Abstract
In order to uncover patterns and processes of segregation of co-existing cytotypes, we investigated a zone in the eastern Alps (Austria) where diploid and hexaploid individuals of the alpine herb Senecio carniolicus Willd. (Asteraceae) co-occur. Linking the fine-scale distribution of cytotypes to environmental and spatial factors revealed segregation along an ecological gradient, which was also reflected in the cytotype-associated plant assemblages. Compared to diploids, hexaploids are found in more species-rich and denser communities. This may be due to their better competitive ability and lower tolerance of abiotic stress compared to the diploids. The lack of any intermediate cytotypes suggests the presence of strong reproductive isolation mechanisms, whose nature is, however, elusive.
Keywords: contact zone, cytotype mixture, fine-scale distribution, flow cytometry, habitat segregation, polyploidy, spatial structure
Introduction
Polyploidy is widely considered a major driving force for speciation and it is reported that all angiosperms (with the possible exception of Amborella) have at least an ancient polyploid origin (Soltis et al. 2009). Ploidy differences do not only occur between species, but also within them (Weiss et al. 2003, Baack 2004). Intra-populational cytotype mixtures are reported repeatedly (Rothera & Davy 1986, Lumaret et al. 1987, Felber-Girard et al. 1996, Husband & Schemske 1998, Halverson et al. 2008) and increasingly detected in various plant groups, not least due to recent advances in comparative measurements of genome size (e.g., Kron et al.2007) allowing rapid screening of a large number of samples and consequently a more detailed assessment of cytotype distributions. In contrast to the predictions from theory (minority cytotype exclusion, Levin 1975), mixed ploidy populations seem to be fairly stable and frequently occur in nature (e.g., Lumaret et al. 1987). Hence, there is an increasing interest in understanding the factors that enable the coexistence of cytotypes, such as reproductive isolation or divergent adaptations to environmental conditions (Mable 2003).
Ecological requirements of polyploids often differ from those of the parental cytotypes (Ehrendorfer 1979, Petit & Thompson 1997) and numerous studies report the ecological differentiation of cytotypes in a wide variety of species. For example, in Dactylis glomerata sympatric diploid and tetraploid individuals differ in habitat preferences, with diploids mainly found growing in low density forest floor vegetation and tetraploids in various habitats but showing a preference for open areas and disturbed sites (Lumaret et al. 1987). Jay & Blaise (1991) found diploid individuals of Lotus corniculatus mainly in bare, unprotected habitats while tetraploids preferred more sheltered habitats. In contrast, no significant ecological differentiation was found for Ranunculus adoneus (Baack & Stanton 2005) and Aster amellus (Mandáková & Münzbergová 2006). Likewise, habitat segregation is not significant in Centaurea jacea, but cytotypes segregated altitudinally with diploids occurring mostly above and tetraploids below 500 m a.s.l. (Hardy et al. 2000). An influence of altitude on the distribution of cytotypes is also recorded for Lotus corniculatus (Gauthier et al. 1998), Taraxacum section Ruderalia (Meirmans et al. 2003) and Chamerion (Epilobium) angustifolium (Husband & Schemske 1998).
Altitudinal differentiation of cytotypes is also observed in Senecio carniolicus (Asteraceae), a plant of alpine to subnival grasslands, moraines and stable screes (Ellenberg 1996) occurring at altitudes up to 3300 m a.s.l. (Reisigl & Pitschmann 1958) in the Eastern Alps and the Carpathians. Alpine populations consist of three main cytotypes (2x, 4x, 6x), the most widespread ones (diploids and hexaploids) occurring in mixed populations over a significant portion of the Alpine range (Suda et al. 2007). At a finer scale, these cytotypes are altitudinally segregated with hexaploids restricted to lower altitudes and diploids occupying the whole altitudinal range (Schönswetter et al. 2007). This differentiation is hypothesized to be a result of ecological niche differentiation, but the design of this study prevented the separation of altitudinal from other ecological effects.
