Abstract
Ectopic pregnancy occurs when the embryo failed to transits to the uterus and attached to the luminal epithelium of Fallopian tube. Tubal ectopic pregnancy (EP) is a common gynecological emergency and more than 95% of EP occurs in the ampullary region of the Fallopian tube (FT). In humans, Wnt-activation and down-regulation of Olfactomedin-1 (Olfm-1) occur in the receptive endometrium and coincided with embryo implantation in vivo. Whether similar molecular changes happen in the Fallopian tube leading to EP remains unclear. We hypothesized that activation of Wnt-signaling down-regulates Olfm-1 expression predisposes to EP. We investigated the spatiotemporal expression of Olfm-1 in FT from non-pregnant women and women with EP, and used a novel trophoblastic spheroid (embryo surrogate)-Fallopian tube epithelial cell co-culture model (JAr and OE-E6/E7 cells) to study the role of Olfm-1 on spheroids attachment. Olfm-1 mRNA expression in the ampullary region of non-pregnant FT was higher (p<0.05) in the follicular than theluteal phase. Ampullary tubal Olfm-1 expression was lower in FT from women with EP compared to normal controls at the luteal phase (H-SCORE=1.3±0.2 vs 2.4±0.5; p<0.05). Treatment of OE-E6/E7 with recombinant Olfm-1 (0.2-5 μg/ml) suppressed spheroids attachment to OE-E6/E7 cells; while activation of Wnt-signaling pathway by Wnt3a or LiCl, reduced endogenous Olfm-1 expression and increased spheroids attachment. Conversely, suppression of Olfm-1 expression by RNAi increased spheroids attachment to OE-E6/E7 cells. Taken together, Wnt-activation suppresses Olfm-1 expression and this may predispose a favourable microenvironment of the retained embryo in the Fallopian tube leading to EP in humans.
Keywords: Olfactomedin-1, Fallopian tube, tubal ectopic pregnancy, Wnt-signaling
Introduction
Ectopic pregnancy occurs when an embryo implants outside the uterus. It happens in 1-2% of pregnancies and is associated with significant morbidity and mortality. Interestingly, about 85-95% of the ectopic pregnancies occur in the ampullary region (distal 2/3) of the Fallopian tube (1). Genital pelvic infection and cigarette smoking are the risk factors associated with tubal ectopic pregnancy (2). Moreover, changes in extracellular matrix molecules, cytokines and other growth factors expression are also reported in the Fallopian tube of patients with tubal ectopic pregnancy. Yet, the aetiology of tubal ectopic pregnancy (TEP) is still not fully understood.
The olfactomedins (Olfms) are a family of secretary glycoproteins first identified in the olfactory epithelial tissues of the Xenopus (3). To date, over 50 species including rats, frogs, sea urchins, cats, dogs, zebra fish and humans are found to express Olfms (4). All the well-characterized Olfm proteins share a common Olfm-domain of about 250 amino acids in size at their C-termini (4). In mammals, there are about 13 well characterized Olfm domain-containing proteins (4) and among the best understood Olfms; Olfm-1, -2, -3, -4 and myocilin are known to regulate cellular growth, differentiation and pathological processes.
Olfm-1 is one of the most studied members in the family. The Olfm-1 gene islocated on chromosome 9q34.3. Four variant transcripts are produced by alternative splicing (5). The longest transcript encodes a protein of 464 amino acids with a native molecular weight of 57-kDa. Olfm-1 is expressed in the human brain, eye, liver, lung, heart, as well as uterine tissues. The exact function of Olfm-1 is not known, but proteins containing an Olfm-like domain may play important roles on cell adhesion (6) or act as scaffolding proteins (7, 8). Recently, Olfm-1 was found to modulate the canonical Wnt-signaling pathway, which regulates various cellular functions (9).
Recent microarray data has shown that the Olfm-1 transcript is down-regulated in the endometrium during the window of implantation (10-13) and that up-regulation of Olfm-1 transcript is associated with pathological conditions, including endometriosis (14) and recurrent spontaneous abortions (14, 15). Recently, were ported that Olfm-1 expression in the endometrial epithelium is lower at the luteal phase than at the follicular phase of the menstrual cycle, suggesting that progesterone may suppress Olfm-1 expression (16). In addition, using a trophoblastic spheroids (JAr)-endometrial epithelial cells (17) co-culture model, we have shown that Olfm-1 dose-dependently down-regulates JAr spheroid attachment onto Ishikawa cells(16).
We hypothesized that aberrant expression of Olfm-1 in the Fallopian tube maybe one of the predisposing factors leading to ectopic pregnancy in humans. Olfm-1 interacts with Wnt-signaling inhibitor (WIF-1) (9), and therefore modulates Wnt-regulated genes important for embryo implantation and early development (18, 19). In the present study, we compared the spatiotemporal expression of Olfm-1 in Fallopian tube from non-pregnant women and from women with ectopic pregnancies, and used a novel trophoblast spheroids (embryo surrogate)-Fallopian tubal epithelial cell co-culture model (JAr and OE-E6/E7 cells) to study the role of Olfm-1 in regulating Wnt-signaling and tubal-embryo attachment.
Material and Methods
Patient Samples
All non-pregnant Fallopian tubes (ipsilateral and contralateral side) were obtained from 29 patients (age: 36-51 years; mean±SD: 45±4.9 years) who had undergone hysterectomy, had regular menstrual cycle (28-30 days) and no known tubal pathological conditions with written consent. The phase of menstrual cycle was determined by the date of the last menstrual period (LMP). Fallopian tubes with implantation site at the ampullary region were retrieved from our archive samples from 10 patients (age: 26-42 years; mean±SD: 33±5.2 years, gestational age: 7 to 10 weeks) who had undergone salpingectomy for treatment of tubal ectopic pregnancy.The study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW10-109).
