Abstract
Effective, long-lasting immune responses largely depend upon T cell reponses. Antigen-specific T lymphocytes are activated and differentiate into effector T cells after antigen presentation by professional antigen presenting cells (APCs). However, T cell responses are tightly regulated to prevent T cell hyperactivation which may end up in autoimmune pathology. One of these regulatory mechanisms is ligand-induced TCR down-modulation, a process by which TCRs are removed from the T cell surface shortly after engagement with their cognate antigenic peptide associated to MHC molecules on the APC. TCR down-modulation is a complicated process. Here we briefly describe the three main models that attempt to clarify this mechanism in the context of T cell activation and function.
Keywords: TCR, Cbl, down-modulation, PD-L1, PD1
One of the key roles of the immune system is to protect the organism against infectious diseases and cancer, while avoiding autoimmune damage. This relies on the discrimination between pathogenic and innocuous antigens. The induction of long-lasting, protective immunity largely relies on the activities of T lymphocytes. However, uncontrolled hyperactive T cells can cause significant tissue damage which could eventually lead to autoreactive disease. Thus, T cell activation and effector activities are regulated at several levels, especially during antigen presentation.
T cell activation and ligand-induced TCR down-modulation
T cells recognise specific antigenic peptides in association with major histocompatibility complex molecules (p-MHC) on the surface of antigen presenting cells. This recognition is mediated through their specific T cell receptors (TCR), together with simultaneous engagement of a wide range of ligand-receptor interactions that provide either positive or negative signals to T cells (Figure 1). The integration of these signals will determine the extent and type of effector T cell responses [1]. A key co-stimulatory interaction is mediated by binding between CD80 on the APC surface with CD28 on the T cell surface. Antigen recognition by the TCR in combination with CD80-CD28 co-stimulation effectively activates T cells, which proliferate and acquire cytotoxic activities. These cytotoxic T cells expand, recognise their cognate antigens on the target cells and destroy them in a TCR-dependent recognition manner. Surprisingly, TCRs are removed from the T cell surface shortly after p-MHC engagement, by a process called ligand-induced T cell receptor down-modulation [2-7]. Although this phenomenon has been well studied for a relatively long time, its physiological role still remains elusive [8]. Some experimental evidence supports the theory that TCR down-modulation is required for effective T cell activation [9]. Other studies suggest that it limits signal transduction and prevents hyperactivation [7,8,10-12]. How and why T cells down-modulate their TCRs shortly after activation is a critical question in T cell physiology. To date, three models have been put forward attempting to provide plausible explanations; serial TCR triggering, TCR co-modulation, and regulation by extrinsic signalling.
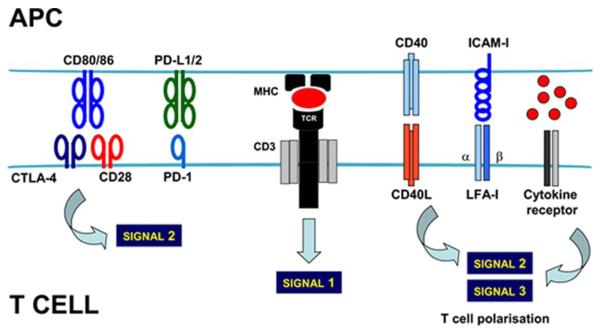
Figure 1. Antigen recognition by T cells at the immunological synapse.