Here, the effect of altitude was minimized and the contact zone of diploids and hexaploids in the mixed diploid/hexaploid stretch of the previously investigated transect was studied (Schönswetter et al. 2007). According to the hypothesis of exclusively altitudinal differentiation, cytotypes should be distributed randomly in their contact zone. Alternatively, spatial structure may be caused by ecological factors, such as habitat differences, which have been identified as important factors in other studies and were also invoked as an explanation in our previous study.
Material and methods
Field work
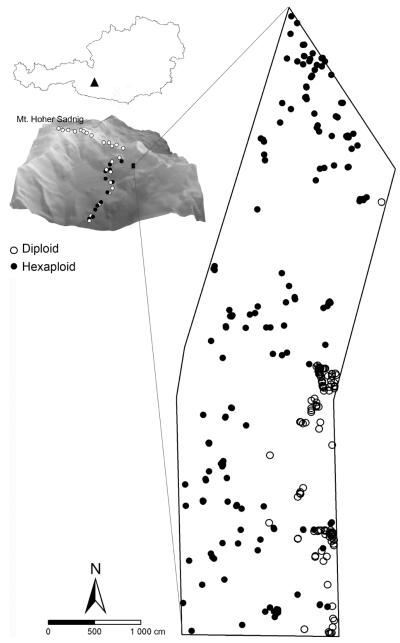
The study area is situated in the mountain range of Goldberggruppe in the Austrian Alps (Fig. 1). Based on a previous investigation (Schönswetter et al. 2007), a ca 990 m2 study plot on the south-eastern slope of Mt Hoher Sadnig (46.94°N, 12.99°E, ca. 2460 m a.s.l.), which is located within the contact zone between di- and hexaploid S. carniolicus, and thus likely to contain both cytotypes, was chosen. In order to control for possible effects of altitude, the study plot was selected to cover only a negligible altitudinal range (ca 20 m). To characterize the fine-scale distribution of cytotypes, the relative positions of all individuals of S. carniolicus occurring within the plot were mapped by triangulation. Leaf samples for flow-cytometric determination of ploidy level were collected from each plant and dried by placing them in a container with silica gel.
Fig. 1.
Fine-scale distribution of diploid and hexaploid cytotypes of Senecio carniolicus in the study plot, located within the contact zone (insert; modified from Schönswetter et.al. 2007) on Mt Hoher Sadnig, Carinthia, Austria.
All vascular plant species growing within a radius of 20 cm around each sampled individual were recorded together with their abundance classified as < 25%, 25–50%, 50–75% or 75–100%. Bryophytes and lichens were not recorded because they are difficult to identify. Additionally, total vegetation and rock cover were estimated in terms of percentage cover. Nomenclature of vascular plants follows Fischer et al. (2008).
Flow cytometry
DNA ploidy levels of samples dried over silica gel were estimated using a 4′,6-diamino-2-phenylindole (DAPI) flow cytometry as described in Suda & Trávníček (2006) with minor modifications (Suda et al. 2007). Pisum sativum ‘Ctirad’ (2C = 9.09 pg; Doležel et al. 1998) was selected as an appropriate internal reference standard. Up to three Senecio samples were pooled together; our previous results (Schönswetter et al. 2007, Suda et al. 2007) indicate that this approach does not affect the reliability of the ploidy estimates. Nevertheless, if there were several peaks, which indicates several levels of ploidy, each plant was reanalysed separately.
Statistical analyses
Analyses were conducted using the package ‘vegan’ (Oksanen et al. 2009) for R (R Development Core Team 2008). Differences in species composition accompanying cytotypes were evaluated using non-metric multidimensional scaling (NMDS). The multidimensional space of accompanying species represented by pairwise Bray-Curtis distances between individuals was reduced to a four dimensional configuration (NMDS-space). The quality of this transformation is indicated by the ‘stress’, which is a non-linear monotone transformation of observed distances and ordination distances (Oksanen 2009). To avoid being trapped in local minima, calculations were repeated twenty times with random starting arrangements and the configuration with the lowest stress for the given number of axes was chosen. To enhance the determinacy of scaling and orientation of NMDS-axes the ordination result was post-processed following the default-options of the function ‘metaMDS’ (Oksanen et al. 2009).
