Abstract
Matrix Metalloproteinase 9 (MMP-9) expression is known to enhance the invasion and metastasis of tumor cells. In previous work based on a proteomic screen, we identified the serpin Protease nexin-1 (PN-1) as a potential target of MMP-9. Here we demonstrate that PN-1 is a substrate for MMP-9 and establish a link between PN-1 degradation by MMP-9 and regulation of invasion. PN-1 levels increased in prostate carcinoma cells after downregulation of MMP-9 and in tissues of MMP-9 deficient mice, consistent with MMP-9 acting to degrade PN-1. We identified three MMP-9 cleavage sites in PN-1 and demonstrated that mutations in those sites made PN-1 more resistant to MMP-9. Urokinase plasminogen activator (uPA) is inhibited by PN-1. MMP-9 augmented uPA activity in the medium of PC3-ML cells by degrading PN-1. Prostate cancer cells, overexpressing PN-1 or treated with MMP-9 shRNA, had reduced cell invasion in matrigel. PN-1 siRNA restored uPA activity and the invasive capacity. PN-1 mutated in the serpin inhibitory domain, the reactive centre loop (RCL), failed to inhibit uPA and failed to reduce matrigel invasion. Taken together, this study demonstrates a novel molecular pathway in which MMP-9 regulates uPA activity and tumor cell invasion through cleavage of PN-1.
Introduction
Matrix metalloproteinase-9 (MMP-9) has been long recognized as a key enzyme for the proteolytic degradation of extracellular matrix (ECM) during tumor invasion and metastasis (1). Its expanding roles include regulating cancer progression, activating angiogenesis, and recruiting macrophages or other bone marrow derived myeloid cells to the pre-existing metastatic niche (1), (2). These varied functions of MMP-9 have made it an extremely promising target for preventing metastasis in cancer patients (3), (4). However, in the last decade, clinical trails of MMP inhibitors have failed to produce breakthrough results (3). This may be attributed to the lack of specificity of the inhibitors used with more global MMP inhibition resulting in unacceptable side effects. If the particular proteolytic substrates of this enzyme could be identified, then potentially more precise inhibition profiles could be targeted.
Besides cleaving ECM components such as collagens and fibronectin, MMP-9 can degrade many non-collagenous substrates (1). MMP-9 cleavage alters the biological activity of chemokines and its activity can result in the shedding of cell surface receptors (5). These molecules influence many biological and pathological functions involved in cell adhesion, proliferation, angiogenesis, cell invasion and metastasis (5), (6). MMP-9 has long been known to enhance cancer cell invasion, but the underlying molecular mechanisms of how MMP-9 regulates tumor cell invasion and metastasis remain poorly understood (1), (6). To identify MMP-9 targets and potentially unveil new molecular mechanisms, we previously performed a label free quantitative proteomics to identify MMP-9 substrates in cancer cells (7). A number of novel MMP-9 targets were revealed, including the extracellular matrix protein, protease nexin-1 (PN-1) (7).
PN-1, also called Serpin E2 or Glial-derived Nexin (GDN), belongs to the serpin family of regulatory proteins (8). It is a serine protease inhibitor known to potently and irreversibly inhibit several proteases including thrombin, urokinase (uPA), tPA and trypsin (9), (10). Many of these proteins are involved in tissue remodelling and tumor invasion (11). Although many serpins are found in plasma, PN-1 is found predominantly in tissues and platelets (12),(13). PN-1 is a 43 kDa secreted protein, and can be produced by a multitude of cell types, including endothelial cells, fibroblasts, tumor cells, smooth muscle cells and astrocytes (14), (15), (16). PN-1 is present in the extracellular space where it can bind to glycosaminoglycans (GAGs) (17) and Collagen IV (18).
Notably, PN-1 contains a reactive centre loop (RCL) region at its C-terminus, which is the critical structural feature shared by most serpins and is necessary for inhibitory activity (19), (20). Serpins are usually present in a metastable state with the RCL region exposed. Upon contact with the target protease, the RCL is cleaved, leading to a covalent linkage between a C-terminal portion of the cleaved serpin and the target protease. The protease-serpin complex then reverts to a more stable and energetically favourable state, retaining the covalent, inhibitory linkage to target protease (20). This dramatic conformational change is the structural basis of the inhibitory effect of serpins against most proteases (19), (20).
In mammals, extracellular serpin-protease complexes are rapidly cleared from circulation via low-density lipoprotein receptor-related protein (LRP) mediated endocytosis (21). Serpin-protease complexes bind to the LRP and are internalized, thus triggering subsequent signaling events and finally resulting in transport to the lysosomes (22). For example, PN-1-thrombin and PN-1-uPA complexes are internalised through the LRP (23).
PN-1 mRNA overexpression has been identified in head and neck squamous cell cancers (24) and in colon carcinoma (25). No functional role was described for this overexpression nor confirmation of altered protein levels was provided (24), (25). The regulation of PN-1 itself remains to be characterised. In our previous study, we found that recombinant MMP-9 cleaved PN-1 in a dose- and time-dependent manner and determined possible MMP-9 dependent cleavage sites using a proteomics approach (7). Our current study has confirmed that PN-1 is a direct target of MMP-9 so that its inhibition of proteases, such as uPA, is then under the control of MMP-9. Although PN-1 does not have a clearly defined role in cancer biology, our results suggest that it may have a significant impact on tumor invasion.