Quantitative PCR (qPCR) and analysis
The Fallopian tube from the non-pregnant women who had hysterectomy for non-tubal pathological conditions was dissected longitudinally, and the mucosal layer of different regions (ampulla, infundibullum and isthmus) were carefully peeled off using a forceps as described elsewhere (20). Total RNA was isolated using the Absolutely RNA RT-PCR miniprep kit (Stratagene, La Jolla, CA, USA). The integrity of total RNA was determined by gel electrophoresis and spectrophotometry. Then, 400 ng of total RNA was reverse transcribed to synthesize cDNA using the TaqMan RT Reagents (N8080234, ABI Biosystems, Foster City, CA). Human Olfm-1, -2, -3 and -4 transcript-specific TaqMan probes (supplementary table 1) were used with the ABI 7500 for quantitative analysis of Olfm transcripts. All qPCR assays were performed with TaqMan PCR Master Mix® (Applied BioSystems) and a standard PCR cycling protocol. Human 18S ribosomal RNA was used as the internal control to normalize the Olfm expression in the samples. The relative mRNA expression was quantified using 2−ΔΔct method as described elsewhere (21). The qPCR experiment was repeated three times in duplicates under similar conditions.
Immunohistochemistry (IHC) and Histological Scoring (H-SCORE)
Fallopian tube biopsies obtained from non-pregnant women and from women with ectopic pregnancies were fixed with 4% paraformaldehyde, embedded in paraffin wax and sectioned at 5 μm for IHC studies as previously described (16). Both the non-pregnant and ectopic pregnancy Fallopian tubes were subjected to IHC using polyclonal anti-Olfm-1 antibody (1:100) which bind specifically to the long-form of the Olfm-1 protein (16). To confirm the presence of an intact epithelium in the ectopic pregnancy sections, cytokeratin (1:100, DakoCytomation, Glostrup, Denmark) staining was also performed. DAB Substrate Chromogen (DakoCytomation) was used to detect positive staining. Images were captured using a digital camera mounted on a light microscope (Axioscop, Zeiss, Göttingen, Germany). The intensity of Olfm-1 staining in the Fallopian tube epithelium in a total of 500 cells (5 fields with 100 cells each) in each section (2-4 sections each) were subjected to H-Score analysis by two independent observers as previously described (22). Results are presented as mean±SD.
Western blotting
OE-E6/E7 cells were lysed in RIPA buffer (1 ml) supplemented with protease inhibitors as described (23). Protein loading was normalized using the total protein concentrations as determined by the Bradford assay. Antibodies against Olfm-1 (1:1000, Zymed, San Francisco, CA, USA), β-catenin (1:2500, Cat. No.: 610153, BD Transduction Laboratory, Franklin Lakes, NJ, USA), active-β-catenin (1:1000, Cat. No.: 05-665, Millipore, Temecula, CA), GSK3-β (1:1000, Cat. No.: 610201, BD Transduction Laboratory), E-cadherin (1:1000, Cat No.:1b1416, Abcam, Cambridge, MA, USA) and β-actin (1:10000, Cat. No.: A3854, Sigma-Aldrich, St Louis, MO, USA) were used as primary antibodies; while horseradish peroxidase-conjugated goat anti-rabbit IgG and sheep anti-mouse IgG (1:5000, GE Healthcare, NJ, USA) were used as secondary antibodies. Specific signal was visualized by the enhanced chemoiluminescence (ECL) method.
Trophoblastic spheroids (embryo surrogate)-Fallopian tubal epithelialcell co-culture model
A previously established co-culture model to simulate trophoblast-endometrium attachment was modified (16, 23). A human trophoblastic choriocarcinoma cell line (JAr, HTB-144, ATCC, Manassas, VA, USA) and human immortalized Fallopiantubal epithelial cell line (OE-E6/E7) (24) were cultured at 37°C in a humid atmosphere with 5% CO2 in air. The JAr cells were maintained in RPMI 1640 (Sigma), supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA), 2 mM L-glutamine, penicillin/streptomycin (100 U/ml and 0.1 mg/ml, Gibco, CA, USA). Dulbecco’s Modified Essential Medium/F12 (DMEM/F12) supplemented with 10% FBS, L-glutamine and penicillin/streptomycin were used for culturing the OE-E6/E7 cells. Briefly, OE-E6/E7 cells were treated with or without 1 μg/ml bovine serum albumin (BSA, Sigma), rhOlfm-1 (0.2, 1 and 5 μg/ml), trophoblast differentiation agents 5 μM dibutyryl-cAMP (dbcAMP, Sigma), 5 μM methotrexate(MTX, Sigma), or progesterone receptor antagonist 4 μM RU486 (Tocris) for 24 hours. Cloning, over-expression and purification of rhOlfm-1 were described previously (16). MTX and RU486 were included as negative controls to validate the co-culture assay (16, 25, 26). Multi-cellular spheroids of 60-120 μm in size were generated by shaking the trypsinized JAr cells at 6g for 24 hours. The spheroids were carefully transferred onto the surface of a confluent OE-E6/E7 monolayer and incubated in DMEM/F12 medium with supplements for up to 24 hours. Non-adherent spheroids were removed by agitation at low g-force (15g) for 10 minutes. The medium was removed and refilled, and the attached spheroids were counted under a light microscope and expressed as percentage of the total number of seeded spheroids (% adhesion). Photographs of cultures were taken with a Nikon Eclipse TE300 inverted microscope (Nikon, Tokyo, Japan).