Vegetation and rock cover, and the number of accompanying species, were included in the resulting ordination diagram using the function ‘envfit’, which calculates Pearson’s correlation coefficients with NMDS-axes scores. Indicator species analysis based on a comparison of species abundance and occurrence within and between sites (Dufrȩne & Legendre 1997) was conducted to identify species representative of the habitats of diploid and hexaploid individuals of S. carniolicus. The significance of the indicator value for each species (ranging from 0, i.e. no association, to 1, i.e. perfect association) was calculated using a randomization procedure with 1000 permutations.
A variance partitioning procedure was carried out following the concept of Borcard et al. (1992) to decompose the variation of the cytotype distribution pattern into environmental [a], spatial [c], spatially structured environmental [b] and undetermined [d] fractions, but using generalized linear models (GLM) with binomial or Gaussian error distribution instead of CCA (canonical correspondence analysis). The presence/absence of the diploid cytotype – the presence of a diploid individual equates to the absence of a hexaploid and vice versa – was used as a response variable. The NMDS-axes scores were used as environmental predictors. To create spatially explanatory variables a principal coordinates of neighbour matrices analysis (PCNM; Borcard & Legendre 2002) was applied. PCNM performs an eigenvalue decomposition using principal coordinate analysis (PCoA) of a truncated distance matrix of Euclidean distances among individuals. Thereby, the length of the longest edge of a minimum spanning tree was used as a threshold value for the truncation. Partial regression coefficients of PCNM-axes scores (i.e., eigenvectors) corresponding to positive eigenvalues were tested for significance using the method of permutation of residuals using the full model (Anderson & Legendre 1999). Only PCNM-axes with a significant partial regression coefficient were retained as spatial predictors in the variation partitioning analysis. In order to remove broad-scale linear trends, the response variable was detrended prior to the PCNM analysis (Borcard et al. 2004), i.e. the residuals of a multiple regression of the response variable on the x- and y-coordinates of individuals were used to calculate the eigenvectors.
Fraction [a+b] was determined using a binomial GLM by regressing cytotype on NMDS-axes, while detrended cytotype data were linearly regressed on significant PCNM-axes to obtain the fraction [b+c]. To calculate fraction [a] each detrended NMDS-axis was linearly regressed on all significant PCNM-axes and residuals were computed for each regression. Cytotype data were subjected to multiple regression of the residuals using a binomial GLM. Finally, fraction [b] was obtained by subtracting fraction [a] from fraction [a+b] and fraction [c] by subtracting [b] from [b+c]. The fraction of variation explained was calculated as the difference between the null-model and the residual deviance of the fitted model divided by the deviance of the null-model.
Results
DNA ploidy levels were determined for 275 individuals of S. carniolicus, which revealed the presence of 110 diploids and 165 hexaploids. Flow cytometric analyses yielded high-resolution histograms with distinct G0/G1 peaks for both the sample and standard with negligible background noise. Mean coefficients of variation of sample and standard peaks were 3.12% (range 1.57–4.88) and 2.06% (range 1.21–3.29), respectively. Setting Pisum sativum as the unit value, relative fluorescence intensities (means±SD) for diploids and hexaploids were 0.760±0.009 and 2.123±0.033, respectively.
Individuals of both cytotypes were spatially segregated and overlapped only slightly (Fig. 1). The mean numbers of neighbours within a radius of 1 m for diploid and hexaploid individuals were 10.8 and 2.2, respectively, indicating strong clustering of diploids.