Materials and Methods
Animals
All animal experiments were performed in accordance with UK Home Office regulations. MMP-9 deficient C57/B6 mouse strains were kindly provided by Dr Ghislain Oppendernakker (University of Leuven, Belgium) (26). After sacrifice, organs were harvested, rinsed in PBS and stored at −80°C. The frozen organs were individually weighed, smashed, homogenized and lysed in RIPA buffer (Pierce) containing protease inhibitor cocktail (Roche), 4mM dithiothreitol (DTT) and 0.2mM phenylmethylsulphonyl fluoride (PMSF) at 4°C for 1h. The total protein concentration was determined by BCA assay (Pierce).
Plasmids and Mutagenesis
pcDNA3-PN-1 plasmid was a kind gift from Dr Peter Andreasen’s lab (Aarhus, Denmark). pcDNA3-His-PN-1 was generated by incorporating 10× His at the C-terminus of PN-1. Point mutations were generated based on pcDNA3-PN-1 through site-directed mutagenesis kit (Invitrogen) and each was confirmed by DNA sequencing. The sequence pairs of primers were summarized on Table S1.
Cell culture and treatment
A PC-3ML cell line (prostate cancer cell derived from PC-3) was obtained from Dr Mark Stearns (Drexel University, Philadelphia) (27) and maintained as previously described (7), (27). A MMP-9 stably knockdown PC-3ML cell line was established by us as described (7). Panc1 (#CRL1469), PC-3 (#CRL1435) and TRAMP-C2 (#CRL2731) cell lines were from the American Type Culture Collection (ATCC). HT1080 cells were maintained as previously described (28). All cell lines were regularly tested to ensure the absence of Mycoplasma contamination (MycoAlert, Lonza).
A new stock vial of each cell line was thaw every 3-4 months and cell morphology was regularly checked to ensure no cross-contamination of cell lines. Where indicated, PN-1 plasmids were transfected into cells using FuGENE 6 transfection reagent (Roche). Cell conditioned media were collected and concentrated as described (7).
For siRNA experiments, predesigned siRNA against uPA, PN-1, MMP-9 and siRNA negative control oligos were from Ambion. 10nM or 20nM of each siRNA was transfected into cells using the siPORT NeoFX transfection agent (Ambion). After 48h, cells were washed and the media replaced with serum free media. Conditioned media was collected 24h later. Bone marrow derived cells were flushed from femurs of C57/B6 or MMP-9 KO mice and cultured as described (29).
Immunoblotting and uPA activity assay
Whole cell lysates were extracted as described before (7). Conditioned media were concentrated and normalized according to cell numbers and intracellular proteins. Immunoblots were performed as described previously (7). The following antibodies were used: anti-human PN-1, anti-mouse PN-1 (R&D systems), anti-uPA antibody (American Diagnostica), anti-MMP-9 (Abcam, R&D system) and anti-β-actin (Santa Cruz, Abcam). The uPA activity was measured by an uPA activity assay kit (Chemicon). In brief, concentrated cell conditioned media were mixed with assay buffer and incubated with Chromogenic substrate in 96-well plates at 37°C for 3-6h. The absorbance was read at OD405 and the activity (Units) was extrapolated from a standard curve.
Matrigel invasion assay
1×105 PC-3ML or MMP-9 KD PC-3ML cells, subjected to siRNA or transfection treatment for 24h, were seeded on BD Biocoat Growth Factor Reduced Invasion Inserts (BD #354483). After 48h, invaded cells were stained with Hoescht, nine fields from triplicate experiments were counted.
All statistical analysis and plots were performed with Prism Graphpad 5.0 software.
Results
PN-1 is cleaved by MMP-9 in tumor cells
In our previous work, we identified PN-1 as a putative target for MMP-9 proteolysis (7). We then showed that incubation of purified MMP-9 with recombinant PN-1 protein in solution resulted in PN-1 degradation with demonstrable cleavage intermediates (7) (again confirmed in Fig S1). Now additional experiments were performed to learn whether PN-1 is a natural target of MMP-9 in mammalian cells and animals. PN-1 is secreted by prostate carcinoma PC-3 cells and its derivative PC-3ML (7). After stable knockdown of MMP-9 by shRNA in PC-3ML cells (7), PN-1 levels increased in conditioned media (Fig 1A), consistent with the hypothesis that PN-1 is a substrate for MMP-9. Moreover, increased PN-1 expression was found in additional cell lines after the MMP-9 downregulation (Fig S2). PN-1 expression induced by transfection of a PN-1 expression vector was greater after transfection into cells with downregulated MMP-9 (MMP-9 KD) compared to control cells (Fig 1A).
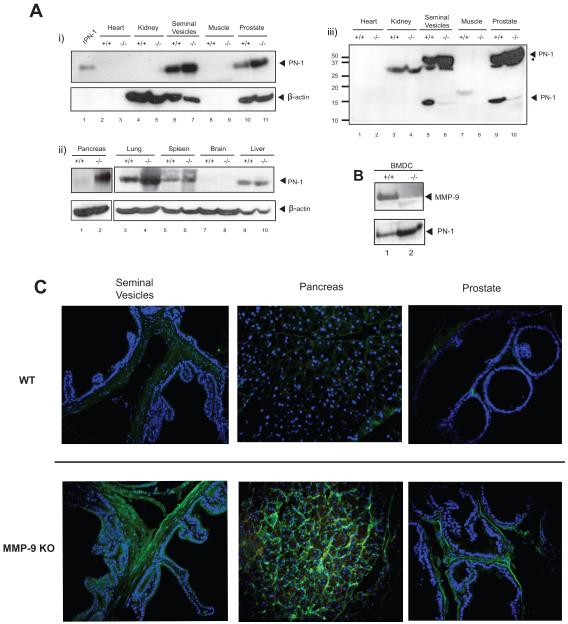
Figure 1. PN-1 is cleaved by MMP-9 in tumor cells.