On top, the membrane of the antigen presenting cell (APC) containing the peptide (red ovoid) associated to the MHC as indicated, binds to the TCR-CD3 complex in the membrane of the T cell. This interaction delivers signal 1. A wide range of receptor-ligand interactions between the APC and the T cell takes place simultaneously. In the figure, the most well studied interactions are shown. The integration of all the signalling from these interactions within the T cell will lead to signal 2, which will determine the level of T cell activation. Other signals such as cytokines (red spheres associated to the cytokine receptor, as indicated) will polarise T cell responses (signal 3) towards cytotoxic, Th2 or regulatory responses. CTLA4, cytotoxic T lymphocyte antigen 4; PD-1, programmed death 1; PD-L1/2, programmed death 1 ligand 1/2; CD40L, CD40 ligand; LFA-I, lymphocyte function-associated antigen 1; ICAM, intercellular adhesion molecule
Serial triggering model of TCR down-modulation
The current view of ligand-induced TCR down-modulation is that intrinsic TCR/CD28 signalling is sufficient for TCR down-modulation. However, TCRs have usually low affinities for their cognate p-MHC complexes. Even so, T cells can be effectively activated by small numbers of p-MHC complexes [13]. In principle, this observation would implicate that only a small number of TCRs engages to p-MHCs in the immunological synapse. Surprisingly, this is difficult to reconcile with the experimental evidence which shows extensive TCR down-modulation in T cells following antigen presentation. Valitutti and cols. proposed the “serial triggering” model, which attempted to clarify this apparent contradiction [9]. In this model, T cells are activated by sequential monovalent binding of many TCR receptors by a small number of p-MHCs [9]. The supporting evidence was based on the specific down-modulation of many TCRs of a given specificity when presented to their cognate p-MHCs; TCRs with a different peptide specificity but in the same T cells did not down-modulate [9]. Therefore, assuming the conditions of this model, it was estimated that a single p-MHC complex can serially engage and trigger around 200 TCRs, and that the TCR engagement is proportional to T cell activation [9]. Importantly, according to serial triggering, non-engaged TCRs would not down-regulatea.
Co-modulation model of TCR down-modulation
The serial triggering model could successfully explain the paradox of low-affinity TCR-pMHC binding with effective T cell activation. However, some key experimental evidence suggested that ligand-induced TCR down-modulation was extensive and included non-engaged TCRs, a process called co-modulation [14,15]. These observations were in clear conflict with the serial triggering model. In a very elegant work, San Jose and cols provided compelling evidence for co-modulation of both engaged and non-engaged TCRs [6]. To solve this issue, the authors constructed a chimeric protein consisting of the extracellular and transmembrane domain of CD25 fused to the cytoplasmic domain of CD3ζ. This chimeric protein behaved as a simplified TCR version for which p-MHC engagement could not be possible. Engagement of the TCR effectively co-modulated CD25-CD3ζ. Conversely, antibody-mediated crosslinking of this chimeric protein could also induce down-modulation of bona fide TCRs. Moreover, the authors demonstrated that engaged and non-engaged TCRs seemed to follow at least two distinct mechanisms for down-modulation. While engaged TCRs were quickly endocytosed, non-engaged TCRs down-modulated following signal transduction from the engaged TCRs (Figure 2B).
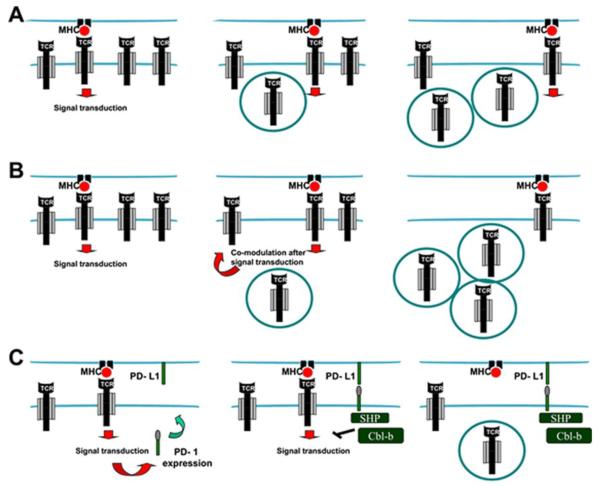
Figure 2. Models for ligand-induced T cell receptor down-modulation.