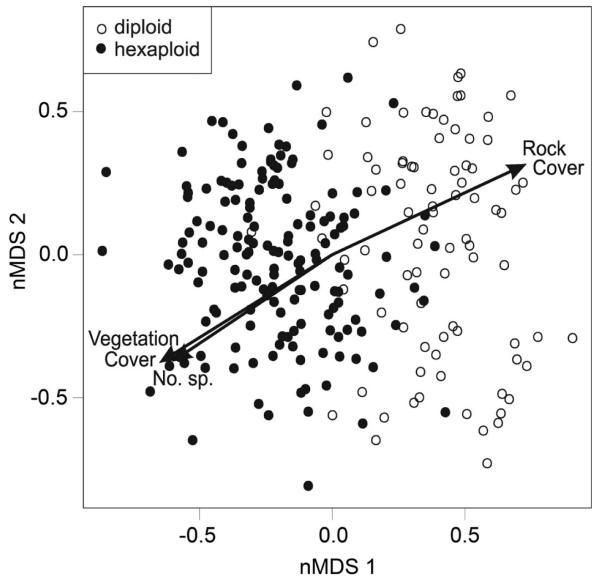
The stress value (16.9%) of the NMDS ordination indicates an acceptable (< 20%; Kruskal 1964) representation of the neighbouring vegetation of Senecio individuals in NMDS-space. Diploid and hexaploid individuals were clearly separated along the first NMDS-axis (Fig. 2). Additionally, a strong correlation of the first NMDS-axis with rock cover (r2 = 0.47, P < 0.001), vegetation cover (r2 = 0.37, P < 0.001) and the number of accompanying species (r2 = 0.43, P < 0.001) was evident. Diploids were linked to high rock covers (t = 14.1, df = 131.5, P < 0.001), while hexaploids were connected to communities with dense vegetation (t = –11.9, df = 207.7, P < 0.001) and high numbers of species (t = –14.8, df = 271.9, P < 0.001).
Fig. 2.
Ordination plot of the first two axes of a four-dimensional non-metric multidimensional scaling (NMDS) performed on the accompanying species of 275 individuals of Senecio carniolicus on Mt Hoher Sadnig, Carinthia, Austria. Arrows indicate correlations of NMDS axes with vegetation cover, rock cover and number of species (No. sp.).
Variance partitioning indicated a large contribution of environmental factors (explained variance of a+b: 74.7%, df = 4, deviance = 276.5, Akaike information criterion [AIC] = 103.61) of which 5.2% were purely environmental (a: df = 4, deviance = 19.3, AIC = 360.82). Spatially structured factors accounted for 73.2% (b+c: df = 21, deviance = 139.4, AIC = 363.86) resulting in a large contribution of spatially structured environmental (b: 69.5%) of which only 3.7% was attributed to spatial factors [c] (Fig. 3). The proportion of unexplained variance [d] was 21.6%.
Fig. 3.
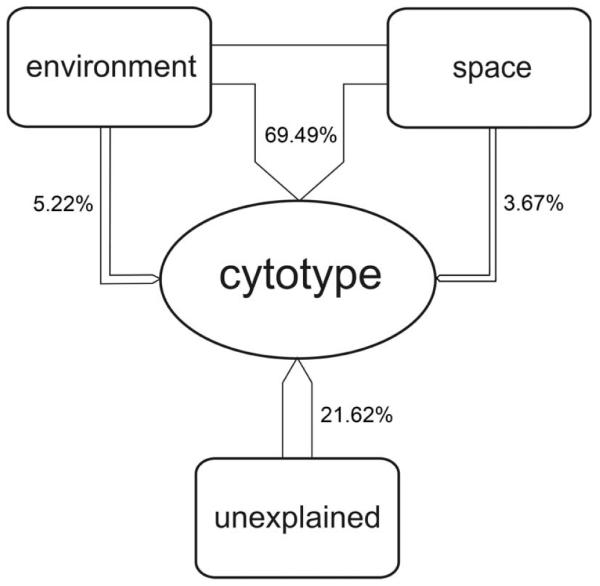
Schematic representation of the partitioning of variance in the distribution of the cytotypes of Senecio carniolicus within the study plot on Mt Hoher Sadnig, Carinthia, Austria, following Borcard (1992), see text for further details. The two predictors, “environment” and “space” are represented by the NMDS– and PCNM-axes, respectively.
