SUMMARY
OBJECTIVE
To investigate mortality rates and risk factors for death among smear-negative tuberculosis (TB) suspects.
DESIGN
Cohort study nested within a cluster-randomised trial of community-based active case finding. Smear-negative TB suspects were followed for 12 months, with home tracing where necessary. We calculated mortality rates and used regression analysis to investigate the relationship between clinical characteristics and death.
RESULTS
Between February 2006 and June 2007, 1195 smear-negative TB suspects were followed for 1136.8 person-years. Human immunodeficiency virus (HIV) prevalence was 63.3%. During follow-up, 139 participants died (11.6%) and mortality rates remained high throughout; 119 (16.5%) HIV-positive individuals and 13 (3.1%) HIV-negative individuals died (HR = 5.8, 95%CI 3.3–10.4, P < 0.001). Advanced immunosuppression was the main risk factor for death among HIV-positive participants, with CD4 count < 50 cells/μl associated with a 13-fold increased risk of death. Anti-retroviral treatment (ART) was initiated by only 106 (14.7%), with long delays in accessing care.
CONCLUSION
HIV-positive smear-negative TB suspects are at high and sustained risk of death. Current guidelines for the management of HIV-infected TB suspects are limited, and this study adds to evidence that specific policies are required to promote earlier HIV and TB diagnosis and reduce delays in ART initiation.
Keywords: TB, HIV, mortality, smear-negative, antiretroviral
WITH OVER 33 MILLION PEOPLE globally infected with the human immunodeficiency virus (HIV),1 there has been an unprecedented increase in the incidence of tuberculosis (TB) cases.2 Sub-Saharan Africa, where over two thirds of HIV-infected people live,1 has seen the largest increase in TB incidence,3 with 79% of the global burden of HIV-associated TB found in the region.2 The proportion of TB diagnosed as smear-negative disease has also increased dramatically.2
The Stop TB Strategy objective of reducing the global TB burden (prevalence and deaths) due to TB by 50% relative to 1990 levels would result in fewer than one million people dying of TB per year by 2015.4,5 Central to the strategy are the 3 I’s (intensified case finding, isoniazid preventive treatment [IPT] and infection control).6,7 However, review of current progress suggests that, in Africa, the planned reduction in mortality is unlikely to be achieved.4,8
Of particular concern is the high mortality rates observed among people with TB-HIV co-infection. With advancing immunosuppression, patients with TB-HIV co-infection are increasingly likely to have smear-negative disease.9 Early antiretroviral treatment (ART) is effective in reducing mortality among TB patients.10 However, the period of time required to diagnose smear-negative TB can be prolonged, with multiple clinic visits and repeated investigations that can delay initiation of ART.11,12
We have previously reported on the diagnosis of TB in a large cohort of smear-negative suspects in Harare, Zimbabwe.11 In the present study, we report on mortality over 12 months of follow-up. The aims were to describe patterns of mortality, identify risk factors that were associated with death, particularly in HIV-positive individuals, and make policy recommendations to improve treatment outcomes of TB suspects.
STUDY POPULATION AND METHODS
This was a cohort study nested within a cluster-randomised trial of intensified community-based case finding for TB.13 Participants came from 46 neighbourhood clusters in Harare and were recruited between February 2006 and July 2007 (during the first half of the cluster-randomised trial). The study methods have been described in detail elsewhere.11,13 In brief, participants in the trial had access to community outreach microscopy services and submitted two sputum smears to a mobile laboratory. Participants who were found to have two negative sputum smears and had ongoing symptoms of cough or weight loss, night sweats or a history of haemoptysis within the last year were invited to attend the study clinic based within the city public hospital and were given a redeemable voucher for transport costs to facilitate access.
Clinic assessments were made at 0, 1, 8 and 12 months, with active home tracing for participants who did not attend follow-up visits. At each clinic assessment, participants underwent assessment of TB symptoms and were offered HIV testing and counselling (HTC). Participants who declined HTC were asked to consent to provide an anonymous sample for HIV testing. At each visit, participants with TB symptoms also underwent chest radiography (CXR) (read by the attending physician), repeat sputum smears and TB culture. Participants diagnosed with TB were referred immediately for treatment.