(A) Serial triggering model. In the scheme, p-MHC interactions with TCRs trigger TCR signal transduction (left), leading to endocytosis of the triggered TCR (center). Then, the same p-MHC complex triggers a second and third receptor, leading to their subsequent internalisation (right). (B) Co-modulation model. As in (A), but signal transduction from the triggered and endocytosed TCR induces internalisation of non-engaged TCRs (Center), which amplifies TCR down-modulation (right). (C) Extrinsic signal model. In addition to down-modulation following the two previous models, signal transduction from engaged TCRs leads to PD-1 surface expression (left). PD-1 engages with PD-L1 on the surface of the APC, recruits SHP phosphatases and leads to Cbl-b expression (center). Both SHP and Cbl-b terminate TCR signal transduction and lead to TCR internalisation (right). Red arrows indicate activatory signal transduction; SHP, anti-Src homology phosphatases.
Extrinsic signal model of TCR down-modulation
Both the serial triggering and co-modulation models rely exclusively on intrinsic TCR/CD28 signalling as the main driving force for TCR down-modulation. However, this view was inconsistent with some relatively recent experimental observations. E3 ubiquitin ligases of the casitas-B lymphoma family (Cbl) are important negative regulators of T cell activation [16]. Intriguingly, T cells from Cbl-b knockout (KO) mice are hyperactive and show a remarkable inhibition of TCR down-modulation following antigen presentation [16-18]. Therefore, this experimental evidence indicated that TCR/CD28 signalling per se is not the only cause for TCR down-modulation. Although Cbl-b KO T cells showed persistent TCR signal transduction, their ligand-induced TCR down-modulation was clearly inhibited [18].
So, can Cbl-b expression shed light on the mechanism of ligand-induced TCR down-modulation? Cbl proteins are quickly up-regulated after TCR/CD28 signalling, but their transcriptional up-regulation in T cells requires programmed death 1 (PD-1) engagement with its ligand PD-L1 on the APC surface [1]. PD-L1 belongs to the B7 family of co-stimulatory/inhibitory molecules, expressed in a wide range of cells including APCs. PD-1 is transiently up-regulated in activated T cells during antigen presentation [19]. Recently, our group provided compelling evidence on the role of PD-L1 co-stimulation in ligand-induced TCR down-modulation [10]. CD8 T cells exhibited decreased TCR down-modulation when PD-L1 was silenced in antigen-presenting cells, or when PD-L1/PD-1 interaction was blocked by antibodies. This was caused by a block in Cbl expression, particularly Cbl-b [10]. Interference with PD-L1 co-stimulation during antigen presentation resulted in hyperactive pro-inflammatory TCRhigh CD8 T cells. Accordingly, we proposed the following model for TCR down-modulation (Figure 2C); first, p-MHC is engaged by specific TCRs which deliver activatory signalling to T cells. After T cell activation, PD-1 is expressed on the surface of T cells, where it engages with PD-L1 on the APC’s surface. PD-1 engagement results in Cbl-b up-regulation, which in turn stops signal transduction and contributes to TCR down-modulation. Our data demonstrated the participation of at least one extrinsic signal in the immunological synapse on TCR down-modulation.
E3 ubiquitin ligases of the Cbl family as central regulators of TCR down-modulation
All the recent evidence including our own research highlights Cbl E3 ubiquitin ligases, namely c-Cbl and Cbl-b, as key regulators of TCR down-modulation. Both c-Cbl and Cbl-b participate in regulating TCR levels in activated T cells, with Cbl-b playing a prominent role [10,17,18,20]. However, what is the precise role of ubiquitylation in the regulation of TCR levels? It is well established that monoubiquitylation and K63 polyubiquitylation modulate signal transduction, receptor-mediated endocytosis and intracellular transport [20-24]. Moreover, CD3ζ and CD3δ intracellular chains are ubiquitylated after T cell activation [25]. Overall, all this evidence points out to a key role for ubiquitylation in the regulation of TCR surface levels and signal transduction. On the other hand, it is yet unclear how Cbl-b may actually regulate TCR trafficking after antigen presentation. Interestingly, in an elegant work, Wang and cols have described a ubiquitin-dependent mechanism of TCR down-modulation in immature CD4+ CD8+ (double-positive, DP) thymocytes, which could point out the direction [26]. DP thymocytes exhibit low TCR surface levels in contrast to single positive thymocytes. The authors of this recent paper demonstrated that constitutive Lck activity present in DP thymocytes results in CD3 and c-Cbl phosphorylation. Subsequently, c-Cbl leads to CD3ζ ubiquitylation, TCR endocytosis and transport to lysosomes in a dynamin-dependent mechanism [26]. Although this process is not per se ligand-induced TCR down-modulation, it would be tempting to speculate that Cbl-b could play a similar role in mature, TCR-activated lymphocytes. Therefore, the precise mechanism of PD-1-dependent Cbl-b transcriptional up-regulation and its activation is a key question in T cell physiology.