Discussion
Diploid and hexaploid individuals of Senecio carniolicus were non-randomly distributed in the study plot, indicating that altitude plays at best a minor role in determining the spatial segregation of cytotypes. Diploids occurred exclusively towards the plot’s eastern margin, whereas hexaploids were widespread throughout the plot, but absent from areas densely inhabited by diploids. Hence, the distribution of cytotypes overlapped only slightly (Fig. 1). Although neither cytotype is capable of clonal growth (R. Flatscher et al., unpubl.), population densities differed between the cytotypes. While hexaploid S. carniolicus was fairly evenly distributed throughout the plot, diploids were highly clustered. So far, only a few studies provide insights into the small-scale structure of contact zones between cytotypes. Felber-Girard et al. (1996) and Baack (2004) found similarly sharp contact zones for Anthoxanthum alpinum and Ranunculus adoneus, respectively.
Cytotypes of S. carniolicus showed clear habitat segregation. Variance partitioning revealed that cytotype-associated differentiation of species assemblages accounted for 74.7% of the overall variation. Relating NMDS-axes to ploidy level using logistic regression yielded a remarkably high value of Nagelkerke’s R2 (0.86, P < 0.001). Habitats of hexaploids were characterized by a higher species number and denser vegetation (Fig. 2). Typical accompanying species were Avenula versicolor, Loiseleuria procumbens, Scorzoneroides helvetica and Vaccinium vitis-idaea (Table 1), which are characteristic of alpine acidophilic meadows and dwarf shrub communities (Mucina et al. 1993). Carex curvula, the dominant graminoid of acidophilic alpine grasslands in the Eastern Alps, co-occurred with both cytotypes, but higher abundances were associated with hexaploids than diploids (Table 1). In contrast, habitats of diploids were characterized by a higher rock cover, sparse and prostrate vegetation and a low number of accompanying species due to the absence of many elements of alpine grassland vegetation. Only the high-alpine Phyteuma globulariifolium and the chionophobic Saponaria pumila were characteristic accompaniers (Table 1). The number of species specifically accompanying diploid S. carniolicus is low, because several species typical of the subnival ecotone, which were previously recorded to be consistent accompanying species of the diploid cytotype (Schönswetter et al. 2007), did not occur at our study plot. The affinity of diploids for open habitats is also recorded in Lotus corniculatus/L. alpinus (Gauthier et al. 1998).
Table 1.
Indicator values for species best representing the habitat surrounding each of the two cytotypes of Senecio carniolicus on Mt Hoher Sadnig, Carinthia, Austria. Higher values (in bold) indicate a stronger preference for a certain habitat.
| Accompanying species | Diploid | Hexaploid | P-value |
|---|---|---|---|
| Agrostis alpina (Poaceae) | 0.34 | 0.15 | 0.002 |
| Agrostis rupestris (Poaceae) | 0.16 | 0.03 | 0.001 |
| Anthoxanthum alpinum (Poaceae) | 0.00 | 0.19 | 0.001 |
| Avenula versicolor (Poaceae) | 0.00 | 0.59 | 0.001 |
| Carex curvula (Cyperaceae) | 0.24 | 0.60 | 0.001 |
| Juncus trifidus (Juncaceae) | 0.03 | 0.22 | 0.004 |
| Loiseleuria procumbens (Ericaceae) | 0.10 | 0.52 | 0.001 |
| Phyteuma globulariifolium (Campanulaceae) | 0.13 | 0.02 | 0.003 |
| Potentilla aurea (Rosaceae) | 0.00 | 0.23 | 0.001 |
| Saponaria pumila (Caryophyllaceae) | 0.33 | 0.13 | 0.003 |
| Scorzoneroides helvetica (Asteraceae) | 0.01 | 0.81 | 0.001 |
| Vaccinium vitis-idaea (Ericaceae) | 0.01 | 0.45 | 0.001 |
Environmental factors that determine the segregation of the two cytotypes of S. carniolicus are strongly spatially structured (Fig. 3). This is probably due to the presence of an east-west oriented ecological gradient, which appears to reflect microtopography. The study plot extends from the eastern slope of a shallow depression eastwards to a weakly developed ridge. Microtopography is regarded as one of the major forces determining the small-scale distribution of alpine plant communities (Körner 2003), because it strongly affects soil depth, duration of snow cover, differences in wind and temperature regimes and thus water and nutrient supply.