A confirmed TB case was defined as a positive smear (including scanty positive) or one or more positive cultures for Mycobacterium tuberculosis. Smear- and culture-negative TB was defined by a clinical or radiological illness consistent with TB that did not respond to broad-spectrum antibiotics but did respond to 1 month of anti-tuberculosis treatment, or where treatment was started independently by an outside provider.
All participants who were HIV-positive on HTC were prescribed cotrimoxazole. Those who met national ART criteria (CD4 count < 200 cells/μl or World Health Organization [WHO] Stage 4 defining condition) were referred for ART at the public clinic located in the neighbouring building. From May 2006, CD4 counts were measured among HIV-positive participants who underwent HTC and analysed using CyFlow® (Partec UK Ltd, Canterbury, UK).
Statistical methods
We expressed baseline participant characteristics as proportions and compared HIV-positive and HIV-negative participants using Fisher’s exact test and Wilcoxon rank sum tests. Time to death was calculated as time from first clinical assessment at the study clinic to date of death when known, or censored on date of last follow-up assessment.
Due to the small number of deaths in HIV-negative individuals, we investigated risk factors for death in HIV-positive individuals only. We used Cox proportional hazard regression accounting for clustering to assess associations between clinical characteristics and death.
Statistical analysis was undertaken using STATA 11.1 (Stata Corp, College Station, TX, USA).
Ethical approval
This study was approved by the Ethics Committees of the London School of Hygiene & Tropical Medicine, the Medical Research Council of Zimbabwe and the Biomedical Research and Training Institute, Harare.
RESULTS
Participant characteristics
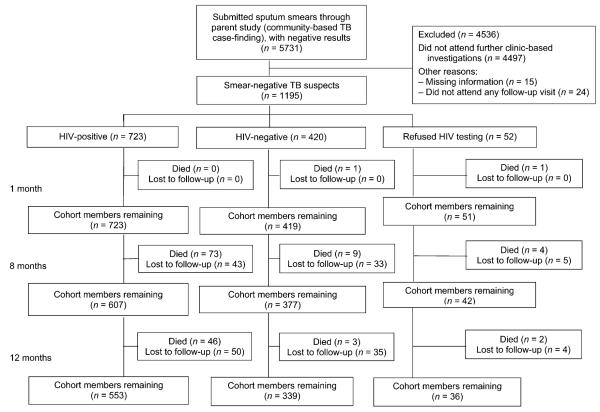
Between February 2006 and June 2007, 5731 adult participants (age ≥16 years) in the parent cluster-randomised trial had two negative sputum smears and were invited to attend the study clinic for further investigation. Of these, 1234 (21.5%) attended and form the starting cohort; 39 (3.2%) were excluded from this analysis, leaving 1195 participants who contributed 1136.8 person-years (py) of follow-up (Figure 1). Participant characteristics are shown in Table 1.
Figure 1.
Cohort flow chart. TB = tuberculosis; HIV = human immunodeficiency virus.
Table 1.
Baseline characteristics and TB diagnostic outcomes of study participants*
| Characteristic | HIV-positive n (%) or median [IQR] |
HIV-negative n (%) or median [IQR] |
P value |
|---|---|---|---|
| Total | 723 (60.5) | 420 (35.1) | |
| Male sex | 253 (35.0) | 177 (42.1) | 0.019 |
| Age, years | 36 [30.0–42.0] | 39 [30.0–54.0] | <0.001 |
| Previously treated for TB | 138 (19.1) | 35 (8.3) | <0.001 |
| CD4 count, cells/μl | 149 [69–255] | — | |
| Duration of TB symptoms, weeks | 11.5 [4.0–20.0] | 12 [4.0–24.0] | 0.506 |
| Diagnosed with smear- or culture- positive TB during study |
67 (9.3) | 17 (4.0) | 0.001 |
| Diagnosed with smear- and culture-negative TB during study† |
110 (15.2) | 17 (4.0) | <0.001 |
| TB case definitions not met, but treated for TB |
5 (0.7) | 0 | 0.090 |
HIV status unknown in 52 (4.4%).
Two additional participants were diagnosed with smear- and culture-negative TB who did not undergo HIV testing.
TB = tuberculosis; HIV = human immunodeficiency virus; IQR = interquartile range.
