Abstract
Phytomyxea (plasmodiophorids) is an enigmatic group of obligate biotrophic parasites. Most of the known 41 species are associated with terrestrial and freshwater ecosystems. However, the potential of phytomyxean species to influence marine ecosystems either directly by causing diseases of their hosts or indirectly as vectors of viruses is enormous, although still unexplored. In all, 20% of the currently described phytomyxean species are parasites of some of the key primary producers in the ocean, such as seagrasses, brown algae and diatoms; however, information on their distribution, abundance and biodiversity is either incomplete or lacking. Phytomyxean species influence fitness by altering the metabolism and/or the reproductive success of their hosts. The resulting changes can (1) have an impact on the biodiversity within host populations, and (2) influence microbial food webs because of altered availability of nutrients (e.g. changed metabolic status of host, transfer of organic matter). Also, phytomyxean species may affect their host populations indirectly by transmitting viruses. The majority of the currently known single-stranded RNA marine viruses structurally resemble the viruses transmitted by phytomyxean species to crops in agricultural environments. Here, we explore possible ecological roles of these parasites in marine habitats; however, only the inclusion of Phytomyxea in marine biodiversity studies will allow estimation of the true impact of these species on global primary production in the oceans.
Keywords: biodiversity, biotrophic interaction, environmental monitoring, plant pathology, plasmodiophorid, Plasmodiophora, protist, zoospores
Introduction
Phytomyxea is defined as a group of obligate biotrophs (Karling 1968; Braselton 2001; Neuhauser et al. 2010). The phytomyxean species live as endobiotic parasites in a wide range of organisms, including flowering plants, brown algae, diatoms and oomycetes. Phytomyxean parasites are notorious as agents of plant diseases or vectors for the transmission of viruses that cause plant diseases, among them clubroot disease of brassicas (Dixon 2009), powdery scab of potatoes and potato mop top virus (Merz 2008), and more than 15 cereal and other plant viruses (Kanyuka et al. 2003; Rush 2003; Rochon et al. 2004). Some of these economically important parasites of agricultural crops have been studied in depth by plant pathologists; however, our knowledge of most phytomyxean species in both freshwater and marine environments is currently limited to brief published records.
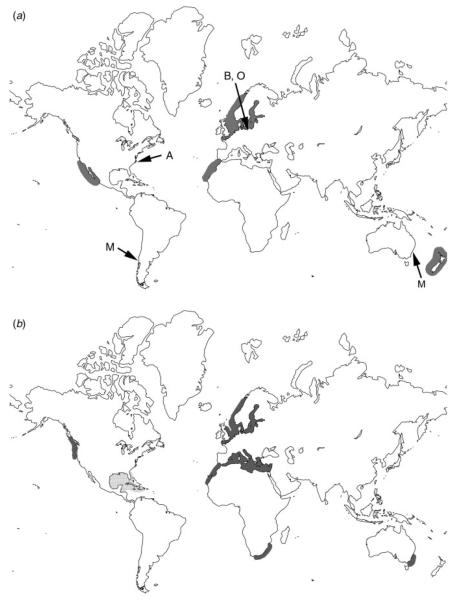
This imbalance of information is also mirrored in the number of species described; most of the currently known species are parasites of plants in terrestrial habitats or of freshwater hosts and only a few species are described from marine hosts. However, the few species known from marine habitats are parasites of important primary producers such as brown algae, diatoms and seagrasses. Johnson and Sparrow (1961) listed Tetramyxa parasitica, Plasmodiophora bicaudata, Pl. maritima, Pl. halophilae and Pl. diplantherae as common parasites of seagrasses and Phagomyxa algarum as a parasite of brown algae. Recently, Maier et al. (2000), Parodi et al. (2010) and Walker and Campbell (2009) investigated two phytomyxean species from marine environments, Maullinia ectocarpii and Pl. bicaudata, which are pathogens of brown algae and seagrasses respectively. Phytomyxean parasites of marine diatoms have only recently been described (Schnepf et al. 2000). Johnson and Sparrow (1961), den Hartog (1989), Schnepf et al. (2000) and Maier et al. (2000) all have suggested that some phytomyxean species with marine hosts have a worldwide distribution, a view also reflected by the specimens listed in Karlings’ monograph (Karling 1968; Fig. 1). Although there is minimal information on distribution because phytomyxids with marine hosts have been poorly studied, we suspect that these organisms may be common parasites in many marine ecosystems worldwide; however, at present there are only anecdotal data available to support this hypothesis.
Fig. 1.
Global distribution of records of marine Phytomyxea. The figure is based on the origin of the specimens listed (Karling 1968; den Hartog 1989; Maier et al. 2000; Schnepf et al. 2000, and the references therein), although the distribution of some of the species is likely to be much wider. For (a), grey shades indicate the distribution of Tetramyxa parasitica. M=Maullina ectocarpi, A Phagomyxa algarum, B=Phagomyxa bellerochae, O=Phagomyxa odontellae. For (b), dark grey=Plasmodiophora bicaudata, dotted light grey=Plasmodiophora diplanthere.