This gradient appears to override altitudinal segregation. In fact, the present results suggest that the apparent altitudinal segregation of cytotypes found in the same area by Schönswetter et al. (2007) is most probably determined by the same factors as in our study plot. Specifically, the lower part of the previously investigated transect, where di- and hexaploids co-occurred, provided a mosaic of habitat types similar to those covered by the present study, whereas the upper part of the transect, where only the diploids occurred, was on an exposed ridge.
Habitat segregation between di- and hexaploid cytotypes of S. carniolicus is likely to be driven by cytotype-specific requirements and life history traits. The low-growing diploids apparently are pioneers and as such experience little competitive pressure, especially on rocky wind-exposed sites with short or even no snow cover and growing in bare lithosols or pure rock habitats. Hexaploids are taller (R. Flatscher et al., unpubl.) and are therefore likely to be more competitive and productive. Similarly, a number of case studies report that the polyploids are larger and more productive than the diploids when measured in terms of cell size (Lumaret et al. 1987, Lindner & Garcia 1997), biomass production and inflorescence size (Petit & Thompson 1997), seed yield (Lindner & Garcia 1997, Burton & Husband 2000) and the growth rate of the seedlings (Baack & Stanton 2005). In S. carniolicus, the presumed higher resource requirements of the polyploids appear to be correlated with sensitivity to mechanical stress or frost drought. Differences in abundances between cytotypes might merely reflect the different availability of suitable (open) habitat.
The absence of tetraploid individuals, likely products of inter-cytotype hybridization, among the 563 individuals from Mt Hoher Sadnig investigated (present study, Schönswetter et al. 2007, M. Sonnleitner et al., unpubl.), suggest the presence of strong barriers to crossing. The nature of these barriers remains unknown, but the occurrence of di- and hexaploids in close spatial proximity suggests that habitat segregation cannot be the only reason for the highly restricted gene flow between cytotypes. Studies investigating other pre- and postzygotic mechanisms and their relative importance in the reproductive isolation of the cytotypes are currently being undertaken.
Acknowledgements
We thank the Austrian Science Fund (FWF) for financing the flow-cytometry (project P20736–B16 to P.S.), Roman Eckstein, Pedro Escobar García, Veronika Fontana, Markus Hofbauer, Iris Schönbrunner and Christiana Staudinger for helping with the field work. Additional support was supplied by the Ministry of Education, Youth and Sports of the Czech Republic (MSM 0021620828) and the Academy of Sciences of the Czech Republic (AV0Z60050516). We are grateful to two anonymous reviewers for their valuable comments on the draft and to Tony Dixon for improving our English.