HIV prevalence was 63.3% (95% confidence interval [CI] 60.3–66.1); among those who tested HIV-positive, 494 (68.3%) underwent HTC and 229 (31.7%) anonymous testing. HIV-positive participants were more likely to be male and were younger than HIV-negative participants, but had a similar duration of TB symptoms. A greater proportion of HIV-positive (19.1%) than HIV-negative participants (8.3%, P < 0.001) had been previously treated for TB.
Overall, 218 (18.2%) participants were diagnosed with TB during the 12-month follow-up period, including 182 (25.2%) HIV-positive participants, 34 (8.1%) HIV-negative participants and two who did not undergo HIV testing.
CD4 counts were obtained in 391 (79.1% of HTC acceptors and 54.1% of all HIV-positive patients). Among the 137 HIV-positive HTC acceptors diagnosed with TB, 105 (76.6%) had CD4 counts measured. The overall median CD4 count was 149 cells/μl (interquartile range [IQR] 69–255); it was 126 cells/μl (IQR 70–209) in participants diagnosed with TB. CD4 counts were <200 cells/μl in 246 of 391 (62.9%) patients and <350 cells/μl in 331/391 (84.6%).
Mortality during cohort follow-up
Overall, there were 139 deaths, giving a crude mortality rate of 122.3/1000 py (95%CI 104.4–144.3). Of the 139 participants who died, 119 (85.6%) were HIV-positive and 13 (9.4%) were HIV-negative, giving mortality rates of respectively 179.2 (95%CI 149.7–214.5) and 30.7 (95%CI 17.8–52.9) per 1000 py. Seven (5.0%) deaths occurred in participants whose HIV status was unknown.
Figure 2 shows Kaplan-Meier plots stratified by HIV status. Rates of mortality for both HIV-positive and HIV-negative participants were relatively constant throughout the 12 months of follow-up, with mortality risk 6-fold higher in HIV-positive participants (hazard ratio [HR] 5.8, 95%CI 3.3–10.4, P < 0.001).
Figure 2.
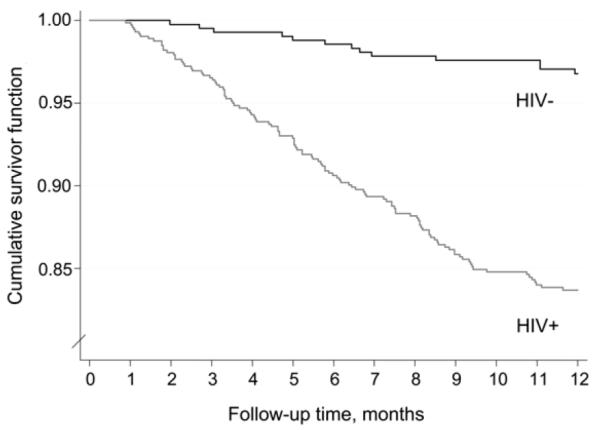
Kaplan-Meier plot of mortality of smear-negative tuberculosis suspects stratified by HIV status. HIV− = human immunodeficiency virus negative; HIV+ = human immunodeficiency virus positive.
When mortality rates were stratified by CD4 count, a trend towards increased risk of death with advancing immunosuppression was shown, with participants with a CD4 count of <50 cells/μl having the highest mortality rate (483.6/1000 py, 95%CI 340.1–687.6, Figure 3). Participants who tested positive for HIV but did not have a CD4 count measured and participants who underwent anonymous HIV testing for study purposes had similar mortality rates.
Figure 3.
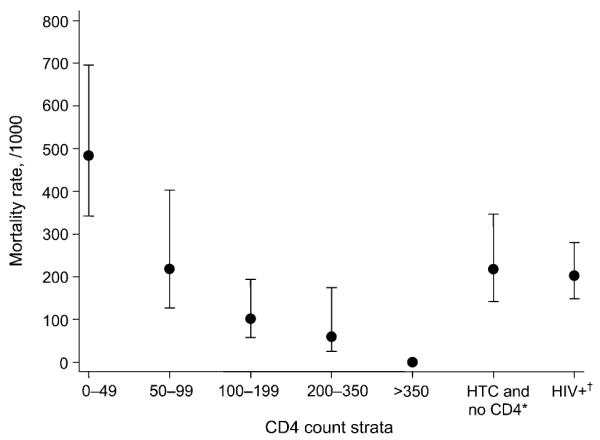
Mortality rate in HIV-positive participants stratified by CD4 count strata and HIV test. *Participant underwent HIV testing and counselling but did not have CD4 count measured. †Participant underwent anonymous HIV testing for study purposes and did not have CD4 count measured. HTC = HIV testing and counselling; HIV+ = human immunodeficiency virus positive.