In the present review, we will focus on the biology of marine and estuarine species of phytomyxids, their life cycles and possible biodiversity. We also consider their roles as parasites of important marine primary producers, the nature of their interactions with viruses, potential impacts on ecosystem stability and dynamics in marine and estuarine communities, including both health and biodiversity of the plant communities and their possible influences on the (microbial) food web.
Classification
The class Phytomyxea includes a monophyletic group of eukaryotes. Currently, this class is considered to be part of the protist supergroup Rhizaria (Cavalier-Smith 1993; Bulman et al. 2001; Burki et al. 2010) and has been placed within the phylum Cercozoa and the Endomyxa (Bass et al. 2009). On the basis of 18S rDNA data, the class Phytomyxea comprises the following two distinct orders: Plasmodiophorida, which comprises mainly parasites of green plants, and the Phagomyxida, which comprises parasites of diatoms and brown algae. Because the taxonomic classification of this group has been unclear since its discovery, the informal term ‘plasmodiophorids’ has been used to include the whole group during the past few decades (Braselton 2001). Phytomyxean species are characterised by cruciform nuclear division, which has been observed in all species described so far, a complex extrusome for infecting host cells (Rohr and Stachel), and a distinctive life cycle. Currently, there are 41 known species belonging to 12 genera (Neuhauser et al. 2010).
The taxonomic placement of the marine species within Phytomyxea is difficult. The five species parasitic on seagrasses are currently classified as Plasmodiophorida, mainly on the basis of morphological characteristics and because their full life cycles have been documented. However, the true taxonomic position is yet to be proven because molecular data are completely missing for these species. The parasites of brown algae and diatoms are currently classified together in the family Phagomyxida. This classification is based primarily on 18S rDNA data, and the full life cycle including resting spores has been documented only recently for one species (Parodi et al. 2010).
Life cycles
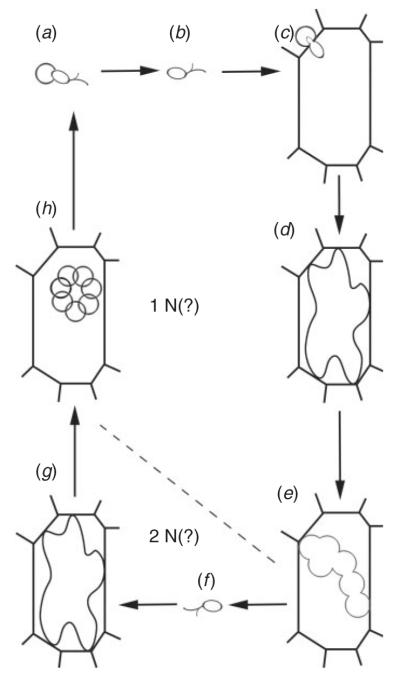
In general, the complete life cycle (Fig. 2) of Phytomyxea includes two types of intracellular plasmodia (sporogenic and sporangial), a resting spore with a chitin cell wall and two types of zoospores (primary and secondary; Karling 1968; Dixon 2009). The zoospores possess two whiplash flagella of unequal length, a characteristic they share with many other species in the Cercozoa. The details of the life cycle of some species of phytomyxids remain largely unknown. Until recently, the resting spores were thought to be absent in the Phagomyxida (species parasitic in brown algae and diatoms); however, Parodi et al. (2010) identified the resting stage of the brown algal parasite Maullinia ectocarpii, disproving the theory that the life cycle of this species is incomplete. Several stages of the life cycle have still not been observed in the parasites of diatoms (Schnepf et al. 2000).
Fig. 2.
Generalised phytomyxean life cycle. Not all stages have yet been reported for some phytomyxean species, and the life cycle may be complicated in some species. (a) Release of primary zoospores from resting spores. (b) Free primary zoospore. (c) Infection of a suitable host. (d) Primary plasmodium inside a host cell. (e) Lobed zoosporangia. (f) Secondary zoospore. (g) Secondary plasmodium formed after infection of a suitable host with at secondary zoospore. (h) Resting spores.
The zoospores are the only part of the life cycle outside the host, and are the main mode of dissemination. Both types of zoospores can move either by the two flagella in liquid media or by lobose pseudopodia on the surface of a suitable host (Karling 1968; Maier et al. 2000; Dixon 2009). It is not known whether zoospores of Phytomyxea can use exogenous energy resources while swimming before attachment to their hosts; however, because these microbes are obligate biotrophic pathogens, it is generally thought that the energy needed by the zoospores is derived entirely from the host cells.
Once inside the host cells, phytomyxean parasites can cause distinct hypertrophy of host tissues, and there is strong evidence that phytomyxea can adversely affect reproduction in both brown algae and seagrasses (den Hartog 1989; Walker and Campbell 2009). The interactions between phytomyxids and their hosts are completely compatible, as no defence reactions of the hosts have been documented (Karling 1944; Dixon 2009; Siemens et al. 2009). The plasmodia inside the host cells, especially those of species forming marked hypertrophies of their host, are known to change the metabolic status of the host plant, leading to an accumulation of energy-rich compounds (reviewed by Ludwig-Müller et al. 2009). However, species not causing marked hypertrophies of their marine hosts such, as phytomyxean parasites of marine diatoms, also live phagotrophically inside the host cell (Schnepf et al. 2000).