References
- Anderson MJ, Legendre P. An empirical comparison of permutation methods for tests of partial regression coefficients in a linear model. J. Statist. Comput. Simul. 1999;62:271–303. [Google Scholar]
- Baack EJ. Cytotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus: Ranunculaceae) Am. J. Bot. 2004;91:1783–1788. doi: 10.3732/ajb.91.11.1783. [DOI] [PubMed] [Google Scholar]
- Baack EJ, Stanton ML. Ecological factors influencing tetraploid speciation in snow buttercups (Ranunculus adoneus): niche differentiation and tetraploid establishment. Evolution. 2005;59:1936–1944. [PubMed] [Google Scholar]
- Borcard D, Legendre P. All-scale analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Model. 2002;153:51–68. [Google Scholar]
- Borcard D, Legendre P, Avois-Jaquet C, Toumisto H. Dissecting the spatial structure of ecological data at multiple scales. Ecology. 2004;85:1826–1832. [Google Scholar]
- Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology. 1992;73:1045–1055. [Google Scholar]
- Burton TL, Husband BC. Fitness differences among diploids, tetraploids, and their triploid progeny in Chamerion angustifolium: mechanisms of inviability and implications for polyploid evolution. Evolution. 2000;54:1182–1191. doi: 10.1111/j.0014-3820.2000.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Lucretti S, Meister A, Lysák MA, Nardi L, Obermayer R. Plant genome size estimation by flow cytometry: inter-laboratory comparison. Ann. Bot. 1998;82:17–26. [Google Scholar]
- Dufrȩne M, Legendre P. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 1997;67:345–366. [Google Scholar]
- Ehrendorfer F. Polyploidy and distribution. Basic Life Sci. 1979;13:45–60. doi: 10.1007/978-1-4613-3069-1_3. [DOI] [PubMed] [Google Scholar]
- Ellenberg H. Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. Ed. 5 Ulmer; Stuttgart: 1996. [Google Scholar]
- Felber-Girard M, Felber F, Buttler A. Habitat differentiation in a narrow hybrid zone between diploid and tetraploid Anthoxanthum alpinum. New Phytol. 1996;133:531–540. [Google Scholar]
- Fischer MA, Oswald K, Adler W. Exkursionsflora für Österreich, Liechtenstein und Südtirol. Ed. 3 Biologiezentrum der Oberösterreichischen Landesmuseen; Linz: 2008. [Google Scholar]
- Gauthier P, Lumaret R, Bédécarrats A. Genetic variation and gene flow in Alpine diploid and tetraploid populations of Lotus (L. alpinus (D.C.) Schleicher/L. corniculatus L.). 1. Insights from morphological and allozyme markers. Heredity. 1998;80:683–693. [Google Scholar]
- Halverson K, Heard SB, Nason JD, Stireman JO. Origins, distribution, and local co-occurrence of polyploid cytotypes in Solidago altissima (Asteraceae) Am. J. Bot. 2008;95:50–58. doi: 10.3732/ajb.95.1.50. [DOI] [PubMed] [Google Scholar]
- Hardy OJ, Vanderhoeven S, De Loose M, Meerts P. Ecological, morphological and allozymic differentiation between diploid and tetraploid knapweeds (Centaurea jacea) from a contact zone in the Belgian Ardennes. New Phytol. 2000;146:281–290. doi: 10.1046/j.1469-8137.2000.00631.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DW. Cytotype distribution at a diploid-tetraploid contact zone in Chamerion (Epilobium) angustifolium (Onagraceae) Am. J. Bot. 1998;85:1688–1694. [PubMed] [Google Scholar]
- Jay M, Blaise S. Evolution and differentiation of populations of Lotus corniculatus sensu lato (Fabaceae) from the southern French Alps Massif du Ventoux and Montagne de Lure. Can. J. Bot. 1991;69:2286–2290. [Google Scholar]
- Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. Springer; Heidelberg: 2003. [Google Scholar]
- Kron P, Suda J, Husband BC. Applications of flow cytometry to evolutionary and population biology. Ann. Rev. Ecol. Evol. Syst. 2007;38:847–876. [Google Scholar]
- Kruskal JB. Nonmetric multidimensional scaling: a numerical method. Psychometrika. 1964;29:115–129. [Google Scholar]