Risk factors for mortality in HIV-positive participants
Univariable analysis of risk factors for death among HIV-positive participants showed that low CD4 count, a confirmed diagnosis of TB, low weight and an abnormal CXR were associated with an increased risk of death (Table 2). Analysis of TB symptoms found that the presence of an increasing number of TB symptoms (cough > 3 weeks, the presence of cough productive of sputum, and chest pain and cough with an abnormal CXR) were all associated with increased risk of death.
Table 2.
Risk factors for mortality in human immunodeficiency virus positive study participants
| Deaths/py follow-up |
Unadjusted HR (95%CI) |
P value | Adjusted HR (95%CI) |
P value | |
|---|---|---|---|---|---|
| Total (0–12 months) | 119/664.0 | ||||
| Sex | |||||
| Male | 51/223.6 | 1.00 | 1.00 | ||
| Female | 68/440.4 | 0.68 (0.45–1.02) | 0.061 | 0.77 (0.52–1.16) | 0.213 |
| Age, years | |||||
| <25 | 5/58.5 | 1.00 | 1.00 | ||
| 25–34 | 45/238.0 | 2.19 (0.94–5.12) | 2.45 (1.03–5.82) | ||
| 35–44 | 47/239.0 | 2.29 (0.99–5.33) | 0.267 2.11 (0.83–5.35) | 0.183 | |
| ≥45 | 22/128.6 | 1.99 (0.85–4.67) | 1.92 (0.77–4.79) | ||
| Weight, kg | |||||
| <50 | 35/116.9 | 2.80 (1.33–5.90) | 1.70 (0.84–3.41) | ||
| 50–59 | 50/278.0 | 1.67 (0.85–3.29) | 0.002 | 0.86 (0.43–1.72) | 0.001 |
| 60–69 | 25/185.4 | 1.25 (0.56–1.83) | 0.93 (0.39–2.23) | ||
| ≥70 | 9/83.7 | 1.00 | 1.00 | ||
| TB symptoms, n | |||||
| 1 | 17/131.9 | 1.00 | |||
| 2 | 21/190.9 | 0.85 (0.44–1.65) | |||
| 3 | 36/154.8 | 1.80 (1.00–3.23) | <0.0001 | ||
| 4 | 45/186.4 | 1.88 (1.13–3.12) | |||
| Cough >3 weeks | |||||
| No | 21/172.3 | 1.00 | |||
| Yes | 98/490.6 | 1.62 (1.09–2.42) | 0.017 | ||
| Cough >3 weeks with sputum and chest pain |
|||||
| No cough | 21/172.3 | 1.00 | |||
| Cough >3 weeks and no sputum or chest pain |
10/92.9 | 0.87 (0.44–1.72) | 0.004 | ||
| Cough >3 weeks and at least one of sputum or chest pain |
88/397.6 | 1.80 (1.20–2.71) | |||
| CXR | |||||
| Normal | 28/205.0 | 1.00 | |||
| Abnormal | 64/291.2 | 1.61 (1.06–2.42) | 0.027 | 1.17 (0.76–1.83) | 0.213 |
| Not done | 27/167.8 | 1.18 (0.72–1.93) | 1.45 (0.95–2.21) | ||
| Recent weight loss | |||||
| No | 12/162.3 | 1.00 | 1.00 | ||
| Yes | 107/499.5 | 2.90 (1.44–5.83) | 0.003 | 2.53 (1.25–5.12) | 0.010 |
| Chest pains when coughing | |||||
| No cough | 21/173.4 | 1.00 | 1.00 | ||
| No | 29/196.6 | 1.20 (0.75–1.92) | <0.001 | 1.01 (0.61–1.68) | 0.022 |
| Yes | 69/293.9 | 1.93 (1.29–2.88) | 1.64 (1.02–2.64) | ||
| Night sweats | |||||
| No | 48/250.3 | 1.00 | |||
| Yes | 71/413.7 | 0.90 (0.67–1.23) | 0.495 | ||
| TB diagnosed during study | |||||
| No | 81/499.6 | 1.00 | 1.00 | ||
| Yes | 38/164.4 | 1.43 (1.06–1.92) | 0.018 | 1.17 (0.84–1.61) | 0.354 |
| History of previous TB treatment |
|||||
| No | 91/538.2 | 1.00 | |||
| Yes | 28/125.8 | 1.32 (0.86–2.01) | 0.202 | ||
| CD4 count, cells/μl | |||||
| <50 | 31/64.1 | 14.13 (5.88–33.96) | 12.93 (5.06–33.08) | ||
| 50–99 | 12/55.0 | 6.35 (2.65–15.18) | 6.31 (2.52–15.83) | ||
| 100–199 | 11/108.7 | 2.96 (1.10–7.91) | 2.73 (0.99–7.53) | ||
| ≥200 | 5/146.4 | 1.00 | <0.0001 | 1.00 | <0.0001 |
| Unknown | 60/289.8 | 6.03 (2.62–13.89) | 6.10 (2.63–14.12) | ||
| Initiated ART during study | |||||
| No | 94/567.5 | 1.00 | |||
| Yes | 25/96.5 | 1.57 (0.98–2.50) | 0.060 |
HR = hazard ratio; CI = confidence interval; TB = tuberculosis; CXR = chest X-ray; ART = antiretroviral treatment.
