Abstract
PD-1 engagement on the surface of effector T cells strongly suppresses their cytotoxic function, which constitutes a major obstacle for T cell-mediated anti-tumor activities. Surprisingly, PD-1 is strongly upregulated in T cells, engaging its ligand PD-L1 during antigen presentation. However, our recent published data may provide an explanation for this apparent contradiction.
The ultimate goal of anti-tumor immunotherapy is stimulating the immune system to specifically destroy cancer cells. The expansion of effector cytotoxic T lymphocytes is particularly important. However, immunotherapy relies on the assumption that the immune system can recognize cancer as “foreign” or “non-self.” In reality, tumors are mostly immunologically silent or strongly immunosuppressive; they are usually recognized as “self” to which there is immunological tolerance. To develop effective anti-tumor therapies, we need first to understand these tolerogenic/immunosuppressive mechanisms, in order to interfere with them while minimizing collateral damage.
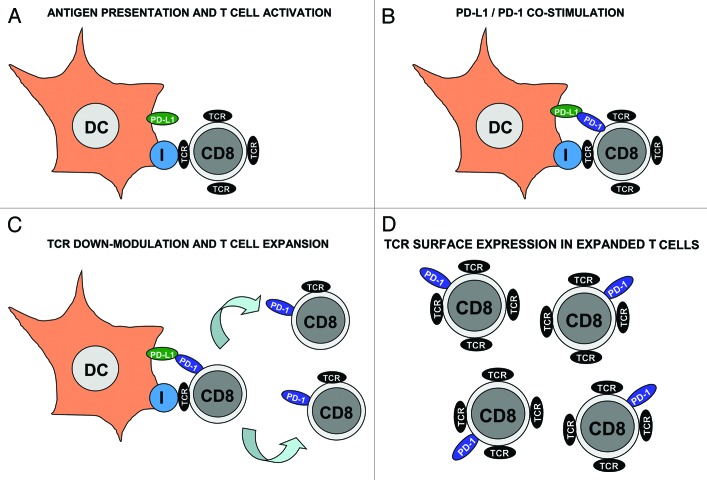
T cell responses are key in the induction of protective and long-lasting immunity. However, if uncontrolled, effector T cells can cause significant autoreactive damage. Thus, T cell activation is regulated at multiple levels, especially during antigen presentation. T cells recognize peptide-MHC complexes expressed on the surface of antigen presenting cells such as dendritic cells (DCs) (Fig. 1A). Simultaneously, a range of co-stimulatory ligand-receptor interactions takes place, providing further signaling to T cells. The final outcome will depend on the integration of “positive” and “negative” stimuli.1 One such interaction is mediated between PD-L1 on antigen presenting cells to PD-1 on T cells, which is critical to maintain peripheral tolerance.2 PD-L1 engagement with PD-1 on effector T cells inhibits their cytotoxic activities by terminating T cell receptor (TCR) signal transduction.3 Intriguingly, PD-1 is strongly upregulated in T cells during antigen-presentation, where it engages with PD-L1 on the surface of antigen-presenting DCs (Fig. 1A and 1B). What is the role of such a suppressive interaction in antigen presentation?
Figure 1.
Regulatory role of PD-1 during T cell activation after antigen presentation. (A) Dendritic cells (DC, as indicated) present antigenic peptide associated with MHC class I (encircled “I”) to specific CD8 T cells. DCs also express PD-L1 (green ovoid) on their surface. CD8 T cells associate with DCs through their TCR (black ovoid) as shown in the figure. These CD8 T cells are TCRhigh and do not express PD-1. (B) After antigen recognition, T cells express PD-1 on their surface (blue ovoid), where it engages with PD-L1 on the DC surface. Binding of PD-1 reduces TCR signal transduction and (C) down-modulates TCR levels in CD8 T cells. Activated CD8 T cells proliferate as indicated by arrows. Please note that these proliferating CD8 T cells are TCRlow PD-1+. It is tempting to speculate that low TCR and high PD-1 may inhibit autoreactive cytotoxicity while effector CD8 T cells are undergoing expansion. (D) Expanded effector CD8 T cells gradually recover their TCR surface expression while keeping PD-1 expression. It could be argued that this increase in TCR levels may “arm” cytotoxic T cells against their targets.