Olfm-1 RNAi knockdown
Olfm-1 siRNA from Dharmacon (L-012203-00, 20 μM) and Applied Biosystems (4392420, 20 μM) was mixed at a 1:1 ratio and diluted in Opti-MEM medium.Briefly, 8 × 105 cells in 800 μl/well were mixed with 200 μl of transfection mixture containing Lipofectamine 2000 and siRNA at a final concentration of 10 nM. After 6 hours of transfection, the transfected cells were attached in a 12-well plate and the medium were changed to DMEM/F12 with supplement. The cells were >90% confluency after overnight culture. To increase the transfection efficiency of OE-E6/E7 cells, the cells were transfected again. The media was changed to Opti-MEM and a mixture of siRNA with Lipofectamine 2000 was added to the attached cells. The medium were changed and the cells were ready for co-culture after 24 hours of incubation. Negative control siRNA (Dharmacon and Applied Biosystems) was transfected using the same protocol. Western blotting was used to check the level of Olfm-1 knockdown in the OE-E6/E7 cells using the anti-Olfm-1 antibody described above.
Activation of the Wnt-signaling pathway
Wnt3a-conditioned medium (Wnt3a-CM) was obtained from cultured mouse L-Wnt 3A cells (ATCC CRL-2647) stably secreting Wnt3a as previously described (27, 28), and used to induce the Wnt-signaling pathway in the OE-E6/E7 cells. Briefly, the mouse fibroblast L cells were cultured in DMEM medium supplemented with 10% FBS, L-glutamine and penicillin/streptomycin until confluent. The conditioned medium was collected at 48 hours after confluency and tested for the presence of Wnt3a by Western blotting. The conditioned media obtained from normal mouse fibroblast L cells was used as negative control for the spheroids attachment assay. All conditioned medium was filter sterilized and stored at −20°C until used. LiCl (Sigma) stock solution at a concentration of 5 M was prepared, dissolved in distilled water, filter sterilized and stored at room temperature until use. Wnt3a-CM at 1:2 (v: v) and 1:1 ratio and 40 mM LiCl in normal culture medium were used for Wnt-signaling activation.
Statistical Analysis
All results were expressed as means±SD. Statistical comparisons were performed by one way ANOVA followed by the Scheffe’s or Tukey test where appropriated. The H-SCORE data were analyzed using Mann–Whitney U test and Kruskall-Wallis tests for multiple comparison. A probability of p<0.05 was used to indicate a significant difference. A paired t-test was used for comparison of H-SCOREs from different observers. The data were analyzed using the statistical software SPSS (ver15.1 for windows; SPPSS Inc., Chicago, IL) for Windows.
Results
Expression of Olfm transcripts in the non-pregnant Fallopian tubes
The expression of Olfm-1, -2, -3 and -4 transcripts were studied in non-pregnant Fallopian tube. Tissues obtained from the follicular or luteal phases were dissected into 3 regions: infundibullary, ampullary and isthmic. The expression of Olfm-1 transcript was higher in the follicular than in the luteal phase in all three regions. A significantly higher (p<0.05) expression of ampullary Olfm-1 in the follicular phase was observed (Figure 1A). Similarly, a significantly higher infundibullary Olfm-2 (p<0.05) expression was found in the follicular than in the luteal phase. Interestingly, the expression of infundibullary Olfm-3 in the follicular phase was significantly higher (p<0.05) than that in the ampullary or isthmic regions in the follicular phase, as well as the infundibullary region in the luteal phase. While the overall expression of Olfm-4 was very low in the Fallopian tube, there was a significant (p<0.0001) increase of its transcript in the isthmic region in the follicular phase when compared to that in the infundibullary and ampullary regions.
Figure 1.
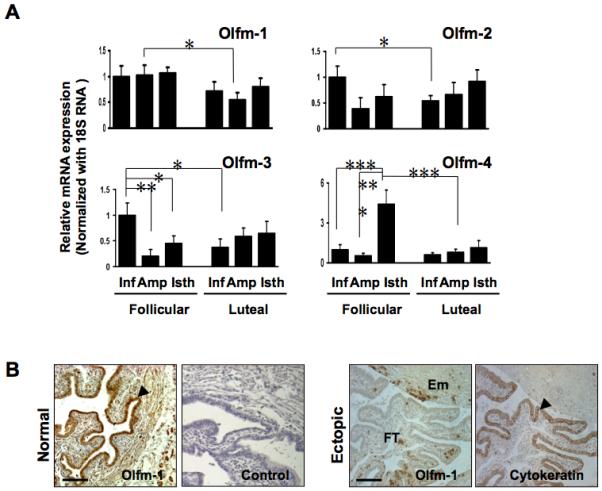
Olfactomedin transcripts are differentially expressed in non-pregnant Fallopian tube and Fallopian tube from women with ectopic pregnancy. (A) The expression of Olfm (Olfm-1, -2, -3 and -4) transcripts in different regions of non-pregnant Fallopian tube in the follicular (n=14) and luteal (n=15) phases of the menstrual cycle was quantified by real-time PCR. The expression of each Olfm transcript at the infundibulum region at the follicular phase of the cycle was arbitrary set to a value of 1. Inf: Infundibulum, Amp: Ampulla, Isth: Isthmus, * p<0.05, **p<0.01 and ***p<0.005. (B) Olfm-1 protein is differentially expressed in the ampullary region of non-pregnant Fallopian tube at the luteal phase and Fallopian tube from women with ectopic pregnancy. The negative control section was included by using pre-absorbed antibody with blocking peptide (left panel). The arrows show the localization of Olfm-1 protein in the luminal epithelial cells of the ampullary region (x400). The right panel illustrates the Olfm-1 and cytokeratin staining in the intact epithelium of the same TEP sample. FT: Fallopian tube, Em: Embryonictissues. Scale bar = 100 μm.