Participants who initiated ART during the study period were noted to be at increased risk of death (HR 1.57, 95%CI 0.98–2.50). However, those who initiated ART had more advanced immunosuppression, and ART initiation was likely a marker of more severe ill health: HIV-positive participants who initiated ART and died had a median CD4 count of 31 cells/μl (IQR 16–86), whereas those who did not initiate ART and died had a median CD4 count of 52.5 cells/μl (IQR 23–136).
In multivariable analysis, the main risk factor for death was low CD4 count, with a CD4 count of <50 cells/μl associated with a 13-fold increased risk of death (HR 12.9 95% CI 5.1–33.1, Table 2). HIV-positive participants who did not have their CD4 count measured had a 6-fold increased risk of death compared to participants with a CD4 count of >200 cells/μl (HR 6.1, 95%CI 2.6–14.1). A positive TB diagnosis during the study was not associated with death on multivariable analysis.
Uptake of ART
During the study period, 106 patients (14.7% of all HIV-positive participants) were started on ART at a time when access through the public health care system was problematic. Median time to initiation of ART was 3.0 months (IQR 1.2–7.7). The median CD4 count at ART initiation was 72.5 cells/μl.
DISCUSSION
The main findings of this large cohort of smear-negative TB suspects were the high mortality (11.6%) at 12 months follow-up, with the majority of deaths occurring in HIV-positive participants. While the high risk of death in HIV-positive individuals diagnosed with TB is well recognised,14 prompting guidelines to recommend initiation of ART as soon as possible and without waiting for CD4 count measurement,15 there has been little research into the outcomes of HIV-positive TB suspects. Our findings demonstrate that TB suspects are vulnerable to death through a complex set of factors that contribute to delayed initiation of ART, including programme constraints, and compounded by the need to concurrently complete TB investigations and ART initiation steps.
We found that 85% of HIV-positive TB suspects had a CD4 count of <350 cells/μl and would qualify for ART on the basis of their advanced immunosuppression alone. CD4 counts of HIV-positive TB suspects have rarely been reported in Southern Africa. In a study of newly diagnosed HIV-positive individuals in Ethiopia, the median CD4 count in all participants was 181 cells/μl, and it was 110 cells/μl in those diagnosed with TB.16 In a study from South-East Asia, 66.7% of HIV-positive, smear-negative TB suspects screened had a CD4 count of <350 cells/μl.17 A confirmed TB diagnosis prompts immediate referral for ART under current guidelines,18 and our data add to the evidence that this policy should be extended to TB suspects.