Integrating the models for TCR down-modulation
So far, there is evidence supporting all of the three mechanisms. Differences in interpretation and the specific experimental conditions (use of primary T cells, T cell clones, hybridomas, the type of TCR stimulation) may account for the apparent contradictions. Here we propose an integrative model that would include serial triggering, co-modulation and extrinsic signalling. Serial triggering could take place during the early stages of TCR down-modulation, soon after antigen recognition (Figure 2A). This would lead to internalisation of a significant number of engaged TCRs. Signal transduction from these engaged TCRs would follow and eventually lead to down-modulation of non-engaged TCRs (co-modulation) (Figure 2B). Signal transduction will also result in PD-1 expression, and the extrinsic signal model could explain a prolonged, reinforced TCR down-modulation (Figure 2C).
Physiological relevance of ligand-induced TCR down-modulation
Even though TCR down-modulation has been extensively studied, its physiological importance is yet unclear [8]. According to most of the experimental evidence TCR down-modulation seems to act at different levels to prevent autoimmunity during the onset of immune responses. Firstly, at the immunological synapse, where PD-1 is strongly up-regulated and engages with PD-L1 on the surface of antigen-presenting cells. The role of a suppressive interaction during antigen presentation possibly resides on the necessity for an early control of T cell activation [10]. PD-L1 co-stimulation would contribute to the removal of TCRs shortly after T cell activation, limiting signal transduction and avoiding excessive responses [6-8,10-12]. Accordingly, the elimination/inhibition of any component of the PD-L1/PD-1/TCR down-modulation pathway such as Cbl-b, PD-L1, PD-1 or Rab5 ends up with enhanced T cell responses and spontaneous development of autoimmune disorders [4,16,17,27,28].
Interestingly, TCR down-modulation could also protect the organism from autoreactive disease at another level. While most of the studies address TCR down-modulation during short intervals, we observed that it proceeds steadily up to 3-4 days after antigen presentation [10]. After that, TCR surface levels recover progressively [10]. If this is the case in vivo, TCR down-modulation would take place during the exponential phase of clonal expansion. It is tempting to speculate that TCR removal from the surface of highly proliferating activated T cells could prevent their untimely cytotoxic activity until a “critical mass” is reached, approximately one week after antigen presentation. Then, TCR surface expression would recover, “arming” the effector T cells after they have reached their destination (the infection site, or tumour). Thus, ligand-induced TCR down-modulation could well be a temporal/spatial regulatory mechanism of T cell functions. In support of this idea, we observed that T cell-mediated anti-tumour activities started significantly earlier using PD-L1-silenced dendritic cells (DCs) as antigen presenting cells [10].