- Legendre P, Legendre L. Numerical ecology. Ed. 2 Elsevier; Amsterdam: 1998. [Google Scholar]
- Levin DA. Minority cytotype exclusion in local plant populations. Taxon. 1975;24:35–43. [Google Scholar]
- Lindner R, Garcia A. Genetic differences between natural populations of diploid and tetraploid Dactylis glomerata ssp. izcoi. Grass Forage Sci. 1997;52:291–297. [Google Scholar]
- Lumaret R, Guillerm JL, Delay J, Loutfi AAL, Izco J, Jay M. Polyploidy and habitat differentiation in Dactylis glomerata L. from Galicia (Spain) Oecologia. 1987;73:436–446. doi: 10.1007/BF00385262. [DOI] [PubMed] [Google Scholar]
- Mable BK. Breaking down taxonomic barriers in polyploidy research. Trends Plant Sci. 2003;8:582–590. doi: 10.1016/j.tplants.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Mandáková T, Münzbergová Z. Distribution and ecology of cytotypes of the Aster amellus aggregates in the Czech Republic. Ann. Bot. 2006;98:845–856. doi: 10.1093/aob/mcl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirmans PG, Vlot EC, Den Nijs JCM, Menken SBJ. Spatial ecological and genetic structure of a mixed population of sexual diploid and apomictic triploid dandelions. J. Evol. Biol. 2003;16:343–352. doi: 10.1046/j.1420-9101.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- Mucina L, Grabherr G, Ellmauer T. Die Pflanzengesellschaften Österreichs. Gustav Fischer Verlag; Jena: 1993. [Google Scholar]
- Oksanen J. Multivariate analysis of ecological communities in R: vegan tutorial. 2009 URL: [ http://cc.oulu.fi/~jarioksa/opetus/metodi/vegantutor.pdf]
- Oksanen J, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, Stevens MHH, Wagner H. Vegan: community ecology package. 2009 URL: [ http://cran.r-project.org/, http://vegan.r-forge.r-project.org] [Google Scholar]
- Petit C, Thompson JD. Variation in phenotypic response to light availability between diploid and tetraploid populations of the perennial grass Arrhenatherum elatius from open and woodland sites. J. Ecol. 1997;85:657–667. [Google Scholar]
- R Development Core Team R: a language and environment for statistical computing. 2008 URL: [http://www.R-project.org]
- Reisigl H, Pitschmann H. Obere Grenzen von Flora und Vegetation in der Nivalstufe der zentralen Ötztaler Alpen (Tirol) Plant Ecol. 1958;8:93–129. [Google Scholar]
- Rothera SL, Davy AJ. Polyploidy and habitat differentiation in Deschampsia cespitosa. New. Phytol. 1986;102:449–467. [Google Scholar]
- Schönswetter P, Lachmayer M, Lettner C, Prehsler D, Rechnitzer S, Reich DS, Sonnleitner M, Wagner I, Huelber K, Schneeweiss GM, Trávníček P, Suda J. Sympatric diploid and hexaploid cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps are separated along an altitudinal gradient. J. Plant Res. 2007;120:721–725. doi: 10.1007/s10265-007-0108-x. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng CF, Sankoff D, dePamphilis CW, Wall PK, Soltis PS. Polyploidy and angiosperm diversification. Am. J. Bot. 2009;96:336–348. doi: 10.3732/ajb.0800079. [DOI] [PubMed] [Google Scholar]
- Suda J, Trávníček P. Reliable DNA ploidy determination in dehydrated tissues of vascular plants by DAPI flow cytometry: new prospects for plant research. Cytometry Part A. 2006;69A:273–280. doi: 10.1002/cyto.a.20253. [DOI] [PubMed] [Google Scholar]
- Suda J, Weiss-Schneeweiss H, Tribsch A, Schneeweiss GM, Trávníček P, Schönswetter P. Complex distribution patterns of di-, tetra-, and hexaploid cytotypes in the European high mountain plant Senecio carniolicus (Asteraceae) Am. J. Bot. 2007;94:1391–1401. doi: 10.3732/ajb.94.8.1391. [DOI] [PubMed] [Google Scholar]
- Weiss H, Stuessy TF, Grau J, Baeza CM. Chromosome reports from South American Hypochaeris (Asteraceae) Ann. Miss. Bot. Garden. 2003;90:56–63. [Google Scholar]