As expected, we found that advanced immunosuppression was the key risk factor for death. We had anticipated a high early mortality rate, as seen in HIV-negative TB patients and in HIV-positive ART initiators with TB.19,20 For example, in a South African ART clinic, HIV-positive TB suspects and individuals with confirmed HIV and TB co-infection had high early mortality rates.20 In contrast, in this study, mortality rates remained relatively constant during the 12-month follow-up period. If confirmed by other studies, this would suggest that interventions are required throughout the routine care pathway, from identification as a TB suspect to HIV testing and initiation of ART. Currently, routine management of TB suspects in health services is suboptimal,21 with high rates of mortality22 and missed opportunities for HIV testing23 and referral for ART.24
To reduce mortality, this study highlights the importance of improving the management of TB suspects in a number of areas (Table 3). First, we recommend that monitoring and reporting systems for TB suspects be strengthened to include the routine measurement of numbers of TB suspects (using a ‘cough register’ approach) and their outcomes, including HIV test uptake, TB treatment commencement and preliminary HIV care outcomes (e.g., ART referral).12 This could be extended to indicate the availability and uptake of standard and new TB diagnostics26,27 and provide a useful resource for impact and equity evaluations.
Table 3.
Current policy and future priority areas for integrated HIV and TB management
| Current policy recommendations |
|
| Future priority areas for reducing mortality in TB suspects |
|
HIV = human immunodeficiency virus; TB = tuberculosis; HTC = HIV counselling and testing; WHO = World Health Organization; ART = antiretroviral treatment; CXR = chest X-ray; IPT = isoniazid preventive treatment; ACF = active case finding.
Provider-initiated HTC is recommended for all individuals presenting to health services with symptoms of TB.28 Recent figures show that approximately 45% of TB patients in Africa know their HIV status.23 In addition to promoting provider-initiated HIV testing for TB suspects, home-based HIV testing programmes (perhaps linked to TB active case finding13) offer a feasible and acceptable approach to increase the uptake of HIV testing in the community.29
The WHO recommends that all TB patients should be referred for ART as soon as possible, without waiting for CD4 count measurement.18 On the basis of our findings of advanced immunosuppression and high and sustained risk of mortality, we suggest that TB suspects should also be referred for ART initiation without delay for CD4 count measurement (analogous to WHO Stage 3 or 4).
Despite numerous calls for the integration of HIV and TB services, care is often still delivered through parallel clinic systems. This means that HIV-positive TB suspects inevitably have to register at two separate clinics to receive comprehensive care. We suggest that HIV and TB care could be structured to allow initiation of ART from within TB clinics, or treatment and monitoring of TB from within ART clinics. This would ensure that TB suspects are managed by a single clinical team, reducing unnecessary clinic and hospital visits and allowing more rapid initiation of ART. Improvements in infection control and delivery of IPT would be needed. Further decentralisation of ART provision from hospital to primary health care clinics could allow delivery of combined ART and TB care in the community.25
There are a number of limitations to this study. Although the parent study undertook intensified case finding for TB within urban areas in Harare, this cohort relied upon re-presention to the study clinic for further diagnostic assessment. Approximately one fifth of the individuals screened in the community attended. It is therefore possible that the cohort may have selected individuals who were more symptomatic or worried about their illness. Alternatively, very unwell individuals may not have been able to return to the clinic due to debility or death. Mortality rates could then have been either over- or underestimated, although the higher mortality rates observed in routinely presenting TB suspects in a separate study in Harare suggest that members of this cohort are more healthy than those identified in clinical settings.22
Ascertainment of death was undertaken by community-based tracing of non-attenders, including interviews with surviving relatives. However, additional information on causes of death from postmortem examination was not available. Loss to follow-up was 14.2% in this cohort, and previous studies of smear-positive TB patients30 and ART initiators31 who were lost to follow-up have shown high rates of mortality.
CONCLUSIONS
Despite a similarly high prevalence of HIV and advanced immunosuppression, current policies for TB suspects do not provide the same level of holistic care as for TB patients. To reduce mortality, TB suspects should be included in the same series of recommendations that have been developed for HIV-positive TB patients, and with emphasis on earlier HIV diagnosis, more rapid initiation of ART, increased use of cotrimoxazole preventive treatment, and increased integration of HIV and TB care. Further studies in different country and clinical settings are required to confirm our findings of advanced immunosuppression, prolonged risk of mortality and poor access to ART among HIV-positive TB suspects.
Acknowledgements
PM receives funding from the Wellcome Trust Clinical PhD Programme at Liverpool School of Tropical Medicine. ELC was funded by a Wellcome Trust Senior Fellowship in Clinical Tropical Medicine.