Expression of Olfm-1 protein in non-pregnant Fallopian tubes and in Fallopian tubes from women with Ectopic Pregnancy
Olfm-1 protein was mainly localized to the luminal epithelium and some scattered staining was found in the stroma of the Fallopian tube (Figure 1B left panel). The expression of Olfm-1 protein in the Fallopian tube with tubal ectopic pregnancy was reduced and localized to the stromal part of the Fallopian tube (Figure 1B right panel). An intense cytokeratin 17 staining was observed in the ampullary epithelial cells, suggesting the epithelium was intact in all of the ectopic sections studied. Table 1 summarizes the histological scoring (H-SCORE) of Olfm-1 protein expression non-pregnant Fallopian tubes and in Fallopian tubes from women with ectopic pregnancy. The expression of Olfm-1 protein was significantly higher (p<0.05) in the infundibullary region than in the isthmic and ampullary regions in both the follicular (H-SCORE=3.6±0.3 vs 2.5±0.3 vs 2.3±0.7, respectively, n=8) and luteal (H-SCORE=3.5±0.2 vs 2.4±0.5 vs 2.3 ± 0.6, respectively, n=10) phases of the cycle. The expression of Olfm-1 protein in the ampullary ectopic sections was significantly (p<0.05) lower than that in the ampullae of non-pregnant Fallopian tube (H-SCORE=1.3±0.4 vs 2.4±0.5, n=10; Table 1).
| Fallopian tube region | H-SCORE (mean±s.d.) | |
|---|---|---|
| Follicular phase (n=8) | Luteal phase (n=10) | |
| Infundibullary | 3.6±0.3a | 3.5±0.2a |
| Ampullary | 2.5±0.3b | 2.4±0.5b |
| Isthmic | 2.3±0.7b | 2.3±0.6b |
| Ampullary TEP | 1.3±0.4c (n=10) | |
a-bSignificant difference (P<0.01) between regions.a-c,b-cSignificant difference (P<0.05) between normal and ectopic Fallopian tubes.
H-SCORE=ΣPi(i+1), where i represents 0 (none) to 3 (very strong) and Pi=percentage of cells for each intensity (0-100%).
Effect of Olfm-1 on JAr spheroids attachment onto OE-E6/E7 cells
The JAr cells were used to produce spheroids of sizes of about 60-200 μm (Figure 2A) for the attachment studies. The spheroids attached to the OE-E6/E7 monolayer and the attachment rate increased with time. At 1, 2 and 3 hours, the attachment rates were 25%, 48% and 65%, respectively. Incubation of spheroids for 6 hours or longer did not further increase the attachment rate (range: 65-75%, Figure 2B). Therefore, a 4-hour co-culture period was selected for the assay. Interestingly, trophoblastic cells invasion like phenotype could be observed after prolonged (>24 hours) coculture (Figure 2A).
Figure 2.
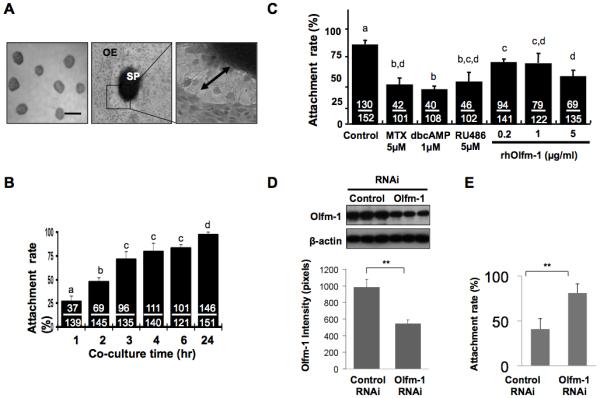
OE-E6/E7 monolayer and JAr trophoblastic spheroids are used in co-culture assay. (A) JAr spheroids of sizes (60-120 μm) were selected (left), and co-cultured with an OE-E6/E7 monolayer (middle, OE) showing invasion of trophoblastic cells (right, double arrows). (B) The attachment rate of JAr spheroids onto OE-E6/E7 monolayer increased with time in the coculture assay. (C) The effect of MTX, dbcAMP, RU486 and Olfm-1 recombinant protein (0.2, 1 and 5μg/ml) on attachment rate after 4hrs coculture were determined. a-d denotes significant different from each other at p<0.05. (D) Knockdown of Olfm-1 expression in OE-E6/E7 cells by siRNA suppresses JAr spheroids attachment. Olfm-1 siRNA significantly down-regulated Olfm-1 protein expression (>47%) in OE-E6/E7 monolayer when compare to the control siRNA (left panel). Knockdown of Olfm-1 expression in OE-E6/E7 monolayer significantly increased (p<0.05, 2-fold) JAr spheroids attachment onto OE-E6/E7 monolayer after 2hr coculture when compared to the cells transfected with control RNAi (right panel).
To test the effect of human recombinant Olfm-1 (rhOlfm-1) on spheroids attachment onto OE-E6/E7 cells, we first detected the binding of rhOlfm-1 onto both cell types in vitro. It was found that AlexaFlour 488-labelled rhOlfm-1 bound to both the JAr (16) and the OE-E6/E7 cells (data not shown). Treatment of OE-E6/E7 cells with rhOlfm-1 for 24 hours dose-dependently decreased the JAr spheroids attachment on to the OE-E6/E7 monolayer (Figure 2C), and the reduction was significantly different (p<0.01) from the untreated control. In addition, rhOlfm-1 at 5 μg/ml significantly (p<0.05) suppressed attachment when compared with the 0.2 μg/ml group.
Knockdown of Olfm-1 in OE-E6/E7 cells stimulates JAr spheroids attachment
To determine whether the down-regulation of Olfm-1 stimulated JAr spheroids attachment onto OE-E6/E7 cells, we used Olfm-1 specific siRNA for transfection study (Figure 2D). Olfm-1 siRNA significantly (p<0.05) down-regulated >45% of Olfm-1 expression when compared to OE-E6/E7 cells transfected with control siRNA. The JAr spheroids attachment rate onto the transfected OE-E6/E7 monolayer was significantly increased (p<0.001) by 2-fold (39% vs 82%) after coculture for 2 hours when compared to the control (control siRNA, Figure 2D).