Exploiting PD-L1 co-stimulation and TCR down-modulation for cancer immunotherapy
The exacerbated CD8 T cell responses observed experimentally upon PD-L1/PD-1 interference or Cbl-b abrogation could be exploited to achieve effective anti-tumour immunotherapy. In fact, one of the major problems for cancer immunotherapy is to break tolerance towards tumour associated antigens (TAAs). TAAs are frequently self-proteins or quasi-self-proteins to which there is existing systemic tolerance. In fact, interference with PD-L1/PD-1 signalling has already been attempted as an anti-cancer therapy, either with blocking antibodies or small-interfering RNA (siRNA). Surprisingly, these strategies were not as effective as anticipated. To achieve significant therapeutic activities, they had to be combined with other immune-stimulatory treatments [10,29-31]. Our own experience confirms all these observations. We delivered a PD-L1-targeted shRNA to DCs using lentivectors [10] taking advantage of their efficacy to transduce antigen-presenting DCs [32-34]. Abrogation of PD-L1 co-stimulation differentiated TCRhigh CD8 T cells with a hyperactivated, pro-inflammatory phenotype. Interestingly, these effector T cells significantly increased the lifespan of tumour-bearing mice by accelerating immune responses [10]. However, to achieve significant therapeutic efficacy, PD-L1 interference had to be combined with selected molecular DC activators [10]. More specifically, a constitutive activator of mitogen activated protein kinase (MAPK) p38 [35], and an inhibitor of MAPK extracellularly-regulated protein kinase (ERK) [36].
Why is PD-L1/PD-1 blockade relatively inefficient for anti-tumour immunotherapy? A very plausible explanation may reside on the fact that in many cases tumour cells up-regulate PD-L1 to inhibit cytotoxic PD-1+ T cells [37]. Therefore, while PD-1 expression occurs as a physiological regulatory mechanism during antigen presentation, it can also become an obstacle as it is still expressed in activated T cells [10,38]. Although inhibition of PD-L1 co-stimulation results in high TCR surface levels and sustained activating signalling, it might not be sufficient to improve T cell effector capacities.
CONCLUSIONS
Ligand-induced T cell receptor down-modulation depends on many factors. Firstly, it relies on direct TCR engagement with its cognate p-MHC on the surface of antigen presenting cells. Secondly, TCR signal transduction reinforces down-modulation of non-engaged TCRs. Thirdly, extrinsic signalling pathways between T cells and APCs at the immunological synapse contribute to TCR down-modulation. While we have shown that PD-L1/PD-1 co-stimulation is one of these extrinsic pathways, we cannot discard the contribution of other regulatory interactions during antigen presentation that may regulate TCR down-modulation. However, the physiological role of ligand-induced TCR down-modulation is still unclear, although most of the experimental evidence suggests that it prevents T cell hyperactivation. Interference with PD-L1/PD-1 co-stimulation is not as effective therapeutically as previously expected. Thus, more insight into the detailed regulatory mechanisms of this key process is necessary in order to exploit it for immunotherapy.
ACKNOWLEDGEMENTS
David Escors is funded by an Arthritis Research UK Career Development Fellowship (18433). The Oxford Structural Genomics Consortium is a registered UK charity (number 1097737) that receives funds from the Canadian Institutes of Health Research, The Canadian Foundation for Innovation, Genome Canada through the Ontario Genomics Insitute, GlaxoSmithKline, Karolinska Institutet, the Knut and Alice Wallenberg Foundations, the Ontario Innovation Trust, the Ontario Ministry for Research and Innovation, Merck & Co., Inc., the Novartis Research Foundation, the Swedish Foundation for Strategic Research and the Wellcome Trust.