References
- 1.Joint United Nations Programme on HIV/AIDS . AIDS epidemic update. UNAIDS; Geneva, Switzerland: 2009. [Google Scholar]
- 2.World Health Organization . Global tuberculosis control: epidemiology, strategy, financing. WHO; Geneva, Switzerland: 2009. WHO/HTM/TB/2009.411. [Google Scholar]
- 3.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges and change in the era of antiretroviral treatment. Lancet. 2006;367:926–937. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]
- 4.Stop TB Partnership . The global plan to stop TB, 2006–2015. WHO; Geneva, Switzerland: 2006. [Google Scholar]
- 5.Marais BJ, Raviglione MC, Donald PR, et al. Scale-up of services and research priorities for diagnosis, management, and control of tuberculosis: a call to action. Lancet. 2010;375:2179–2191. doi: 10.1016/S0140-6736(10)60554-5. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO Three ‘I’s Meeting: intensified case finding (ICF), isoniazid preventive therapy (IPT) and TB infection control for people living with HIV. WHO; Geneva, Switzerland: 2008. [Google Scholar]
- 7.Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic—when will we act? Lancet. 2010;375:1906–1919. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]
- 8.Lönnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 9.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 10.Karim S S Abdool, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimairo M, Macpherson P, Bandason T, et al. The risk and timing of tuberculosis diagnosed in smear-negative TB suspects: a 12-month cohort study in Harare, Zimbabwe. Plos ONE. 2010;5:e11849. doi: 10.1371/journal.pone.0011849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harries AD, Zachariah R, Lawn SD. Providing HIV care for co-infected tuberculosis patients: a perspective from sub-Saharan Africa. Int J Tuberc Lung Dis. 2009;13:6–16. [PubMed] [Google Scholar]
- 13.Corbett EL, Bandason T, Duong T, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376:1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–333. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. WHO; Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 16.Shah S, Demissie M, Lambert L, et al. Intensified tuberculosis case finding among HIV-infected persons from a voluntary counseling and testing center in Addis Ababa, Ethiopia. J Acquir Immune Defic Syndr. 2009;50:537–545. doi: 10.1097/QAI.0b013e318196761c. [DOI] [PubMed] [Google Scholar]
- 17.Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. WHO; Geneva, Switzerland: 2009. [Google Scholar]
- 19.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 20.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 21.Botha E, den Boon S, Lawrence KA, et al. From suspect to patient: tuberculosis diagnosis and treatment initiation in health facilities in South Africa. Int J Tuberc Lung Dis. 2008;12:936–941. [PubMed] [Google Scholar]
- 22.Dimairo M, Mativenga S, Dauya E, et al. The fate of sputum smear-negative TB suspects managed by routine clinical services in Harare, Zimbabwe. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. February 8–11, 2009; [Abstract 778] [Google Scholar]
- 23.World Health Organization . Global tuberculosis control: a short update to the 2009 report. WHO; Geneva, Switzerland: 2009. [Google Scholar]
- 24.Meintjes G, Schoeman H, Morroni C, Wilson D, Maartens G. Patient and provider delay in tuberculosis suspects from communities with a high HIV prevalence in South Africa: a cross-sectional study. BMC Infect Dis. 2008;8:72. doi: 10.1186/1471-2334-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harries AD, Zachariah R, Lawn SD, Rosen S. Strategies to improve patient retention on antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2010;15(Suppl 1):70–75. doi: 10.1111/j.1365-3156.2010.02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaisson RE, Martinson NA. Tuberculosis in Africa—combating an HIV-driven crisis. N Engl J Med. 2008;358:1089–1092. doi: 10.1056/NEJMp0800809. [DOI] [PubMed] [Google Scholar]
- 27.Chaisson RE, Harrington M. How research can help control tuberculosis. Int J Tuberc Lung Dis. 2009;13:558–568. [PubMed] [Google Scholar]
- 28.World Health Organization . Guidance on provider-initiated HIV testing and counselling in health facilities. WHO; Geneva, Switzerland: 2007. [Google Scholar]
- 29.Ganguli I, Bassett IV, Dong KL, Walensky RP. Home testing for HIV infection in resource-limited settings. Curr HIV/AIDS Rep. 2009;6:217–223. doi: 10.1007/s11904-009-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Squire SB, Belaye AK, Kashoti A, et al. ‘Lost’ smear-positive pulmonary tuberculosis cases: where are they and why did we lose them? Int J Tuberc Lung Dis. 2005;9:25–31. [PubMed] [Google Scholar]
- 31.Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are ‘lost to follow-up’ in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]