A) Conditioned media (CM) and whole cell lysates (WL) collected from PC-3ML (-) or MMP9 KD PC-3ML (KD) cells with or without transfection of the pcDNA3-PN-1 expression vector, were subjected to SDS-PAGE electrophoresis and blotted with anti-PN-1, anti-MMP-9 or anti-Actin antibodies. B) Within the square an immunoblot after limited cleavage of PN-1 by recombinant MMP-9 in solution. Sizes of cleaved fragments as described (7). Schematic illustration of PN-1: with the black arrow heads indicating the putative cleavage sites and the altered amino acids in the mutants. Known functional domains such as LRP and heparin binding domains, reactive centre loop (RCL) domain are indicated. C) PN-1 in conditioned media collected from PC-3ML cells (i upper panel, ii and iii) or MMP-9 KD PC-3ML cells (i bottom panel), transfected with either WT PN-1 or indicated mutants. was analysed by immunoblot. The most resistant PN-1 mutants against MMP-9 cleavage were marked with asterisks *.
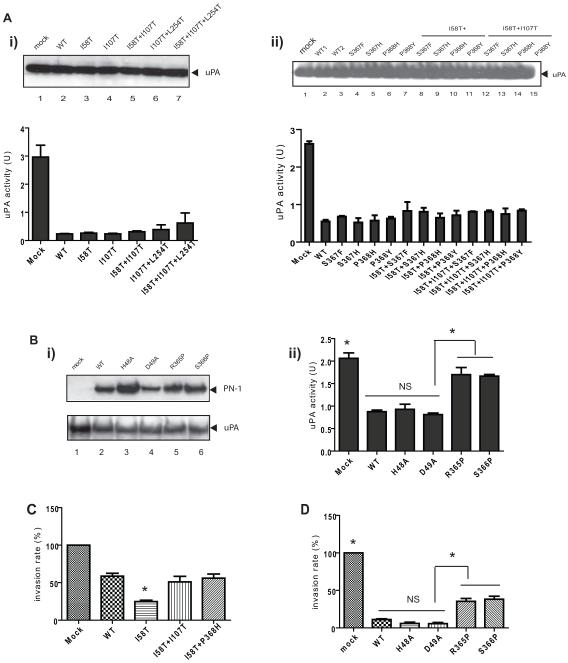
MMP-9 does not recognize substrates through recognition of an exact linear sequence; instead substrates must fit into a groove adjacent to the catalytic site (30). Previously, we identified numerous cleavage sites of PN-1 by MMP-9 through mass spectrometry after extensive digestion (7). More limited digestions allowed the identification of preferred cleavage sites (7). Based on the size of these cleavage fragments as determined by MS/MS, four putative cleavage sites were identified shown with arrowheads in Fig 1B. Only one of these sites at position 58/59 contained a sequence that corresponded to the major MMP-9 specific cleavage consensus sequence P-X-X-|Hy-S/T (Hy: hydrophobic amino acid) as described previously (31). Mutations of the proline at position 55 (P55A) or position 58 from a T to an I (I58T) were generated in PN-1 expression vectors. Transfection of P55A into PC-3ML cells and MMP-9 KD PC-3ML cells did not lead to accumulation of PN-1, suggesting that this mutation had destabilized the protein. However transfection of the I58T mutation led to increased amounts of PN-1 in the conditioned media of the wild type cells compared to the more moderate accumulation of WT PN-1 (Fig 1C). This effect was dependent upon MMP-9 since PN-1 levels after transfection into MMP-9 KD PC-3ML cells were equivalent for both (Fig 1C). This data is consistent with position 58 of PN-1 affecting susceptibility to MMP-9 cleavage. Similar results were obtained with the PN-1 mutation I107T (Fig 1C). Other mutations at the positions 58, 107 and 368 all led to accumulation of PN-1 in PC-3ML cells (Fig 1C). Thus these three sites appear to be dominant cleavage sites in PN-1 for MMP-9. Collectively, out of 21 mutations we generated and tested, mutations including I58T, I58T+I107T, I58T+P368H, I58T+P368Y appeared to be substantially more resistant to MMP-9 cleavage than the wild type (Fig 1C).
PN-1 is targeted by MMP-9 in vivo
The hypothesis that PN-1 levels are controlled through degradation by MMP-9 leads to the prediction that PN-1 levels should be higher in MMP-9 deficient mice. The expression of PN-1 was screened in homogenates made from different organs of WT or MMP-9 KO mice (Fig 2A). PN-1 was most abundantly expressed in seminal vesicles, consistent with previous reports (32). We also readily detected PN-1 in the lung, liver, spleen and prostate. The levels of PN-1 were greatly increased in the lung, prostate and pancreas and slightly increased in the seminal vesicles of the MMP-9 KO mice compared to the wild type (Fig 2A). With longer exposure, a PN-1 degradation fragment was apparent in the lysates from the seminal vesicles and the prostate (Fig 2A-iii), consistent in size with cleavage at aa 107 or at aa 368. In contrast, in the liver and spleen, PN-1 was equally expressed in both WT and MMP-9 KO mice suggesting that other enzymes might be more important for PN-1 degradation in these organs (Fig 2A). Thus in prostate, lung and pancreas, MMP-9 would appear to play a major role in degradation of PN-1. There was more PN-1 in conditioned media of bone marrow derived cells (BMDC) from MMP-9 KO than WT mice (Fig 2B).
