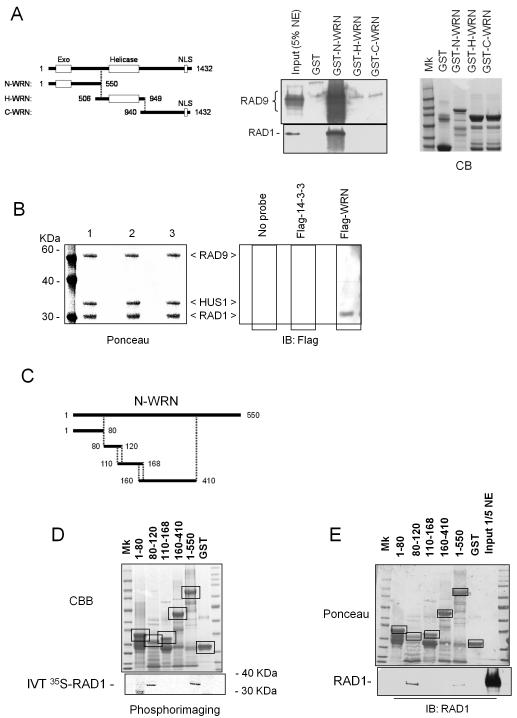
Figure 4. Interaction between the N-terminal region of WRN and the 9.1.1 complex via RAD1.
(A) The N-terminal region of WRN interacts with RAD1. GST-tagged peptides corresponding to the N-, H-, and C- regions of the WRN protein were purified from E. coli and incubated with 5μg of HeLa nuclear extracts (NE). After separation on SDS-PAGE, the presence of RAD9 and RAD1 in the pull-down material was assessed by immunoblotting using the corresponding antibodies. The 5% of total NE was loaded as input. Coomassie Blue (CB) staining was used to show the equal input of the GST-tagged WRN fragments. (B) Far western analysis of the WRN interaction with the 9.1.1 complex. Recombinant 9.1.1 complex was separated using SDS-PAGE and blotted onto nitrocellulose membrane. Ponceau staining shows equal loading and transfer between the lanes (left panel). Single lanes were incubated with: no probe (lane 1), purified Flag-14-3-3 (lane 2) or purified Flag-WRN (lane 3) and subjected to immunoblotting using anti-Flag antibody to detect association of WRN to 9.1.1 complex (right panel). (C) Schematic representation of the N-terminal sub-fragments of WRN used to map the 9.1.1 interaction site. (D) Association between the different N-terminal sub-fragments of WRN and RAD1. GST-tagged peptides corresponding to the five different N-terminal sub-fragments were purified from E. coli and incubated with in-vitro-translated (IVT) 35S-labelled RAD1. After separation on SDS-PAGE and blotting, the presence of RAD1 in the pull-down material was assessed by autoradiography. Ponceau red staining of the blot shows the amount of N-terminal sub-fragments used in the pull-down analysis. Incubation of IVT 35S-labelled RAD1 with GST or beads alone was used as a control. Boxes indicate the identity and position of each fragment. (E) GST-tagged peptides corresponding to the five different N-terminal sub-fragments were purified from E. coli and incubated with 2μg of HeLa nuclear extracts. After separation on SDS-PAGE, the presence of RAD1 in the pull-down material was assessed by immunoblotting using an anti-RAD1 antibody. The 1/5 of total NE was loaded as input. Ponceau red staining of the blot shows the amount of N-terminal sub-fragments used in the pull-down analysis. Boxes indicate the identity and position of each fragment.