Ecology
The effects of moisture, salinity and temperature on the survival of any stage of the phytomyxean life cycle are largely unknown. Phytomyxid parasites have been reported from tropical (>24°C) and temperate (4–24°C) regions of the ocean (Fig. 1). It is very likely that the resting spores can survive cold water temperatures in the winter (den Hartog 1989) or survive periods when the host organisms are absent or rare in the ecosystem.
Although nothing is known about seasonally dependent effects in the life cycle, a seasonal pattern following that of the host is inevitably tied to the obligate biotrophic nature of the interaction and probably occurs in nature. Most known genera of seagrasses that contain compatible host species are ephemeral (Zostera spp., Ruppia spp., Halodule spp., Halophila spp.), diatom hosts form recurring blooms, and brown algal hosts are either ephemeral or persistent depending on the stage of the life cycle. Maier et al. (2000) noted that the temperature range for cultivation of brown algal host in the laboratory may differ from the temperature range for the growth of the parasite; however, they did not observe temperature-dependent influences on the development of the parasite.
Phytomyxea in marine environments
Although only nine marine species have been described, marine phytomyxids are parasites of three major groups of primary producers in the oceans, namely brown algae, seagrasses and diatoms. Because these three groups of host organisms differ markedly in their ecological roles within the oceans, the parasites of these groups will be discussed separately.
Parasites of brown algae
Maullinia ectocarpii is a parasite of Ectocarpus siliculosus and other species of brown algae. The complete life cycle is described by Maier et al. (2000) and Parodi et al. (2010). Initially, this parasite was identified from specimens at La Pasada, Chile and at Flinders, Victoria, Australia. Maier et al. (2000) used infected filaments of E. fasciculatus to produce zoospores for infection experiments, and successfully infected 10 species of brown algae from worldwide geographical origins in culture (from the orders Ectocarpales, Scytothamnales, Desmarestiales and Laminariales), demonstrating a broad spectrum of susceptible hosts. Infection by M. ectocarpii caused hypertrophy of host cells in filaments of both the sporophyte and gametophyte stages in the life cycle of E. fasciculatus (Maier et al. 2000). Microscopic gametophytes of brown algae with heteromorphic life cycles such as Desmarestia and Macrocystis also became infected. Some somatic filament cells in the gametophytes became hypertrophied; in Macrocystis pyrifera, the parasite entered young oogonia in the female gametophyte and reduced the host’s reproductive potential.
Phagomyxa algarum (hosts Bachelotia antillarum and Hincksia mitchelliae), also a phytomyxid of brown alga, has been reported only from the Shackleford Banks, North Carolina (Karling 1944; Johnson and Sparrow 1961). Morphologically, its life cycle differs markedly from that of Maullinia. Ph. algarum aggressively attacks its host, mainly the algal thallus. After approximately 2 days, when the parasite’s life cycle is completed, the host cells quickly disintegrate, consequently making morphological detection of the parasite difficult (Karling 1944). Although the infections were sometimes heavy, the total number of diseased plants never reached a level sufficient to endanger or eradicate the host population (Karling 1944). However, the parasite mediates a change from brown to green in the colour of the host brown alga, which possibly reflects a change of the photosynthetic status of the host capable of influencing its fitness and reproduction rate.
Parasites of seagrasses
Four Plasmodiophora species parasitic in marine hosts are known. Pl. maritima and Pl. halophilae have been found only once, whereas Pl. diplantherea and Pl. bicaudata have been documented repeatedly. Walker and Campbell (2009) recently reported Pl. diplantherae in specimens of Halodule wrightii collected at three sites in northern central Gulf of Mexico. This pathogen has been found on the eastern coast of Florida (Braselton and Short 1985) and along the shores of Saint Croix, Danish West Indies (Karling 1968), indicating a wide distribution in the Caribbean. This pathogen causes enlarged shoot galls in seagrasses and is likely to occur across the entire pan-tropical distribution of the genus Halodule. Pl. bicaudata is parasitic in species of the seagrass Zostera. Infected plants form characteristic galls at the internodes and show reduced root growth. Den Hartog (1989) found a worldwide distribution of this pathogen (Fig. 1).
Gall formation is conspicuous in shoots of both Halodule wrightii and Zostera spp. infected with a phytomyxean parasite. Infected plants appear dwarfed because infected internodes appear unable to elongate and swell up. Leaf growth appears relatively normal in plants infected with marine Plasmodiophora species, whereas inflorescences have not been found in plants infected with Pl. diplantherae (den Hartog 1989). The dwarfing of infected plants is accompanied by a reduced number of and reduced growth of roots (den Hartog 1989; Walker and Campbell 2009).