Figure 2. PN-1 is targeted by MMP-9 in vivo.
A) Tissue lysates of indicated organs from C57/B6 WT (+/+) or MMP-9 KO (−/−) mice were resolved by SDS-PAGE gel and blotted with anti-mouse PN-1 or anti-β-actin antibody (i and ii). 20ng recombinant mouse PN-1 protein (rPN-1) was a control. Please note β-actin is not present in heart or muscle. Panel iii) shows Panel i) after longer exposure. A PN-1 degradation fragment was now apparent in the lysates from the seminal vesicles and the prostate. B) Primary bone marrow derived cells (BMDC) were grown from C57/B6 WT (+/+) or MMP-9 KO (−/−) mice, serum free conditioned media was subjected to immunoblot with anti-MMP-9 or anti-PN-1 antibody. C) Immunohistochemistry showing the expression of PN-1 (green) in seminal vesicles, pancreas and prostate from WT or MMP-9 KO mice. Cell nuclei were stained by Hoechst (blue).
We examined the expression of PN-1 in situ by immunohistochemistry. The images taken from seminal vesicles, prostate and pancreas showed the accumulation of PN-1 in MMP-9 KO mice (Fig 2C). In particular, the staining for PN-1 was mainly in the extracellular matrix surrounding the glands of the seminal vesicles, pancreas and prostate (Fig 2C). MMP-9 staining is shown in Fig S3. Thus PN-1 is localized to the extracellular matrix and its amount is regulated by MMP-9. Taken together, these results indicate that PN-1 appears to be degraded by MMP-9 in cell culture and in mice in the lung, pancreas and prostate.
MMP-9 regulates uPA activity through cleavage of PN-1
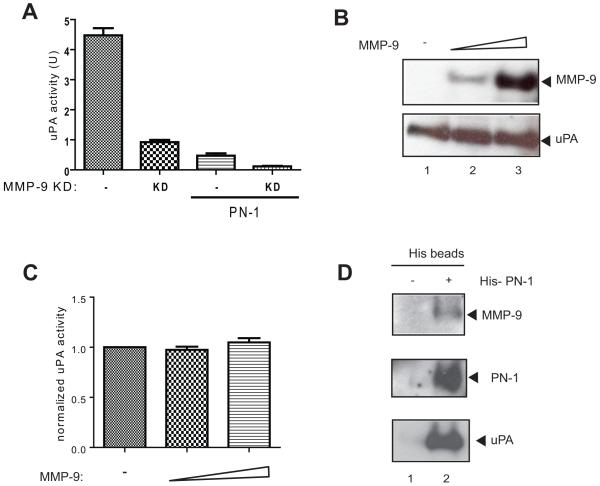
uPA is an inhibitory target of PN-1(9). Increased expression of PN-1 due to transfection with an expression vector led to decreased uPA activity (Fig 3A). Downregulation of MMP-9 led to increased PN-1 amounts and to inhibition of uPA activity (Fig 3A). Both manipulations together led to even lower uPA activity (Fig 3A). To determine whether MMP-9 has a direct impact on uPA level and activity, we incubated these two proteins together. uPA remained intact and active after incubation with MMP-9 (Fig 3B, C). Thus the effect of MMP-9 on increased uPA activity must be indirect and could be mediated by MMP-9 degradation of PN-1.
Figure 3. MMP-9 regulates uPA activity through cleavage of PN-1.
A) The uPA activity of conditioned media collected from PC-3ML cells (-) or MMP-9 KD PC-3ML (KD) cells, with or without PN-1 transfection, were measured. Triplicate experiments were plotted and subjected to one-way ANOVA statistical analysis, P<0.0001. B) Recombinant uPA was incubated with active MMP-9 at 37°C for 3h. All lanes have 10U uPA. Lane 1 has no MMP-9, lane 2 has 50ng and lane 3 has 100ng MMP-9. The reaction products were resolved by 15% SDS-PAGE and immunoblotted with anti-MMP-9 or anti-uPA antibody. C) Reactions as in B): uPA activity was assayed and normalized against uPA control. P>0.05 by one-way ANOVA test. D) Conditioned media collected from control (-) or His-PN-1 transfected (+) PC-3ML cells were incubated with Ni beads for 3h and washed. The protein complexes were then resolved by SDS-PAGE gel and immunoblotted with indicated antibodies.
PN-1 is known to form a SDS-stable covalent complex with its target proteases such as uPA (23). Here we showed uPA was present in the PN-1 complex isolated using the His-tag engineered into PN-1 (Fig 3D). In addition, complexes associated with PN-1 also contained MMP-9 supporting the hypothetical regulation axis of MMP-9/PN-1/uPA.
MMP-9 and PN-1 regulate tumor cell invasion by targeting uPA
As uPA is widely recognized to be integral to migration, metastasis and tumor dormancy (11), we examined the effect of MMP-9 and PN-1 on uPA dependent tumor cell invasion. The MMP-9 KD PC-3ML cell line had a marked reduction in matrigel invasion compared to wildtype PC-3ML cells (Fig 4A), consistent with the established role of MMP-9 in promoting cell invasion. Importantly, PN-1 overexpression in PC-3ML cells significantly reduced invasiveness. When PN-1 was overexpressed in MMP-9 KD PC-3ML cells, invasion was even more substantially inhibited (Fig 4A). Therefore, MMP-9 and PN-1 can oppose each other in the regulation of tumor cell invasion.