In our recent publication,4 we argue that the answer resides on the necessity for an early control of T cell activation. For the first time we show that PD-L1 co-stimulation contributes to ligand-induced TCR down-modulation, a fundamental immunological process that regulates TCR signaling. TCRs are removed from the surface shortly after activation, limiting signal transduction and avoiding excessive responses.5 TCR down-modulation is a transient early event in antigen presentation, and interestingly coincides with the early stages of the exponential T cell expansion4 (Fig. 1). Our results indicate that PD-L1/PD-1 co-stimulation contributes to ligand-induced TCR down-modulation, by upregulating the expression of Cbl E3 ubiquitin ligases in activated T cells. Interference with PD-L1 co-stimulation led to hyperactivated pro-inflammatory TCRhigh CD8 T cells. These effector T cells can exert autoimmune damage if directed toward an auto-antigen, but on the other hand they significantly accelerated anti-tumor immune responses. Vaccination with PD-L1-silenced DCs strongly inhibited tumor growth in a mouse model of lymphoma, and increased the lifespan of tumor-bearing mice.4 Overall, our observations expanded the role of PD-L1 in immune regulation to a critical step in T cell activation, and highlighted its interference as a key therapeutic target.
However, things were not that simple, and these accelerated anti-tumor responses did not increase cure rates in our experimental model. In fact, many tumor cells upregulate PD-L1 expression to counteract cytotoxic PD-1+ T cells.6 So, PD-1 expression that occurs as a physiological regulatory mechanism in antigen presentation ends up backfiring and becomes an obstacle. As a matter of fact, there is limited therapeutic efficacy in PD-L1/PD-1 interference unless combined with other strategies, as others and we have observed.4,7 Reduced PD-L1 co-stimulation may lead to sustained TCR signaling in the immunological synapse, but does not necessarily improve T cell effector capacities. Only after a combination with selected modulators of DC signaling pathways delivered with lentivectors, PD-L1 silencing was clearly effective. These modulators consisted of a constitutive activator of mitogen activated protein kinase (MAPK) p38,8 and an inhibitor of MAPK ERK.9 These were chosen according to their capacity to induce DC maturation by upregulating expression of co-stimulatory and adhesion molecules such as CD80, CD40 and ICAM I. Particularly, when the MAPK ERK inhibitor was expressed in PD-L1-silenced DCs, we increased survival and reduced DC vaccination doses up to 1000-fold compared with standard experimental protocols.4
Concluding, our results highlight the contribution of PD-L1/PD-1 co-stimulation to ligand-induced TCR down-modulation in the immunological synapse. Interference with this pathway results in hyperactivated TCRhigh CD8 T cells. These CD8 T cells significantly accelerate anti-tumor immune responses, but are nevertheless insufficient to increase long-term survival. According to our data, and from a therapeutic point of view, clinically relevant PD-L1/PD-1 blocking antibodies could be co-administered in combination with, for example, inhibitors of the Ras/Raf/MEK/ERK pathway. Certain kinase inhibitors currently used in human chemotherapy already possess strong adjuvant capacities.10 The combination of these inhibitors with PD-L1/PD-1 interference may lead to effective anti-tumor immunotherapy. However, would therapeutic strategies such as these cause autoimmune disease?
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/17824
References
- 1.Nurieva R, Thomas S, Nguyen T, Martin-Orozco N, Wang Y, Kaja MK, et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623–33. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–6. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karwacz K, Bricogne C, Macdonald D, Arce F, Bennett C, Collins M, et al. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med 2011; doi:10.1002/emmm.201100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.San José E, Borroto A, Niedergang F, Alcover A, Alarcón B. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity. 2000;12:161–70. doi: 10.1016/S1074-7613(00)80169-7. [DOI] [PubMed] [Google Scholar]
- 6.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–14. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilon-Thomas S, Mackay A, Vohra N, Mulé JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184:3442–9. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escors D, Lopes L, Lin R, Hiscott J, Akira S, Davis RJ, et al. Targeting dendritic cell signaling to regulate the response to immunization. Blood. 2008;111:3050–61. doi: 10.1182/blood-2007-11-122408. [DOI] [PubMed] [Google Scholar]
- 9.Arce F, Breckpot K, Stephenson H, Karwacz K, Ehrenstein MR, Collins M, et al. Selective ERK activation differentiates mouse and human tolerogenic dendritic cells, expands antigen-specific regulatory T cells, and suppresses experimental inflammatory arthritis. Arthritis Rheum. 2011;63:84–95. doi: 10.1002/art.30099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]