Tetramyxa parasitica is a parasite of Zanichellia spp. and Ruppia spp. Karling (1968) indicated a worldwide distribution of this pathogen. T. parasitica induces marked production of galls in its host plant. In the original description, Goebel (1884) described that galls were white when young. Microscopic observations revealed that the outer part of the gall consisted of sponge-like tissue, whereas the inner part of the gall was dark and filled with resting spores of the parasite. In autumn, the galls of senescent plants became darker until the galls disintegrated and the spores were released.
Parasites of diatoms
Phagomyxa bellerochaea and Phagomyxa odontellae are parasites of marine diatoms (Schnepf et al. 2000). The host for Pl. bellerochaea is Bellerochea malleus and for Ph. odontellae it is Odontella sinensis. In these species, only an incomplete life cycle without secondary zoospores and secondary plasmodia is known. Like all phagomyxids, both Phagomyxa species have phagotrophic nutrition inside the host cell. Inside the host cells, the plasmodia have a single large central digestion vacuole in Ph. bellerochaea and numerous small digestion vacuoles in Ph. odontellae. The host cytoplasm, including chloroplasts, is digested within the digestion vacuoles. Nothing is known about the influence of infection on the reproductive status of their hosts.
Influence on the ecology and biodiversity of the hosts
Besides anecdotal reports, very little information is available on the influence of marine phytomyxean parasites on their hosts. Nonetheless, from what is known about their interactions with their hosts, the disease symptoms, and their life cycles, conclusions can be made about possible consequences of this parasitic symbiosis for both host populations and ecosystem function and stability.
The negative impact on the reproductive potential and/or growth of the host caused by all marine phytomyxids is evident. The reproductive potential of brown algae infected by M. ectocarpii and Ph. algarum was reduced compared with uninfected individuals (Karling 1944; Maier et al. 2000). Infections with Plasmodiophora spp. and T. parasitica reduce the growth of the host plant (Johnson and Sparrow 1961; den Hartog 1989; Walker and Campbell 2009), and probably reduce the formation of inflorescences (den Hartog 1989). The effects of a reduced formation of inflorescences on the competitiveness and fitness of the hosts is not clear, because some seagrasses such as Zostera can reproduce both sexually through the formation of seeds and asexually through the formation of rhizomes (ramets), the latter process being very important on a smaller spatial scale (Becheler et al. 2010).
Phytomyxean parasites also cause increased uprooting of their angiosperm hosts as a consequence of reduced root growth. In areas where seagrass-restoration projects are ongoing, this uprooting can subsequently damage seagrass beds (Walker and Campbell 2009) and there is also a considerable risk of floating plants spreading this pathogen to other populations of seagrasses.
Phytomyxean parasites are abundant in diverse habitats (terrestrial, marine, freshwater). Therefore, phytomyxean parasites probably do not influence the α-biodiversity (i.e. the biodiversity within a certain area) in estuarine or marine habitats; however, the way they influence their hosts can have a strong impact on the β-biodiversity (i.e. how species composition changes with distance). Phytomyxean species are obligate biotrophic parasites and depend on their hosts for survival. Therefore, we assume that they will not have a strong impact on species biodiversity (=α biodiversity) within a certain area. However, phytomyxids can change the abundance of certain organisms within a limited area, creating an environmental gradient (=β biodiversity) within the limited area where they occur. Along this gradient, the impact on biodiversity is probably massive because of the changed metabolic status of the host (e.g. substances excreted by the plant, changed chemical composition of the plant), and because of the amount of energy that is available at the lower levels of the food web.
The currently known marine hosts usually are dominant in a limited spatial area, such as monotypic seagrass meadows (Becheler et al. 2010). The increased uprooting described for seagrasses infected by Plasmodiophora spp. (den Hartog 1989; Walker and Campbell 2009), resulting in a considerable number of floating (i.e. removed) plants, can influence the host-plant density within the plots where it is established. The uprooting and removal of infected plants can regulate the plant density, thereby preventing the plants from becoming locally too abundant and can promote biodiversity by increasing the available space. The high diversity of species within and the energy produced by seagrass meadows together with their role in stabilising sediments makes seagrasses key organisms in marine coastal habitats (Orth et al. 2006), and the impact of phytomyxean species on these environments needs to be studied to properly maintain these habitats.
The potential role of marine phytomyxids as vectors for viruses
Symptoms of disease such as morphological alteration in the host plant (e.g. galling) are caused by the phytomyxean parasites themselves, and in terrestrial ecosystems, phytomyxids can also act as vectors for sometimes devastating viral diseases (Kanyuka et al. 2003; Rush 2003; Rochon et al. 2004). The presence of viruses is independent of the formation of hypertrophies of the host caused by certain phytomyxid species.