Figure 4. PN-1 and MMP-9 regulate tumor cell invasion by targeting uPA.
A) 1× 105 PC-3ML or MMP-9 KD PC-3ML cells, mock treated or transfected with 2μg PN-1 vector for 24hrs, were seeded on matrigel invasion inserts. After 48hrs, invaded cells were stained by Hoechst (Shown in Fig 4A-i). Cells from triplicate experiments were counted, normalized against mock control and plotted (ii), followed by ANOVA test and P<0.0001. B) i)-Diagram showing the experimental design. 2×105 PC-3ML cells alone (mock), or treated with Ambion predesigned negative control siRNA (siNEG) or siRNA uPA (siuPA), were allowed to grow for 24hrs on 6-well plates before transfection with 1μg PN-1 vector. 24hrs after transfection, cells were washed and incubated with serum free medium (SFM) for 48hrs, the conditioned media was then collected, subjected to uPA activity assay (ii) and immunoblotting (iii). In parallel, cells were trypsinized, seeded onto matrigel invasion inserts, and allowed to invade for 48hrs. The invaded cells were stained and counted as shown in iv), followed by a two-way ANOVA test (P<0.0001) and Bonferroni posttests for between groups. Comparison of control (Ctrl) and PN-1 overexpression (PN-1), P<0.001 in Mock or siNEG (marked as *), P>0.05 in siuPA. A two-way ANOVA plus Bonferroni posttests were used for analysing uPA activity. P<0.001 in Mock or siNEG (*), P>0.05 in siuPA.
To understand whether MMP-9 and PN-1 induce this effect through targeting uPA, we knocked down the expression of uPA using siRNA (Fig 4B ii, iii). uPA downregulation greatly reduced invasion (Fig 4B iv). Ectopic expression of PN-1 also inhibited uPA activity and invasion. With the effector molecule uPA depleted by siRNA, overexpressed PN-1 did not further reduce invasion (Fig 4B iv). This result suggests that PN-1 acts to inhibit invasion through inhibition of uPA and further suggests that MMP-9 can enhance invasion through degradation of PN-1.
We tested whether MMP-9 resistant PN-1 mutants retained inhibitory effect against uPA. By measuring uPA activity in cell conditioned media, we found each of the mutant forms tested was capable of uPA inhibition (Fig 5A). The expression levels of uPA in the conditioned media remained unchanged after transfection with the expression vectors for PN-1 and its mutant forms (Fig 5A). Because the inhibitory function of serpins depends upon conformational changes at many regions of the molecules, mutagenesis was based on structural data to attempt to best preserve the overall structure of PN-1 (33). Based on homology to PAI-1, the initial cleavage of PN-1 triggered by the proteases would be anticipated to be between R365 and S366 (34). R365P and S366P mutations appeared to prevent inhibition of uPA activity (Fig 5B).
Figure 5. The impact of PN-1 mutations on uPA activity and cell invasiveness.
A) PC-3ML cells were transfected with the expression vectors for WT-PN-1 and the indicated mutants. uPA levels in the conditioned media were determined by immunoblot (upper panel). The lower panel shows the uPA activity in the condition media from the transfected cells. The reading of uPA activity was analysed by one-way ANOVA followed by Tukey’s multiple comparison test. Comparison of mock with each mutant PN-1 ectopic expression (P<0.05), however there was no significant difference on uPA activity between WT PN-1 and each mutant. B) Conditioned media were collected from cells transfected with expression vectors for WT PN-1, LRP binding mutants (H48A or D49A), or mutations in the RCL (R365P or S366P), and immunoblotted for PN-1 and uPA levels (i) or assayed for uPA activity (ii). P>0.05 between WT and H48A or D49A (NS), or between mock and R365 or S366P. P<0.05 between WT, H48A or D49A and R365P or S366P (*). C) and D) show invasion arrays after transfection with the indicated plasmids. Invaded cells were counted and plotted. In Fig 5C, no significant difference between WT and I58T+I107T, or I58T+P368H, P<0.05 comparing I58T with the rest of groups. In Fig 5D, P<0.05 between Mock, R365P or S366P and WT, H48A, D49A (*); no significance between WT and H48A or D49A (NS).
The effects on invasion of various PN-1 mutations were then tested. MMP-9 resistant mutations I58T+I107T and I58T+P368H displayed similar inhibition of cell invasion as wild type PN-1 (Fig 5C). Unexpectedly, despite inhibiting uPA activity in the same fashion as WT PN-1, I58T mutant was even more effective in reducing cell invasive capacity (Fig 5C). In contrast, R365P and S366P had greatly reduced ability to inhibit invasion, in accordance with their reduced uPA inhibitory activity (Fig 5D). Mutations at H48A and D49A, were reported to impair PN-1 binding to LRP (23), (35). These PN-1 mutants retained the ability to inhibit uPA (Fig 5B ii) and inhibited tumor cell invasion to the same extent as WT PN-1 (Fig 5D). These data indicate that the inhibitory activity of PN-1 is crucial for its effect on tumor cell invasion.