Wnt-activation suppresses Olfm-1 expression and increases JAr spheroids attachment onto OE-E6/E7 cells
To determine whether activation of the Wnt-signaling pathway could restore Olfm-1 suppression and spheroids attachment, we used Wnt3a conditioned medium (Wnt3a-CM, 1:1 ratio) and LiCl (40 mM) to treat the OE-E6/E7 cells for 24 hours and studied the changes in expression of Wnt-signaling molecules and spheroids attachment rates (Figure 3A). Both Wnt3a-CM and LiCl activated Wnt-signaling as demonstrated by up-regulation of β-catenin expression in the treated OE-E6/E7 cells. Interestingly, the treatments were associated with a significant decrease in Olfm-1 protein expression at 1:1 ratio of Wnt3a-CM:DMEM/F12 and 40 mM of LiCl (Figure 3A). Treatment of OE-E6/E7 cells with either Wnt3a-CM or LiCl stimulated spheroids attachment significantly when compared to the controls (Figure 3B). Wnt3a-CM and LiCl significantly (p<0.001) increased (3-fold and 2-fold, respectively) spheroids attachment rates after co-culture with OE-E6/E7 for 1 hour. Addition of the Wnt-inhibitor Dkk-1 (0.1 μg/ml) or rhOlfm-1 (0.2 μg/ml) to the OE-E6/E7 cells for 24 hour nullified the stimulatory effect of Wnt3a-CM or LiCl on JAr spheroids attachment (Figure 3B).
Figure 3.
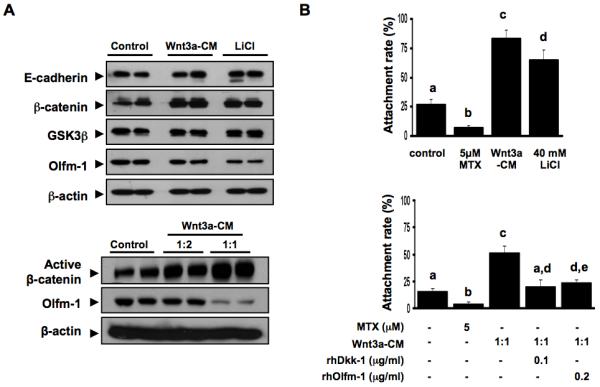
Activation of Wnt-signaling down-regulates Olfm-1 expression and reverses the suppressive effect of Olfm-1 on spheroids attachment. (A) Upper panel shows the activation of Wnt-signaling pathway by Wnt3a-conditioned medium (1:2 ratio) or LiCl (40mM) resulted in an elevated total β-catenin but reduced Olfm-1 expression. No change in the expression of GSK-3β and β-actin were found. The lower panel depicts the changes of active-β-catenin and Olfm-1 in the OE-E6/E7 cells treated 24h with Wnt3a-CM. (B) Wnt3a-CM and LiCl stimulate JAr spheroids attachment onto OE-E6/E7 monolayer after 1 hr coculture (upper panel). The increased JAr spheroids attachment by Wnt3a-CM can be nullified by co-treatment of Wnt3a-CM with rhOlfm-1 or rhDkk-1 (lower graph). a, b, c and d denotes significant different from each other at p<0.05.
Discussion
This study provides fundamental data on the spatiotemporal expression and regulation of Olfm-1 in the human Fallopian tube and is the first report of the association of activation of Wnt-signaling and suppression of Olfm-1 in ectopic pregnancy. We demonstrate that Olfm-1 expression is reduced in the epithelium of the Fallopian tube of women with ectopic pregnancy compared to Fallopian tube from non-pregnant women. In addition, our novel in-vitro model of tubal-embryo attachment, demonstrates that Olfm-1 suppresses trophoblastic spheroids attachment onto Fallopian tubal epithelial cells whereas Wnt-signaling activation, or RNAi knockdown of Olfm-1, increases the attachment.
In the non-pregnant human Fallopian tube, the expression of the Olfm-1 to -4 mRNAs were significantly higher in the follicular phase than in the luteal phase of the menstrual cycle, suggesting that their expression might be hormonally-regulated. Similarly, Olfm-1 mRNA is also down-regulated in the secretory phase of the human endometrium (16). Interestingly, the expression of ampullary Olfm-1 transcript was significantly lower (p<0.05) at the luteal phase when compared to the follicular phase, though the expression of Olfm-1 protein was similar in the same region at both phases of the cycle. The discrepancy would be due to changes in mRNA stability and protein turnover in response to different hormonal regulation. More than 70% of ectopic pregnancies are found in the ampullary region of the Fallopian tube where as implantation in the infundibullary or isthmic portions is rare (less than 5%) (29). The higher expression of Olfm-1 protein in the infundibullary region than in the ampullary and isthmic regions in non-pregnant human Fallopian tube supports the low percentage of TEP found in the infundibullum region of the Fallopian tube (1).
Bioinformatics was used to analysis the promoter region of the OLFM1 gene and no consensus estrogen responsive element (ERE) or progesterone responsive element was identified. Therefore, it is unlikely that ovarian steroids regulate Olfm-1expression in the Fallopian tube epithelium through ERE or PRE binding. Interestingly, a number of ITF-2 binding sites were identified at the Olfm-1 promoter region. ITF-2 expression is regulated by Wnt/β-catenin signaling pathway (30). Our data also showed that Wnt-activation down-regulated Olfm-1 expression in OE-E6/E7 cells and increased the JAr spheroids attachment in vitro. Further studies on Olfm-1 promoter will help to understand the regulatory mechanism of Olfm-1 expression and also to delineate the interaction of Olfm-1 and Wnt-signaling in the Fallopian tube.
Olfactomedin protein was significantly down-regulated in the Fallopian tube of patients with EP when compared to the non-pregnant patients. However, it was noted that patients in the non-pregnant group is older than the EP group. Moreover, the differences in Olfm-1 expression could be due to the increase in steroid hormone levels in patients with ectopic pregnancy (7-10 wk gestational period). Therefore, comparison between patients with ectopic pregnancy and normal pregnancy at similar gestational period warrants further investigations. However, the collection of Fallopian tube in normal pregnant women at similar gestational period is practically difficult. Despite of this, those pregnant women who seek termination of pregnancy and tubal ligation could be recruited.