At least 20 types of viruses that cause diseases in flowering plants important in agriculture are known to be transmitted by phytomyxid parasites (Kanyuka et al. 2003; Rochon et al. 2004). These are all positive-sense single-stranded RNA (ssRNA) viruses belonging to five virus genera and one currently unassigned virus (Adams 2005). The viruses in the genus Bymovirus (Family Potyviridae) have flexuous filaments with two modal lengths (250–300 nm or 500–600 nm) and a diameter of 13 nm, whereas the viruses in the genus Benyvirus (Family unassigned) and in the genera Pecluvirus, Furovirus and Pomovirus (Family Virgaviridae) have rod-shaped virions that vary in length (60–380 nm) and width (18–21 nm). The watercress yellow spot virus (family unassigned) has icosahedral virions (Walsh et al. 1989). All of these viruses are thought to be carried within the cytoplasm of zoospores (in vivo transmission); however, the mechanisms of uptake and release are still largely unknown. These viruses can be present in the phytomyxids during all stages of the life cycle and they can persist for years in resting spores (Rush 2003; Rochon et al. 2004).
In the marine environment, no correlation between an infection with a phytomyxid and the transmission of viruses has been found and no virus particles have yet been detected in zoospores of marine phytomyxids. However, this is not surprising considering the lack of knowledge of this group. Recently, it was revealed that viruses are the largest source of genetic diversity and (fatal) pathogenicity in marine environments, being responsible for killing an estimated 20% of the marine biomass daily (Suttle 2007; Lang et al. 2009; Kristensen et al. 2010). The mechanisms of transmission of eukaryote-infecting viruses in the marine environment are largely unknown. Although most of the recently characterised ssRNA viruses are transmitted via water on lysis of the host cell (Lang et al. 2009), the vast biodiversity of marine viruses implies that some of these viruses will be transmitted by vectors, especially the ones infecting aquatic angiosperms or brown algae that have to enter the host organism through thick cell walls. Prior to infection, the viral genome must enter the host’s cells, a process that could be facilitated by phytomyxids in the marine environment, as it is in freshwater and terrestrial environments.
This viewpoint is supported by the fact that the most abundant group of RNA viruses in the ocean are ‘picorna-like viruses’. This group is loosely defined as positive-sense ssRNA viruses with an icosahedral capsid. Viruses transmitted by phytomyxids from the genus Bymovirus (e.g. barley yellow mosaic virus, vector Polymyxa graminis) are covered by this definition (Culley and Steward 2007). The structural properties of the watercress yellow spot virus, transmitted by Spongospora nasturtii, are similar to the picorna-like viruses. Therefore, we suspect that marine phytomyxids probably can act as vectors for the most abundant group of viruses in the ocean.
Recently, positive-sense ssRNA viruses were detected in diatoms (Nagasaki 2008; Tomaru et al. 2009); however, the interactions between the viruses and their hosts are still largely unknown. Furthermore, brown algae are infected by ssRNA viruses that probably could be transmitted by phytomyxid hosts. Easton et al. (1997) studied epidemic dieback of the kelp Ecklonia radiata (Laminariales) in New Zealand. Dieback disease of Ecklonia causes massive damage to kelp populations along the coast of parts of New Zealand (Easton et al. 1997). In general, the large number of kelp plants that wash onto the beaches is thought to be caused by storm damage and changing water temperature; however, the role of viral diseases must now be considered. Both straight rod-like and flexuous filamentous viral particles were observed in dieback-affected plants, and the viruses could not be transmitted via water, suggesting that a vector for the transmission might be needed. The long flexuous virus filaments (700–900 nm) were in size similar to potyviruses, and the rods (280 nm) were in size and form similar to tobamoviruses or furoviruses (Easton et al. 1997). Furoviruses can be transmitted by terrestrial members of the phytomyxids (Adams 2005), strengthening the hypothesis that phytomyxean species might play a role as vectors for marine viruses. Because phytomyxean zoospores can infect many species of brown algae, it is possible that both phytomyxean zoospores and ssRNA viruses can come in contact on the surface of at least some brown algae such as E. radiata.
Possible impact on marine and estuarine food webs
We expect that a much wider spectrum of brown algae, seagrasses and diatoms than currently known are hosts for phytomyxean species. Brown algae, diatoms and seagrasses are keystone species in highly productive and complex marine ecosystems. Brown algae dominate many benthic communities, especially along cold-temperate coasts, and seagrasses are prevalent in estuaries worldwide (Orth et al. 2006). Diatoms are prevalent in all aquatic ecosystems.
Large numbers of zoospores may be released by phytomyxids to ensure survival in these ecosystems, because strong currents can carry many of the zoospores far away from their potential hosts. Zoospores that do not attach to hosts possibly provide significant food resources for grazing zooplankton and small filter-feeding invertebrates. As obligate biotrophic parasites (i.e. primary consumers), phytomyxean zoospores provide a link between (otherwise inedible) producers and secondary consumers in freshwater ecosystems, as do other zoosporic microbes (Gleason et al. 2008). We predict the same role in marine ecosystems. The impact of shoot galls as an additional food source for consumers in estuarine ecosystems is unknown, although shoot galls could provide valuable food resources for grazing invertebrates, owing to a changed chemical composition (Neuhauser et al. 2011).
