI. Int. J. Remote Sens. 2002;23:3347–3383. [Google Scholar]
- Lioubimtseva E, Cole R, Adams JM, Kapustin G. Impacts of climate and landcover changes in arid lands of Central Asia. J. Arid Environ. 2005;62:285–308. [Google Scholar]
- Ludwig JA. Primary Productivity in Arid Lands - Myths and Realities. J. Arid Environ. 1987;13:1–7. [Google Scholar]
- Meusel H, Jäger E, Weinert E. Vergleichende Chorologie der zentraleu-ropäischen Flora. Teil 1. Text und Kartenband. Fischer; Jena: 1965. [Google Scholar]
- Milner-Gulland EJ, Lhagvasuren B. Population dynamics of the Mongolian gazelle Procapra gutturosa: an historical analysis. J. Appl. Ecol. 1998;35:240–251. [Google Scholar]
- Mittelbach GG, Steiner CF, Scheiner SM, Gross KL, Reynolds HL, Waide RB, Willig MR, Dodson SI, Gough L. What is the observed relationship between species richness and productivity? Ecology. 2001;82:2381–2396. [Google Scholar]
- Mix HM, Reading RP, Blumer ES, Badamjaviin L. Status and distribution of Wild Bactrian camels in Mongolia. In: Reading R, Dulamtserengiin E, Tuvdendorjiin G, editors. Ecology and conservation of Wild Bactrian Camels. Ulaanbaatar: 2002. [Google Scholar]
- Mucina L, Schaminee JHJ, Rodwell JS. Common data standards for recording releves in field survey for vegetation classification. J. Veg. Sci. 2000;11:769–772. [Google Scholar]
- Mueller-Dombois D, Ellenberg H. Aims and methods of vegetation ecology. John Wiley & Sons; New York, London, Sydney, Toronto: 1974. [Google Scholar]
- Nagendra H. Using remote sensing to assess biodiversity. Int. J. Remote Sens. 2001;22:2377–2400. [Google Scholar]
- Nagendra H, Gadgil M. Biodiversity assessment at multiple scales: Linking remotely sensed data with field information. P. Natl. Acad. Sci. USA. 1999;96:9154–9158. doi: 10.1073/pnas.96.16.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Statistical Office of Mongolia . Mongolian Statistical Yearbook 2002. National Statistical Office of Mongolia; Ulaanbaatar: 2003. [Google Scholar]
- Ni J. Estimating net primary productivity of grasslands from field biomass measurements in temperate northern China. Plant Ecol. 2004;174:217–234. [Google Scholar]
- Oesterheld M, DiBella CM, Kerdiles H. Relation between NOAA-AVHRR satellite data and stocking rate of rangelands. Ecol. Appl. 1998;8:207–212. [Google Scholar]
- Oindo BO, Skidmore AK. Interannual variability of NDVI and species richness in Kenya. Int. J. Remote Sens. 2002;23:285–298. [Google Scholar]
- Paruelo JM, Epstein HE, Lauenroth WK, Burke IC. ANPP estimates from NDVI for the central grassland region of the United States. Ecology. 1997;78:953–958. [Google Scholar]
- Pineiro G, Oesterheld M, Paruelo JM. Seasonal variation in aboveground production and radiation-use efficiency of temperate rangelands estimated through remote sensing. Ecosystems. 2006;9:357–373. [Google Scholar]
- Prince SD, Goward SN. Global primary production: A remote sensing approach. J. Biogeogr. 1995;22:815–835. [Google Scholar]
- Purevdorj T, Tateishi R, Ishiyama T, Honda Y. Relationships between percent vegetation cover and vegetation indices. Int. J. Remote Sens. 1998;19:3519–3535. [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. Vienna, Austria: 2005. http://www.R-project.org. [Google Scholar]
- Reading RP, Bedunah DJ, Amgalanbaatar S. Conserving biodiversity on Mongolian rangelands: Implications for protected area development and pastoral uses. In: Bedunah DJ, McArthur ED, Fernandez-Gimenez M, editors. Rangelands of Central Asia: Proceedings of the Conference on Transformations, Issues, and Future Challenges. Salt Lake City, USA: 2006. pp. 1–17. [Google Scholar]
- Reading RP, Johnstad MD, Batjargal Z, Amgalanbaatar S, Mix HM. Expanding Mongolia’s system of protected areas. Nat. Area J. 1999;19:211–222. [Google Scholar]
- Reading RP, Mix HM, Lhagvasuren B, Feh C, Kane DP, Dulamtseren S, Enkhbold S. Status and distribution of khulan (Equus hemionus) in Mongolia. J. Zool. 2001;254:381–389. [Google Scholar]
- Retzer V, Nadrowski K, Miehe G. Variation of precipitation and its effect on phytomass production and consumption by livestock and large wild herbivores along an altitudinal gradient during a drought, South Gobi, Mongolia. J. Arid Environ. 2006;66:135–150. [Google Scholar]
- Rodrigues ASL, Gaston KJ. How large do reserve networks need to be? Ecol. Lett. 2001;4:602–609. [Google Scholar]
- Rodrigues ASL, Gaston KJ. Optimisation in reserve selection procedures - why not? Biol. Conserv. 2002;107:123–129. [Google Scholar]