To our knowledge, this is the first report of an in vitro model of tubal pregnancy. There are numerous studies using coculture of human embryos and endometrium for the study of endometrial biology (31). However, similar studies using Fallopian tube, designed for further understanding of the aetiology of ectopic pregnancy are lacking due to limited availability of cell lines. Our OE-E6/E7 cells retain most of the Fallopian tube epithelial cell characters including morphological, hormonal responses and expression of receptor (24, 32, 33) and many early implantation-related molecules including Olfm-1, β-catenin, E-cadherin, LIF, Intergrin αV and β3, MUC1, ERK and JNK (data not shown). The trophoblastic JAr cell line was used as embryo surrogate based on its expression of many cytotrophoblastic markers (34, 35). The OE-E6/E7 and JAr cell lines also provide flexibility for gene manipulation (e.g. siRNA or plasmid DNA) in functional studies.We believe that this co-culture model could be used to study the functional role of other proteins in embryo attachment and ectopic pregnancy.
Since Olfm-1 is an integral part of the ECM, down-regulation of Olfm-1 maybe associated with changes in the expression of other ECM molecules thereby affecting cell-cell interaction. A number of other ECM molecules, including integrins (36), mucin1 (37, 38), cadherins (39), laminins and fibronectins (36) have been associated with tubal ectopic pregnancy. For example, a reduced expression of the MUC1 mucin has been noted in the Fallopian tube from women with ectopic pregnancies (38, 40). MUC1 is proposed to function as a barrier for embryo implantation in the endometrium (41-45). Knockdown of Olfm-1 expression with siRNA in the OE-E6/E7 cells increased trophoblast spheroids attachment in the present in vitro model, supporting our hypothesis that Olfm-1 may also function as a barrier for embryo attachment. It is possible that down-regulation of Olfm-1 and MUC1 expression (38) contribute synergistically for ectopic pregnancy.
Activation of the canonical Wnt-signaling pathway by Wnt3a or LiCl down-regulates Olfm-1 expression in the OE-E6/E7 cells and significantly stimulates JAr spheroids attachment. It is possible that the reduced Olfm-1 expression also allows exposure of other adhesion molecules for embryo attachment. Previous studies have shown that activation of the canonical Wnt-signaling molecule β-catenin favours adhesion junction formation through a cadherin-catenin complex (46). In line with this, the implanting embryo secretes canonical Wnt-signaling activators (e.g. Wnt3a), to regulate the expression of genes responsible for ectopic implantation (47, 48). Yet,how activation of canonical Wnt-signaling affects gene expression leading to ectopic pregnancy warrants further investigations.
It is generally believed that pelvic infection triggers an immune response and stimulates secretion of various cytokines including interleukins (49), TNFα (50), TGF-β and interferons that are associated with ectopic pregnancy (51). Interestingly, TNFα and TGF-β down-regulate Olfm-1 mRNA expression in the podocytes (52). Whether pelvic infection, changes in cytokines expression act synergistically with Olfm-1 in the Fallopian tube leading to EP remains to be investigated.
In summary, our data shows that Olfm-1 is differentially expressed in the human Fallopian tube and activation of the Wnt-signaling pathway or suppression of Olfm-1 expression in the Fallopian tubal cells increases spheroids attachment. A better understanding of the role of adhesion-related molecules (e.g. Olfm and MUC1) in the human Fallopian tube may help to delineate the underlying mechanism leading to ectopic pregnancies and better prevention of tubal ectopic pregnancies in the future.
Supplementary Material
Acknowledgment
This work was supported in part by grants from the Committee on Research and Conference Grant, The University of Hong Kong to KFL and Hong Kong Research Grant Council. AWH is supported by an MRC Clinician Scientist Fellowship.
Footnotes
The authors declare no conflict of interest.
Author contribution statement
SK, RTKP, WSBY and KFL conceived the experiments. SK and KFL carried out the experiments. All authors analyzed data. SK and KFL wrote the paper. All authors had final approval of the submitted and published versions.