PN-1 is essential for MMP-9 regulated tumor cell invasion
An important consideration is whether PN-1 is a key mediator that links MMP-9 and uPA. To further address this question, we used PN-1 siRNA to reduce the expression of PN-1 in cells (confirmed by qRT-PCR) and in conditioned media (confirmed by immunoblot) (Fig 6A). Importantly, downregulation of PN-1 led to increased uPA activity (Fig 6B). Downregulation of PN-1 led to similar increases in uPA activity in cells deficient of MMP-9 (Fig 6B), suggesting that MMP-9 has little direct effect on uPA activity itself. PC3-ML cell invasion was enhanced by downregulation of PN-1 (Fig 6C). Likewise, reducing PN-1 led to enhanced uPA activity and invasion in cells with downregulated MMP-9 (Fig 6C). Taken together, the data indicate a novel molecular pathway in which MMP-9 regulates tumor cell invasion through the selective targeting of PN-1, thus affecting uPA activity (Fig 6D).
Figure 6. PN-1 is essential for MMP-9 regulation of tumor cell invasion.
A) Immunoblot (i) showing that 10nM siRNA against PN-1 reduced the expression of PN-1 in conditioned media from both PC-3ML cells (Lane 1-3) and MMP-9KD PC-3ML cells (Lane 4-6). qRT-PCR (ii) showing that the expression of PN-1 RNA in PC-3ML cells was reduced by both of 10nM and 20nM PN-1 siRNA. ANOVA test: P<0.05. B) Conditioned media collected from PC-3ML or MMP-9 KD PC-3ML cells, mock treated or treated with 10nM negative control siRNA (siNeg) or siRNA against PN-1 (siPN-1), were assayed for the uPA activity. The reading from triplicate samples were plotted, analysed by a grouped two-way ANOVA (P<0.05) and Bonferroni posttests. P<0.05 between PC-3ML or MMP-9 KD PC-3ML cells in Mock or siNEG, but P>0.05 in siPN-1. C) In parallel experiments to B), cells were treated with or without 10nM siRNA for 48hrs and then trypsinized, 1×105 cells were seeded on each matrigel chamber. 48hrs later, invaded cells were stained, counted and plotted. Data was analysed a grouped two-way ANOVA (P<0.05) and Bonferroni posttests. P<0.05 between PC-3ML or MMP-9 KD PC-3ML cells in Mock or siNEG, but P>0.05 in siPN-1. D) Schematic model of the pathway that MMP-9 regulates uPA activity and tumor cell invasion through cleaving PN-1. A feedback loop has been suggested between MMP-9 and PN-1 (16). However, we failed to see substantial regulation of MMP-9 by PN-1 (Fig S4).
Discussion
In this study, we have identified a novel pathway through which MMP-9 regulates tumor cell invasion via degradation of PN-1. Consistent with the identification of PN-1 as an enzymatic substrate of MMP-9, PN-1 dramatically accumulated in cell line downregulated in MMP-9 and in many of the organs from mice genetically deficient in MMP-9 (Fig 1, 2). Mutation of any of three predominant sites of MMP-9 cleavage in PN-1 led to resistance to degradation (Fig 1C). Next, we demonstrated that MMP-9 regulation of uPA activity is mediated by control of PN-1 levels by degradation (Fig 3). Finally we showed that negative regulation of tumor cell invasion by PN-1 was mediated by PN-1 protease inhibition since mutations in PN-1 that impaired its inhibitory function abrogated its ability to inhibit migration (Fig 4, 5, 6). These results lay the foundation for a new concept arguing that MMP-9 controls the activity of uPA by cleavage of PN-1.
The invasive capacity of tumor cells is an important aspect of tumor progression and a major factor of cancer morbidity and mortality (36). Importantly, these data reveal a protective role of PN-1 against invasion by a prostatic carcinoma cell line. PN-1 overexpression significantly prevents the invasion of tumor cells, analogous to its inhibition of thrombin dependent migration by vascular smooth muscle cells (37). The extracellular matrix in the prostate proved to be a site of high levels of endogenous PN-1 in the mouse (Fig 2A, C). Other serpins have been well documented in cancer invasion and metastasis, including PAI-1 which has paradoxical roles both in stimulating and inhibiting aspects of tumor progression (38). Whether PN-1 has a similar pro-angiogenic role as PAI-1, in which inhibition of plasmin generation reduces fasL release and endothelial apoptosis (39), remains to be investigated. Maspin, a tumor suppressor, is homologous to serpins but does not have demonstrable protease inhibitor activity (40). PN-1 targets uPA and thrombin, both implicated in tumor progression (41).
uPA is an independent prognostic marker for poorer prognosis in breast, gastric, pulmonary, prostate and ovarian cancer (42). The uPA-uPAR system has great impact on prostate cancer progression and metastasis (36). uPA is secreted as a proenzyme and through binding its membrane receptor uPAR, it efficiently converts inactive plasminogen into the active serine protease plasmin. In addition to triggering a cascade of enzymatic activity, binding to uPAR triggers a signalling cascade including a consequent activation of ERK (36). Downregulation of either uPA or uPAR have been shown to inhibit prostate cancer cell invasion and its tumorigenicity in vivo (43). Some of uPAR effects are due to its interactions with integrins especially documented for alpha3 beta 1 and alpha5beta1 (44). PAI-1 or PN-1 binding to uPA reduces those interactions and alters integrin recycling (45). Thus there are several mechanisms through which altered uPA activity might alter migration. Thrombin is also known to regulate tumor cell invasion and metastasis (41). However, prostate cancer cells and PC-3 in particular produce little thrombin endogenously (46). Thus in this system, PN-1 inhibition of thrombin is less likely to play a significant role than uPA does. In this study, we did not find alterations in uPA levels after changes in PN-1 expression (Fig 5A). In our previous research (7), we found significant changes of uPA and tPA levels after MMP-9 downregulation by proteomics, which, may be due to the changes of PN-1 levels (>10 fold). Decreased uPA could result from LRP-mediated clearance of uPA-PN-1 complexes (Table S2).