Dead marine plants are not easily digested by microbes and, if they sink to the bottom in the water column, their organic matter may not be quickly recycled. Infected plants behave differently from uninfected plants (e.g. increased uprooting of seagrasses). Further, the process of lysis of host cells on infection with phytomyxean parasites or by viruses releases nutrients back into the environment before the plants sink. Both dissolved and particulate organic matter is thus made available as a substrate for microbes in the food web directly at the site where the plants grow and not at the bottom of the ocean. Consequently, microbial parasites of primary producers have a direct influence on the levels of carbon dioxide stored in the ocean. Diseases caused by phytomyxids that result in uprooting of seagrasses and kelps may release new substrates for colonisation by other populations. This could increase biodiversity; however, infected hosts carried by the water currents can spread these diseases as they float into areas free of disease, and this can negatively influence seagrass restoration projects (Orth et al. 2006; Walker and Campbell 2009).
Future directions in marine-phytomyxid research
Recent data suggest that phytomyxean species are widespread in both marine and estuarine ecosystems worldwide, yet these biotrophic parasites have been poorly studied. Parasitism by phytomyxean species may severely reduce primary production globally; however, at the same time, these species might enhance biodiversity. To remedy this lack of quantitative data on the prevalence of infection, the abundance of phytomyxids in marine ecosystems and their biodiversity and host range need to be explored. Without these data, the true role of these enigmatic biotrophic parasites and their impact on their host populations cannot be fully understood.
Are the known phytomyxids examples of emerging diseases? Is it possible that deteriorating environmental conditions and climate change are increasing the rate of spread of these diseases in some ecosystems? The effects of rising temperatures in the ocean on populations of phytomyxean species are unknown. To restore seagrass beds in estuarine and coastal ecosystems and kelp beds in benthic coastal environments to pristine conditions, the roles of phytomyxids and other parasites need clarification. Monitoring programs need to be implemented to track the spread of the diseases caused by phytomyxids. Also, it is possible that phytomyxean species may have a devastating effect on gametophyte cultures of brown algae used in maritime facilities. However, phytomyxids might also have a regulating role in the environment by controlling the population size and density of their hosts, thus increasing biodiversity and health of the ecosystem (Hudson et al. 2006; Lafferty et al. 2008).
Phytomyxean species are not the only eukaryotic parasites of brown algae and seagrasses. For example, members of the Chytridiomycota, the Hyphochytridiomycota and the Oomycota are also parasites of brown algae (Küpper and Müller 1999) and the Labyrinthulomycota (Vergeer and den Hartog 1994) are parasites of seagrasses. Andrews (1976) listed some of the microbial and metazoan parasites of marine algae; however, this list needs to be updated, with new groups of microbes added. The interaction between these parasites and other microbes affects the complexity of these ecosystems.
The recent discovery of viruses causing diseases in primary producers in the marine environment adds another dimension to marine ecology. We have just begun to explore the vast biodiversity of viruses in the sea, and their possible roles in marine ecosystems. Little is known about which organisms are infected by most of these viruses, and data on the transmission of viruses by vectors, as in terrestrial ecosystems, are incomplete. Terrestrial phytomyxean species are notorious for their ability to transmit plant viruses. Because most of marine RNA viruses discovered so far structurally resemble the viruses transmitted by terrestrial phytomyxids, it is very likely that phytomyxean species can serve as vectors for at least some of them in marine ecosystems.
Acknowledgements
We thank the editor, Professor A. Boulton, and the referees (including Professor G. R. Dixon) for their constructive comments. We thank Professor R. Pöder, University of Innsbruck, Austria, for kindly providing his knowledge and expertise for this project. SN was supported by a Hertha-Firnberg research grant (Austrian Science Fund (FWF) grant T379-B16).