References
- 1.DeCherney AH, Boyers SP. Isthmic ectopic pregnancy: segmental resection as the treatment of choice. Fertil Steril. 1985 Sep;44(3):307–12. doi: 10.1016/s0015-0282(16)48852-7. [DOI] [PubMed] [Google Scholar]
- 2.Shaw JL, Dey SK, Critchley HO, Horne AW. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update. 2010 Jul-Aug;16(4):432–44. doi: 10.1093/humupd/dmp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder DA, Rivers AM, Yokoe H, Menco BP, Anholt RR. Olfactomedin: purification, characterization, and localization of a novel olfactory glycoprotein. Biochemistry. 1991 Sep 24;30(38):9143–53. doi: 10.1021/bi00102a004. [DOI] [PubMed] [Google Scholar]
- 4.Tomarev SI, Nakaya N. Olfactomedin domain-containing proteins: possible mechanisms of action and functions in normal development and pathology. Mol Neurobiol. 2009 Oct;40(2):122–38. doi: 10.1007/s12035-009-8076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danielson PE, Forss-Petter S, Battenberg EL, deLecea L, Bloom FE, Sutcliffe JG. Four structurally distinct neuron-specific olfactomedin-related glycoproteins produced by differential promoter utilization and alternative mRNA splicing from a single gene. J Neurosci Res. 1994 Jul 1;38(4):468–78. doi: 10.1002/jnr.490380413. [DOI] [PubMed] [Google Scholar]
- 6.Goldwich A, Scholz M, Tamm ER. Myocilin promotes substrate adhesion, spreading and formation of focal contacts in podocytes and mesangial cells. Histochem Cell Biol. 2009 Feb;131(2):167–80. doi: 10.1007/s00418-008-0518-4. [DOI] [PubMed] [Google Scholar]
- 7.Harland RM. A protein scaffold plays matchmaker for chordin. Cell. 2008 Sep 5;134(5):718–9. doi: 10.1016/j.cell.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Inomata H, Haraguchi T, Sasai Y. Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell. 2008 Sep 5;134(5):854–65. doi: 10.1016/j.cell.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Nakaya N, Lee HS, Takada Y, Tzchori I, Tomarev SI. Zebrafish olfactomedin 1 regulates retinal axon elongation in vivo and is a modulator of Wnt signaling pathway. J Neurosci. 2008 Jul 30;28(31):7900–10. doi: 10.1523/JNEUROSCI.0617-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, et al. Determination of the transcript profile of human endometrium. Mol Hum Reprod. 2003 Jan;9(1):19–33. doi: 10.1093/molehr/gag004. [DOI] [PubMed] [Google Scholar]
- 11.Horcajadas JA, Riesewijk A, Martin J, Cervero A, Mosselman S, Pellicer A, et al. Global gene expression profiling of human endometrial receptivity. J Reprod Immunol. 2004 Aug;63(1):41–9. doi: 10.1016/j.jri.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Lee KF, Ng EH, Yeung WS, Ho PC. Gene expression profiling of human peri-implantation endometria between natural and stimulated cycles. Fertil Steril. 2008 Dec;90(6):2152–64. doi: 10.1016/j.fertnstert.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Riesewijk A, Martin J, van Os R, Horcajadas JA, Polman J, Pellicer A, et al. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003 May;9(5):253–64. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- 14.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003 Jul;144(7):2870–81. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Oh J, Choi E, Park I, Han C, Kim do H, et al. Differentially expressed genes implicated in unexplained recurrent spontaneous abortion. Int J Biochem Cell Biol. 2007;39(12):2265–77. doi: 10.1016/j.biocel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Kodithuwakku SP, Ng PY, Liu Y, Ng EH, Yeung WS, Ho PC, et al. Hormonal regulation of endometrial olfactomedin expression and its suppressive effect on spheroid attachment onto endometrial epithelial cells. Hum Reprod. 2011 Jan;26(1):167–75. doi: 10.1093/humrep/deq298. [DOI] [PubMed] [Google Scholar]
- 17.Funayama T, Mashima Y, Ohtake Y, Ishikawa K, Fuse N, Yasuda N, et al. SNPs and interaction analyses of noelin 2, myocilin, and optineurin genes in Japanese patients with open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006 Dec;47(12):5368–75. doi: 10.1167/iovs.06-0196. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed OA, Dufort D, Clarke HJ. Expression and estradiol regulation of Wnt genes in the mouse blastocyst identify a candidate pathway for embryo-maternal signaling at implantation. Biol Reprod. 2004 Aug;71(2):417–24. doi: 10.1095/biolreprod.103.025692. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005 Jun 14;102(24):8579–84. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung WS, Ng VK, Lau EY, Ho PC. Human oviductal cells and their conditioned medium maintain the motility and hyperactivation of human spermatozoa in vitro. Hum Reprod. 1994 Apr;9(4):656–60. doi: 10.1093/oxfordjournals.humrep.a138566. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Wei Q, St Clair JB, Fu T, Stratton P, Nieman LK. Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertil Steril. 2009 May;91(5):1686–91. doi: 10.1016/j.fertnstert.2008.02.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Kodithuwakku SP, Ng PY, Chai J, Ng EH, Yeung WS, et al. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKK1 in human endometrium and may affect implantation: an in vitro co-culture study. Hum Reprod. 2010 Feb;25(2):479–90. doi: 10.1093/humrep/dep429. [DOI] [PubMed] [Google Scholar]
- 24.Lee YL, Lee KF, Xu JS, Wang YL, Tsao SW, Yeung WS. Establishment and characterization of an immortalized human oviductal cell line. Mol Reprod Dev. 2001 Aug;59(4):400–9. doi: 10.1002/mrd.1046. [DOI] [PubMed] [Google Scholar]
- 25.Aboussahoud W, Bruce C, Elliott S, Fazeli A. Activation of Toll-like receptor 5 decreases the attachment of human trophoblast cells to endometrial cells in vitro. Hum Reprod. 2010 Sep;25(9):2217–28. doi: 10.1093/humrep/deq185. [DOI] [PubMed] [Google Scholar]
- 26.Heneweer C, Kruse LH, Kindhauser F, Schmidt M, Jakobs KH, Denker HW, et al. Adhesiveness of human uterine epithelial RL95-2 cells to trophoblast: rho protein regulation. Mol Hum Reprod. 2002 Nov;8(11):1014–22. doi: 10.1093/molehr/8.11.1014. [DOI] [PubMed] [Google Scholar]