We have shown that MMP-9 can regulate uPA via cleavage of PN-1. This regulation may be more complex. In THP-1 monocytes, uPA was shown to activate MMP-9 expression at the transcriptional level (47). Moreover, a recent report suggests that PN-1 can induce MMP-9 expression in breast cancer cells when it is complexed with its target proteases such as thrombin and t-PA (16). However, even after the addition of large concentrations of recombinant uPA and PN-1, the increase in MMP-9 levels were modest (about 2 fold) (16), (47). Therefore we also tested the effect of additional PN-1 on MMP-9 production in PC-3ML cells (Fig S4). There was a slight elevation of MMP-9 (less than 2-fold) occurred despite greatly increased PN-1 (Fig S4). It is possible that there is a regulatory feedback loop in which PN-1 might induce MMP-9 that would then lead to decreased PN-1, possibly resulting in relatively minor changes in level overall (Fig 6D). The regulation may be a cell type specific response to PN-1. More importantly, in vivo the situation is more complicated since the feedback loops are not only between the same cell type, but also occurring with host stromal cells and PN-1 in the stroma itself.
Clearly, the interaction between tumor cells and stromal environment is crucial during tumor progression and metastasis. This study has demonstrated the regulatory axis along MMP-9, PN-1 and uPA in tumor cells. Since MMP-9, PN-1 and uPA are all secreted into extracellular spaces in tissues, they closely interact with ECM components. It will be crucial to assess the effects of PN-1 in the context of in vivo assays of tumorigenicity and metastasis in order to determine whether these targets represent novel entry points for pharmacological intervention in the future.
Supplementary Material
Acknowledgements
We thank Dr. Sally Hill and Miss Spela Ferjancic for experimental assistance. We are grateful to Dr Ghislain Opdenakker for providing MMP-9 deficient mice, Dr Mark Stearns for PC-3ML cells (Drexel University, Philadelphia) and Dr Peter Andreasen for pcDNA3-PN-1 plasmid. The research is funded by Cancer Research UK.
Reference
- 1.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 4.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–97. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–85. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 6.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 7.Xu D, Suenaga N, Edelmann MJ, Fridman R, Muschel RJ, Kessler BM. Novel MMP-9 substrates in cancer cells revealed by a label-free quantitative proteomics approach. Mol Cell Proteomics. 2008;7:2215–28. doi: 10.1074/mcp.M800095-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer J, Gloor SM, Rovelli GF, et al. cDNA sequence coding for a rat glia-derived nexin and its homology to members of the serpin superfamily. Biochemistry. 1987;26:6407–10. doi: 10.1021/bi00394a016. [DOI] [PubMed] [Google Scholar]
- 9.Baker JB, Low DA, Simmer RL, Cunningham DD. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980;21:37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- 10.Wagner SL, Lau AL, Nguyen A, et al. Inhibitors of urokinase and thrombin in cultured neural cells. J Neurochem. 1991;56:234–42. doi: 10.1111/j.1471-4159.1991.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 11.Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J Pathol. 2008;214:283–93. doi: 10.1002/path.2282. [DOI] [PubMed] [Google Scholar]
- 12.Baker JB, Gronke RS. Protease nexins and cellular regulation. Semin Thromb Hemost. 1986;12:216–20. doi: 10.1055/s-2007-1003554. [DOI] [PubMed] [Google Scholar]
- 13.Boulaftali Y, Adam F, Venisse L, et al. Anticoagulant and antithrombotic properties of platelet protease nexin-1. Blood. 2009;115:97–106. doi: 10.1182/blood-2009-04-217240. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan PJ, Cunningham DD. Regulation of protease nexin-1 synthesis and secretion in cultured brain cells by injury-related factors. J Biol Chem. 1993;268:3720–7. [PubMed] [Google Scholar]
- 15.Bouton MC, Richard B, Rossignol P, et al. The serpin protease-nexin 1 is present in rat aortic smooth muscle cells and is upregulated in L-NAME hypertensive rats. Arterioscler Thromb Vasc Biol. 2003;23:142–7. doi: 10.1161/01.atv.0000047867.98019.2d. [DOI] [PubMed] [Google Scholar]
- 16.Fayard B, Bianchi F, Dey J, et al. The serine protease inhibitor protease nexin-1 controls mammary cancer metastasis through LRP-1-mediated MMP-9 expression. Cancer Res. 2009;69:5690–8. doi: 10.1158/0008-5472.CAN-08-4573. [DOI] [PubMed] [Google Scholar]
- 17.Farrell DH, Cunningham DD. Glycosaminoglycans on fibroblasts accelerate thrombin inhibition by protease nexin-1. Biochem J. 1987;245:543–50. doi: 10.1042/bj2450543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donovan FM, Vaughan PJ, Cunningham DD. Regulation of protease nexin-1 target protease specificity by collagen type IV. J Biol Chem. 1994;269:17199–205. [PubMed] [Google Scholar]