REFERENCES
- 1.Nurieva R, et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krangel MS. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J Exp Med. 1987;165:1141–1159. doi: 10.1084/jem.165.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minami Y, Samelson LE, Klausner RD. Internalization and cycling of the T cell antigen receptor. Role of protein kinase C. J Biol Chem. 1987;262:13342–13347. [PubMed] [Google Scholar]
- 4.Andre P, et al. A dominant-negative mutant of the Rab5 GTPase enhances T cell signaling by interfering with TCR down-modulation in transgenic mice. J Immunol. 1997;159:5253–5263. [PubMed] [Google Scholar]
- 5.Dietrich J, et al. The phosphorylation state of CD3gamma influences T cell responsiveness and controls T cell receptor cycling. J Biol Chem. 1998;273:24232–24238. doi: 10.1074/jbc.273.37.24232. [DOI] [PubMed] [Google Scholar]
- 6.San Jose E, Borroto A, Niedergang F, Alcover A, Alarcon B. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity. 2000;12:161–170. doi: 10.1016/s1074-7613(00)80169-7. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–675. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 8.Cai Z, Kishimoto H, Brunmark A, Jackson MR, Peterson PA, Sprent J. Requirements for peptide-induced T cell receptor downregulation on naive CD8+ T cells. J Exp Med. 1997;185:641–651. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 10.Karwacz K, et al. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med. 2011;3:1–12. doi: 10.1002/emmm.201100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schonrich G, et al. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65:293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- 12.Zanders ED, Lamb JR, Feldmann M, Green N, Beverley PC. Tolerance of T-cell clones is associated with membrane antigen changes. Nature. 1983;303:625–627. doi: 10.1038/303625a0. [DOI] [PubMed] [Google Scholar]
- 13.Weber S, Traunecker A, Oliveri F, Gerhard W, Karjalainen K. Specific low-affinity recognition of major histocompatibility complex plus peptide by soluble T-cell receptor. Nature. 1992;356:793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Miguel G, et al. Multivalent structure of an alphabetaT cell receptor. Proc Natl Acad Sci USA. 1999;96:1547–1552. doi: 10.1073/pnas.96.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niedergang F, Dautry-Varsat A, Alcover A. Peptide antigen or superantigen-induced down-regulation of TCRs involves both stimulated and unstimulated receptors. J Immunol. 1997;159:1703–1710. [PubMed] [Google Scholar]
- 16.Bachmaier K, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 17.Naramura M, Jang IK, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 18.Shamim M, et al. Cbl-b regulates antigen-induced TCR down-regulation and IFN-gamma production by effector CD8 T cells without affecting functional avidity. J Immunol. 2007;179:7233–7243. doi: 10.4049/jimmunol.179.11.7233. [DOI] [PubMed] [Google Scholar]
- 19.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paolino M, et al. Essential Role of E3 Ubiquitin Ligase Activity in Cbl-b-Regulated T Cell Functions. J Immunol. 2011;186:2138–2147. doi: 10.4049/jimmunol.1003390. [DOI] [PubMed] [Google Scholar]
- 21.Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- 23.Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell. 2006;21:737–748. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Wertz IE, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 25.Cenciarelli C, et al. Activation-induced ubiquitination of the T cell antigen receptor. Science. 1992;257:795–797. doi: 10.1126/science.1323144. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, et al. Tonic ubiquitylation controls T-cell receptor:CD3 complex expression during T-cell development. EMBO J. 2010;29:1285–1298. doi: 10.1038/emboj.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latchman YE, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22:265–268. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 29.Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184:3442–3449. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sierro SR, et al. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur J Immunol. 2011;41:2217–2228. doi: 10.1002/eji.201041235. [DOI] [PubMed] [Google Scholar]
- 31.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escors D, Breckpot K. Lentiviral Vectors in Gene Therapy: Their Current Status and Future Potential. Arch Immunol Ther Exp. 2010;58:107–119. doi: 10.1007/s00005-010-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breckpot K, et al. HIV-1 lentiviral vector immunogenicity is mediated by Toll-like receptor 3 (TLR3) and TLR7. J Virol. 2010;84:5627–5636. doi: 10.1128/JVI.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breckpot K, Escors D. Dendritic Cells for Active Anti-cancer Immunotherapy: Targeting Activation Pathways Through Genetic Modification. Endocr Metab Immune Disord Drug Targets. 2009;9:328–343. doi: 10.2174/187153009789839156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escors D, et al. Targeting dendritic cell signalling to regulate the response to immunisation. Blood. 2008;111:3050–3061. doi: 10.1182/blood-2007-11-122408. [DOI] [PubMed] [Google Scholar]
- 36.Arce F, et al. Selective ERK activation differentiates mouse and human tolerogenic dendritic cells, expands antigen-specific regulatory T cells, and suppresses experimental inflammatory arthritis. Arthritis Rheum. 2011;63:84–95. doi: 10.1002/art.30099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]