- Schmidt H, Karnieli A. Remote sensing of the seasonal variability of vegetation in a semi-arid environment. J. Arid Environ. 2000;45:43–59. [Google Scholar]
- ter Braak CJF, Smilauer P. Canoco 4.5 Reference Manual. Biometrics, Wageningen Ceske Budejovice; 2002. [Google Scholar]
- Tichy L. JUICE, software for vegetation classification. J. Veg. Sci. 2002;13:451–453. [Google Scholar]
- Tichy L. New similarity indices for the assignment of releves to the vegetation units of an existing phytosociological classification. Plant Ecol. 2005;179:67–72. [Google Scholar]
- Titlyanova AA, Romanova IP, Kosykh NP, Mironycheva-Tokareva NP. Pattern and process in above-ground and below-ground components of grassland ecosystems. J. Veg. Sci. 1999;10:307–320. [Google Scholar]
- Tucker CJ. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979;8:127–150. [Google Scholar]
- Tucker CJ, Pinzon JE, Brown ME, Slayback DA, Pak EW, Mahoney R, Vermote EF, El Saleous N. An extended AVHRR 8-km NDVI dataset compatible with MODIS and SPOT vegetation NDVI data. Int. J. Remote Sens. 2005;26:4485–4498. [Google Scholar]
- Turner W, Spector S, Gardiner N, Fladeland M, Sterling E, Steininger M. Remote sensing for biodiversity science and conservation. Trends Ecol. Evol. 2003;18:306–314. [Google Scholar]
- Vetter S. Rangelands at equilibrium and non-equilibrium: recent developments in the debate. J. Arid Environ. 2005;62:321–341. [Google Scholar]
- von Wehrden H. Vegetation Mapping in the Gobi Gurvan Saikhan national park and the Great Gobi B special protected area - a comparison of first results. Erforsch. biol. Ress. Mongolei. 2005;9:225–236. [Google Scholar]
- von Wehrden H, Hilbig W, Wesche K. Plant communities of the Mongolian Transaltay. Feddes Repert. 2006a;117:526–570. [Google Scholar]
- von Wehrden H, Wesche K, Reudenbach C, Miehe G. Mapping of large-scale vegetation pattern in southern Mongolian semi-deserts - an application of LAND-SAT 7 data. Erdkunde. 2006b;60:261–272. [Google Scholar]
- von Wehrden H, Wesche K. Mapping the vegetation of southern Mongolian protected areas: an application of GIS and remote sensing techniques. In: Gunin PD, editor. Ecosystems of Mongolia and frontier areas of adjacent countries: natural resources, biodiversity and ecological prospects. Ulaanbataar: 2005. [Google Scholar]
- von Wehrden H, Wesche K. Mapping Khulan habitats - a GIS-based approach. Erforsch. biol. Ress. Mongolei. 2007;10 [Google Scholar]
- von Wehrden H, Wesche K. Plant communities of the Southern Mongolian Gobi. in prep.
- von Wehrden H, Wesche K, Tungalag R. Plant communities of the Great Gobi B strictly protected area. Mongolian J. Biol. Sci. doi: 10.22353/mjbs.2007.05.03. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WDPA Consortium “World Database on Protected Areas” 2006. 2006 http://Maps.geog.umd.edu/WDPA/WDPA_info/English/datacontents.html.
- Waide RB, Willig MR, Steiner CF, Mittelbach G, Gough L, Dodson SI, Juday GP, Parmenter R. The relationship between productivity and species richness. Annu. Rev. Ecol. Syst. 1999;30:257–300. [Google Scholar]
- Weischet W, Endlicher W. Regionale Klimatologie. Teil 2. Die Alte Welt. Teubner; Stuttgart, Leipzig: 2000. [Google Scholar]
- Weiss E, Marsh SE, Pfirman ES. Application of NOAA-AVHRR NDVI time-series data to assess changes in Saudi Arabia’s rangelands. Int. J. Remote Sens. 2001;22:1005–1027. [Google Scholar]
- Wesche K, Miehe S, Miehe G. Plant communities of the Gobi Gurvan Sayhan National Park (South Gobi Aymak, Mongolia) Candollea. 2005a;60:149–205. [Google Scholar]
- Wesche K, Jäger EJ, von Wehrden H, Undrakh R. Status and distribution of four endemic vascular plants in the Gobi Altai. Mongolian J. Biol. Sci. 2005b;3:3–11. [Google Scholar]
- Wesche K, Ronnenberg K, Hensen I. Lack of sexual reproduction within mountain steppe populations of the clonal shrub Juniperus sabina L. in semi-arid southern Mongolia. J. Arid Environ. 2005c;63:390–405. [Google Scholar]
- Wesche K, Ronnenberg K, Retzer V. Effects of herbivore exclusion in southern Mongolian desert steppes. submitted.
- Woinarski JCZ, Fisher A. Conservation and the maintenance of biodiversity in the rangelands. Rangeland J. 2003;25:157–171. [Google Scholar]
- Yu F, Price KP, Ellis J, Feddema JJ, Shi P. Interannual variations of the grassland boundaries bordering the eastern edges of the Gobi Desert in central Asia. Int. J. Remote Sens. 2004;25:327–346. [Google Scholar]
- Zevegmid D, Dawaa N. Die seltenen Großsäuger der Mongolischen Volksrepublik und ihr Schutz. Arch. Naturschutz u. Landschaftsforsch. 1973;13:87–106. [Google Scholar]