References
- Adams MJ. A taxonomic review of the fungally-transmitted viruses. In: Rush CM, editor. Proceedings of the Sixth Symposium of the International Working Group on Plant Viruses with Fungal Vectors. American Society of Sugar Beet Techonologists; Denver, CO.: 2005. pp. 1–4. [Google Scholar]
- Andrews JH. The pathology of marine algae. Biological Reviews of the Cambridge Philosophical Society. 1976;51:211–252. doi:10.1111/J.1469-185X.1976.TB01125.X. [Google Scholar]
- Bass D, Chao EEY, Nikolaev S, Yabuki A, Ishida KI, Berney C, Pakzad U, Wylezich C, Cavalier-Smith T. Phylogeny of novel naked filose and reticulose Cercozoa: Granofilosea cl. n. and Proteomyxidea. Protist. 2009;160:75–109. doi: 10.1016/j.protis.2008.07.002. doi:10.1016/J.PROTIS.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Becheler R, Diekmann O, Hily C, Moalic Y, Arnaud-Haond S. The concept of population in clonal organisms: mosaics of temporally colonized patches are forming highly diverse meadows of Zostera marina in Brittany. Molecular Ecology. 2010;19:2394–2407. doi: 10.1111/j.1365-294X.2010.04649.x. [DOI] [PubMed] [Google Scholar]
- Braselton JP. Plasmodiophoromycota. In: McLaughlin DJ, McLaughlin EG, Lemke PA, editors. The Mycota VII Part A. Systematics and Evolution. Springer-Verlag; Berlin: 2001. pp. 81–91. [Google Scholar]
- Braselton JP, Short FT. Karyotypic analysis of Plasmodiophora diplantherae. Mycologia. 1985;77:940–945. doi:10.2307/3793306. [Google Scholar]
- Bulman SR, Kuhn SF, Marshall JW, Schnepf E. A phylogenetic analysis of the SSU rRNA from members of the Plasmodiophorida and Phagomyxida. Protist. 2001;152:43–51. doi: 10.1078/1434-4610-00042. doi:10.1078/1434-4610-00042. [DOI] [PubMed] [Google Scholar]
- Burki F, Kudryavtsev A, Matz MV, Aglyamova GV, Bulman S, Fiers M, Keeling P, Pawlowski J. Evolution of Rhizaria: new insights from phylogenomic analysis of uncultivated protists. BMC Evolutionary Biology. 2010;10:377. doi: 10.1186/1471-2148-10-377. doi:10.1186/1471-2148-10-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Kingdom Protozoa and its 18 phyla. Microbiological Reviews. 1993;57:953–994. doi: 10.1128/mr.57.4.953-994.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley AI, Steward GF. New genera of RNA viruses in subtropical seawater, inferred from polymerase gene sequences. Applied and Environmental Microbiology. 2007;73:5937–5944. doi: 10.1128/AEM.01065-07. doi:10.1128/AEM.01065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog C. Distribution of Plasmodiophora bicaudata, a parasitic fungus on small Zostera species. Diseases of Aquatic Organisms. 1989;6:227–229. doi:10.3354/DAO006227. [Google Scholar]
- Dixon GR. Plasmodiophora brassicae in its environment. Journal of Plant Growth Regulation. 2009;28:212–228. doi:10.1007/S00344-009-9098-3. [Google Scholar]
- Easton LM, Lewis GD, Pearson MN. Virus-like particles associated with dieback symptoms in the brown alga Ecklonia radiata. Diseases of Aquatic Organisms. 1997;30:217–222. doi:10.3354/DAO030217. [Google Scholar]
- Gleason FH, Kagami M, Lefèvre E, Sime-Ngando T. The ecology of chytrids in aquatic ecosystems: roles in food web dynamics. Fungal Biology Reviews. 2008;22:17–25. doi:10.1016/J.FBR.2008.02.001. [Google Scholar]
- Goebel K. Tetramyxa parasitica. Flora. 1884;67:517–521. [Google Scholar]
- Hudson PJ, Dobson AP, Lafferty KD. Is a healthy ecosystem one that is rich in parasites? Trends in Ecology & Evolution. 2006;21:381–385. doi: 10.1016/j.tree.2006.04.007. doi:10.1016/J.TREE.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Johnson TW, Sparrow FK. Fungi in Oceans and Estuaries. J. Cramer; Weinheim, Germany: 1961. [Google Scholar]
- Kanyuka K, Ward E, Adams MJ. Polymyxa graminis and the cereal viruses it transmits: a research challenge. Molecular Plant Pathology. 2003;4:393–406. doi: 10.1046/j.1364-3703.2003.00177.x. doi:10.1046/J.1364-3703.2003.00177.X. [DOI] [PubMed] [Google Scholar]
- Karling JS. Phygomyxa algarum n.gen., n.sp., an unusual parasite with plasmodiophoralean and protomyxean characteristics. American Journal of Botany. 1944;31:38–52. doi:10.2307/2437666. [Google Scholar]
- Karling JS. The Plasmodiophorales. 2nd edn Hafner Publishing Company; New York: 1968. [Google Scholar]
- Kristensen DM, Mushegian AR, Dolja VV, Konin EV. New dimensions of the virus world discovered through metagenomics. Trends in Microbiology. 2010;18:11–19. doi: 10.1016/j.tim.2009.11.003. doi:10.1016/J.TIM.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper FC, Müller DG. Massive occurrence of the heterokont parasites Anisolpidium, Eurychasma and Chytridium in Pylaiella littoralis (Ectocarpales, Phaeophyceae) Nova Hedwigia. 1999;69:381–389. [Google Scholar]