- 27.Kishida S, Yamamoto H, Kikuchi A. Wnt-3a and Dvl induce neurite retraction by activating Rho-associated kinase. Mol Cell Biol. 2004 May;24(10):4487–501. doi: 10.1128/MCB.24.10.4487-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003 May 22;423(6938):448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 29.Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum Reprod. 2002 Dec;17(12):3224–30. doi: 10.1093/humrep/17.12.3224. [DOI] [PubMed] [Google Scholar]
- 30.Kolligs FT, Nieman MT, Winer I, Hu G, Van Mater D, Feng Y, et al. ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with beta-catenin defects and promotes neoplastic transformation. Cancer Cell. 2002 Mar;1(2):145–55. doi: 10.1016/s1535-6108(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 31.Teklenburg G, Macklon NS. Review: in vitro models for the study of early human embryo-endometrium interactions. Reprod Sci. 2009 Sep;16(9):811–8. doi: 10.1177/1933719109334966. [DOI] [PubMed] [Google Scholar]
- 32.Aboussahoud W, Aflatoonian R, Bruce C, Elliott S, Ward J, Newton S, et al. Expression and function of Toll-like receptors in human endometrial epithelial cell lines. J Reprod Immunol. 2010 Jan;84(1):41–51. doi: 10.1016/j.jri.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Monkkonen KS, Aflatoonian R, Lee KF, Yeung WS, Tsao SW, Laitinen JT, et al. Hormonal regulation of Galphai2 and mPRalpha in immortalized human oviductal cell line OE-E6/E7. Mol Hum Reprod. 2007 Dec;13(12):845–51. doi: 10.1093/molehr/gam075. [DOI] [PubMed] [Google Scholar]
- 34.Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod. 2010 Feb;82(2):235–45. doi: 10.1095/biolreprod.109.077800. [DOI] [PubMed] [Google Scholar]
- 35.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009 May;127(1):26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin L, Wang YL, Bai SX, Xiao ZJ, Herva R, Piao YS. Expression of integrins and extracellular matrix proteins at the maternal-fetal interface during tubal implantation. Reproduction. 2003 Sep;126(3):383–91. doi: 10.1530/rep.0.1260383. [DOI] [PubMed] [Google Scholar]
- 37.Al-Azemi M, Refaat B, Amer S, Ola B, Chapman N, Ledger W. The expression of inducible nitric oxide synthase in the human fallopian tube during the menstrual cycle and in ectopic pregnancy. Fertil Steril. 2010 Aug;94(3):833–40. doi: 10.1016/j.fertnstert.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Al-Azemi M, Refaat B, Aplin J, Ledger W. The expression of MUC1 in human Fallopian tube during the menstrual cycle and in ectopic pregnancy. Hum Reprod. 2009 Jun 27; doi: 10.1093/humrep/dep233. [DOI] [PubMed] [Google Scholar]
- 39.Revel A, Ophir I, Koler M, Achache H, Prus D. Changing etiology of tubal pregnancy following IVF. Hum Reprod. 2008 Jun;23(6):1372–6. doi: 10.1093/humrep/den018. [DOI] [PubMed] [Google Scholar]
- 40.Savaris RF, da Silva LC, Moraes Gda S, Edelweiss MI. Expression of MUC1 in tubal pregnancy. Fertil Steril. 2008 Apr;89(4):1015–7. doi: 10.1016/j.fertnstert.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 41.Carson DD, DeSouza MM, Kardon R, Zhou X, Lagow E, Julian J. Mucin expression and function in the female reproductive tract. Hum Reprod Update. 1998 Sep-Oct;4(5):459–64. doi: 10.1093/humupd/4.5.459. [DOI] [PubMed] [Google Scholar]
- 42.Carson DD, Julian J, Lessey BA, Prakobphol A, Fisher SJ. MUC1 is a scaffold for selectin ligands in the human uterus. Front Biosci. 2006;11:2903–8. doi: 10.2741/2018. [DOI] [PubMed] [Google Scholar]
- 43.DeSouza MM, Surveyor GA, Price RE, Julian J, Kardon R, Zhou X, et al. MUC1/episialin: a critical barrier in the female reproductive tract. J Reprod Immunol. 1999 Dec;45(2):127–58. doi: 10.1016/s0165-0378(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 44.Hey NA, Graham RA, Seif MW, Aplin JD. The polymorphic epithelial mucin MUC1 in human endometrium is regulated with maximal expression in the implantation phase. J Clin Endocrinol Metab. 1994 Feb;78(2):337–42. doi: 10.1210/jcem.78.2.8106621. [DOI] [PubMed] [Google Scholar]
- 45.Hey NA, Meseguer M, Simon C, Smorodinsky NI, Wreschner DH, Ortiz ME, et al. Transmembrane and truncated (SEC) isoforms of MUC1 in the human endometrium and Fallopian tube. Reprod Biol Endocrinol. 2003 Jan 30;1:2. doi: 10.1186/1477-7827-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gooding JM, Yap KL, Ikura M. The cadherin-catenin complex as a focal point of cell adhesion and signalling: new insights from three-dimensional structures. Bioessays. 2004 May;26(5):497–511. doi: 10.1002/bies.20033. [DOI] [PubMed] [Google Scholar]
- 47.Hierholzer A, Kemler R. Beta-catenin-mediated signaling and cell adhesion in postgastrulation mouse embryos. Dev Dyn. 2010 Jan;239(1):191–9. doi: 10.1002/dvdy.22075. [DOI] [PubMed] [Google Scholar]
- 48.Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005 Jul;233(3):1064–75. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- 49.Iyibozkurt AC, Kalelioglu I, Gursoy S, Corbacioglu A, Gurelpolat N, Karahan GE, et al. Evaluation of serum levels of interleukin-10, interleukin-11 and leukemia inhibitory factor in differentiation of eutopic and tubal ectopic pregnancies. Clin Exp Obstet Gynecol. 2010;37(3):217–20. [PubMed] [Google Scholar]
- 50.Laskarin G, Redzovic A, Vukelic P, Veljkovic D, Gulic T, Haller H, et al. Phenotype of NK cells and cytotoxic/apoptotic mediators expression in ectopic pregnancy. Am J Reprod Immunol. 2010 Nov;64(5):347–58. doi: 10.1111/j.1600-0897.2010.00844.x. [DOI] [PubMed] [Google Scholar]
- 51.Tonello A, Poli G. Tubal ectopic pregnancy: macrophages under the microscope. Hum Reprod. 2007 Oct;22(10):2577–84. doi: 10.1093/humrep/dem246. [DOI] [PubMed] [Google Scholar]
- 52.Bohr DC, Koch M, Kritzenberger M, Fuchshofer R, Tamm ER. Increased expression of olfactomedin-1 and myocilin in podocytes during puromycin aminonucleoside nephrosis. Nephrol Dial Transplant. 2010 Jun 30; doi: 10.1093/ndt/gfq366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