- 19.Silverman GA, Bird PI, Carrell RW, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–6. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 20.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 21.Conese M, Nykjaer A, Petersen CM, et al. alpha-2 Macroglobulin receptor/Ldl receptor-related protein(Lrp)-dependent internalization of the urokinase receptor. J Cell Biol. 1995;131:1609–22. doi: 10.1083/jcb.131.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strickland DK, Ranganathan S. Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling. J Thromb Haemost. 2003;1:1663–70. doi: 10.1046/j.1538-7836.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- 23.Crisp RJ, Knauer DJ, Knauer MF. Roles of the heparin and low density lipid receptor-related protein-binding sites of protease nexin 1 (PN1) in urokinase-PN1 complex catabolism. The PN1 heparin-binding site mediates complex retention and degradation but not cell surface binding or internalization. J Biol Chem. 2000;275:19628–37. doi: 10.1074/jbc.M909172199. [DOI] [PubMed] [Google Scholar]
- 24.Gao S, Krogdahl A, Sorensen JA, Kousted TM, Dabelsteen E, Andreasen PA. Overexpression of protease nexin-1 mRNA and protein in oral squamous cell carcinomas. Oral Oncol. 2008;44:309–13. doi: 10.1016/j.oraloncology.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Selzer-Plon J, Bornholdt J, Friis S, et al. Expression of prostasin and its inhibitors during colorectal cancer carcinogenesis. BMC Cancer. 2009;9:201. doi: 10.1186/1471-2407-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois B, Masure S, Hurtenbach U, et al. Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest. 1999;104:1507–15. doi: 10.1172/JCI6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stearns ME, Wang M, Fudge K. Liarozole and 13-cis-retinoic acid anti-prostatic tumor activity. Cancer Res. 1993;53:3073–7. [PubMed] [Google Scholar]
- 28.Bernhard EJ, Stanbridge EJ, Gupta S, et al. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60:6597–600. [PubMed] [Google Scholar]
- 29.Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58:1797–806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aureli L, Gioia M, Cerbara I, et al. Structural bases for substrate and inhibitor recognition by matrix metalloproteinases. Curr Med Chem. 2008;15:2192–222. doi: 10.2174/092986708785747490. [DOI] [PubMed] [Google Scholar]
- 31.Kridel SJ, Sawai H, Ratnikov BI, et al. A unique substrate binding mode discriminates membrane type-1 matrix metalloproteinase from other matrix metalloproteinases. J Biol Chem. 2002;277:23788–93. doi: 10.1074/jbc.M111574200. [DOI] [PubMed] [Google Scholar]
- 32.Mansuy IM, van der Putten H, Schmid P, Meins M, Botteri FM, Monard D. Variable and multiple expression of Protease Nexin-1 during mouse organogenesis and nervous system development. Development. 1993;119:1119–34. doi: 10.1242/dev.119.4.1119. [DOI] [PubMed] [Google Scholar]
- 33.Antalis TM, Lawrence DA. Serpin mutagenesis. Methods. 2004;32:130–40. doi: 10.1016/s1046-2023(03)00204-4. [DOI] [PubMed] [Google Scholar]
- 34.Tucker HM, Gerard RD. Sequence requirements in the reactive-center loop of plasminogen-activator inhibitor-1 for recognition of plasminogen activators. Eur J Biochem. 1996;237:180–7. doi: 10.1111/j.1432-1033.1996.0180n.x. [DOI] [PubMed] [Google Scholar]
- 35.Knauer MF, Hawley SB, Knauer DJ. Identification of a binding site in protease nexin I (PN1) required for the receptor mediated internalization of PN1-thrombin complexes. J Biol Chem. 1997;272:12261–4. doi: 10.1074/jbc.272.19.12261. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Cozzi PJ. Targeting uPA/uPAR in prostate cancer. Cancer Treat Rev. 2007;33:521–7. doi: 10.1016/j.ctrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Richard B, Pichon S, Arocas V, et al. The serpin protease nexin-1 regulates vascular smooth muscle cell adhesion, spreading, migration and response to thrombin. J Thromb Haemost. 2006;4:322–8. doi: 10.1111/j.1538-7836.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- 38.Binder BR, Mihaly J. The plasminogen activator inhibitor "paradox" in cancer. Immunol Lett. 2008;118:116–24. doi: 10.1016/j.imlet.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Bajou K, Peng H, Laug WE, et al. Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell. 2008;14:324–34. doi: 10.1016/j.ccr.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou Z, Anisowicz A, Hendrix MJ, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–9. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 41.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Ulisse S, Baldini E, Sorrenti S, D’Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9:32–71. doi: 10.2174/156800909787314002. [DOI] [PubMed] [Google Scholar]
- 43.Pulukuri SM, Gondi CS, Lakka SS, et al. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J Biol Chem. 2005;280:36529–40. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Chapman HA, Wei Y. Protease crosstalk with integrins: the urokinase receptor paradigm. Thromb Haemost. 2001;86:124–9. [PubMed] [Google Scholar]
- 45.Czekay RP, Loskutoff DJ. Plasminogen activator inhibitors regulate cell adhesion through a uPAR-dependent mechanism. J Cell Physiol. 2009;220:655–63. doi: 10.1002/jcp.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J Biol Chem. 2006;281:26483–90. doi: 10.1074/jbc.M604376200. [DOI] [PubMed] [Google Scholar]
- 47.Menshikov M, Elizarova E, Plakida K, et al. Urokinase upregulates matrix metalloproteinase-9 expression in THP-1 monocytes via gene transcription and protein synthesis. Biochem J. 2002;367:833–9. doi: 10.1042/BJ20020663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.