- Lafferty KD, Allesina S, Arim M, Briggs CJ, DeLeo G, Dobson AP, Dunne JA, Johnson PTJ, Kuris AM, Marcogliese DJ, Martinez ND, Memmott J, Marquet PA, McLaughlin JP, Mordecai EA, Pascual M, Poulin R, Thieltges DW. Parasites in food webs: the ultimate missing links. Ecology Letters. 2008;11:533–546. doi: 10.1111/j.1461-0248.2008.01174.x. doi:10.1111/J.1461-0248.2008.01174.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AS, Rise ML, Culley AI, Steward GF. RNA viruses in the sea. FEMS Microbiology Reviews. 2009;33:295–323. doi: 10.1111/j.1574-6976.2008.00132.x. doi:10.1111/J.1574-6976.2008.00132.X. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J, Prinsen E, Rolfe SA, Scholes JD. Metabolism and plant hormone action during clubroot disease. Journal of Plant Growth Regulation. 2009;28:229–244. doi:10.1007/S00344-009-9089-4. [Google Scholar]
- Maier I, Parodi E, Westermeier R, Müller DG. Maullinia ectocarpii gen. et sp. nov. (Plasmodiophorea), an intracellular parasite in Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) and other filamentous brown algae. Protist. 2000;151:225–238. doi: 10.1078/1434-4610-00021. doi:10.1078/1434-4610-00021. [DOI] [PubMed] [Google Scholar]
- Merz U. Powdery scab of potato – occurrence, life cycle and epidemiology. American Journal of Potato Research. 2008;85:241–246. doi:10.1007/S12230-008-9019-1. [Google Scholar]
- Nagasaki K. Dinoflagellates, diatoms, and their viruses. Journal of Microbiology. 2008;46:235–243. doi: 10.1007/s12275-008-0098-y. doi:10.1007/S12275-008-0098-Y. [DOI] [PubMed] [Google Scholar]
- Neuhauser S, Bulman S, Kirchmair M. Plasmodiophorids: the challenge to understand soil-borne, obligate biotrophs with a multiphasic life cycle. In: Gherbawy Y, Voigt K, editors. Current Advances in Molecular Identification of Fungi. Springer; Heidelberg, Germany: 2010. pp. 51–78. [Google Scholar]
- Neuhauser S, Kirchmair M, Gleason FH. The ecological potentials of Phytomyxea (‘plasmodiophorids’) Hydrobiologia. 2011;659:23–35. doi: 10.1007/s10750-010-0508-0. doi:10.1007/S10750-010-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S, Short FT, Waycott M, Williams SL. A global crisis for seagrass ecosystems. Bioscience. 2006;56:987–996. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. [Google Scholar]
- Parodi ER, Caceres EJ, Westermeier R, Müller DG. Secondary zoospores in the algal endoparasite Maullinia ectocarpii (Plasmodiophoromycota) Biocell. 2010;34:45–52. [PubMed] [Google Scholar]
- Rochon D, Reade R, Kakani K, Robbins M. Molecular aspects of plant virus transmission by Olpidium and plasmodiophorid vectors. Annual Review of Phytopathology. 2004;42:211–241. doi: 10.1146/annurev.phyto.42.040803.140317. doi:10.1146/ANNUREV.PHYTO.42.040803.140317. [DOI] [PubMed] [Google Scholar]
- Rush CM. Ecology and epidemiology of benyviruses and plasmodiophorid vectors. Annual Review of Phytopathology. 2003;41:567–592. doi: 10.1146/annurev.phyto.41.052002.095705. doi:10.1146/ANNUREV.PHYTO.41.052002.095705. [DOI] [PubMed] [Google Scholar]
- Schnepf E, Kühn SF, Bulman S. Phagomyxa bellerocheae sp. nov. and Phagomyxa odontellae sp. nov., Plasmodiophoromycetes feeding on marine diatoms. Helgoland Marine Research. 2000;54:237–241. doi:10.1007/S101520000056. [Google Scholar]
- Siemens J, Bulman S, Rehn F, Sundelin T. Molecular biology of Plasmodiophora brassicae. Journal of Plant Growth Regulation. 2009;28:245–251. doi:10.1007/S00344-009-9091-X. [Google Scholar]
- Suttle CA. Marine viruses – major players in the global ecosystem. Nature Reviews Microbiology. 2007;5:801–812. doi: 10.1038/nrmicro1750. doi:10.1038/NRMICRO1750. [DOI] [PubMed] [Google Scholar]
- Tomaru Y, Takao Y, Suzuki H, Nagumo T, Nagasaki K. Isolation and characterisation of a single-stranded RNA virus infecting the bloom-forming diatom Chaetoceros socialis. Applied and Environmental Microbiology. 2009;75:2375–2381. doi: 10.1128/AEM.02580-08. doi:10.1128/AEM.02580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergeer LHT, den Hartog C. Omnipresence of Labyrinthulaceae in seagrasses. Aquatic Botany. 1994;48:1–20. doi:10.1016/0304-3770(94)90070-1. [Google Scholar]
- Walker AK, Campbell J. First records of the seagrass parasite Plasmodiophora diplantherae from the northcentral Gulf of Mexico. Gulf and Caribbean Research. 2009;21:63–65. [Google Scholar]
- Walsh JA, Clay CM, Miller A. A new virus disease of watercress in England UK. Bulletin OEPP. EPPO Bulletin. European and Mediterranean Plant Protection Organisation. 1989;19:463–470. doi:10.1111/J.1365-2338.1989.TB00420.X. [Google Scholar]