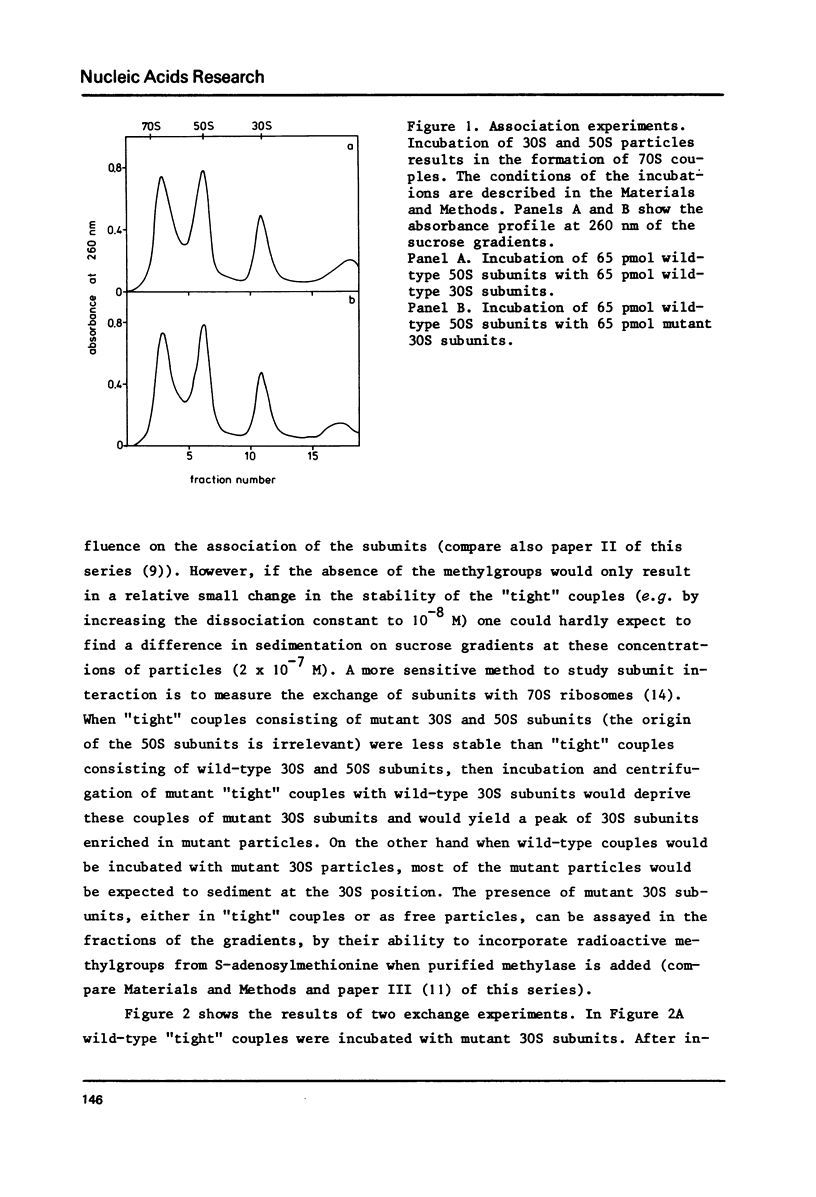
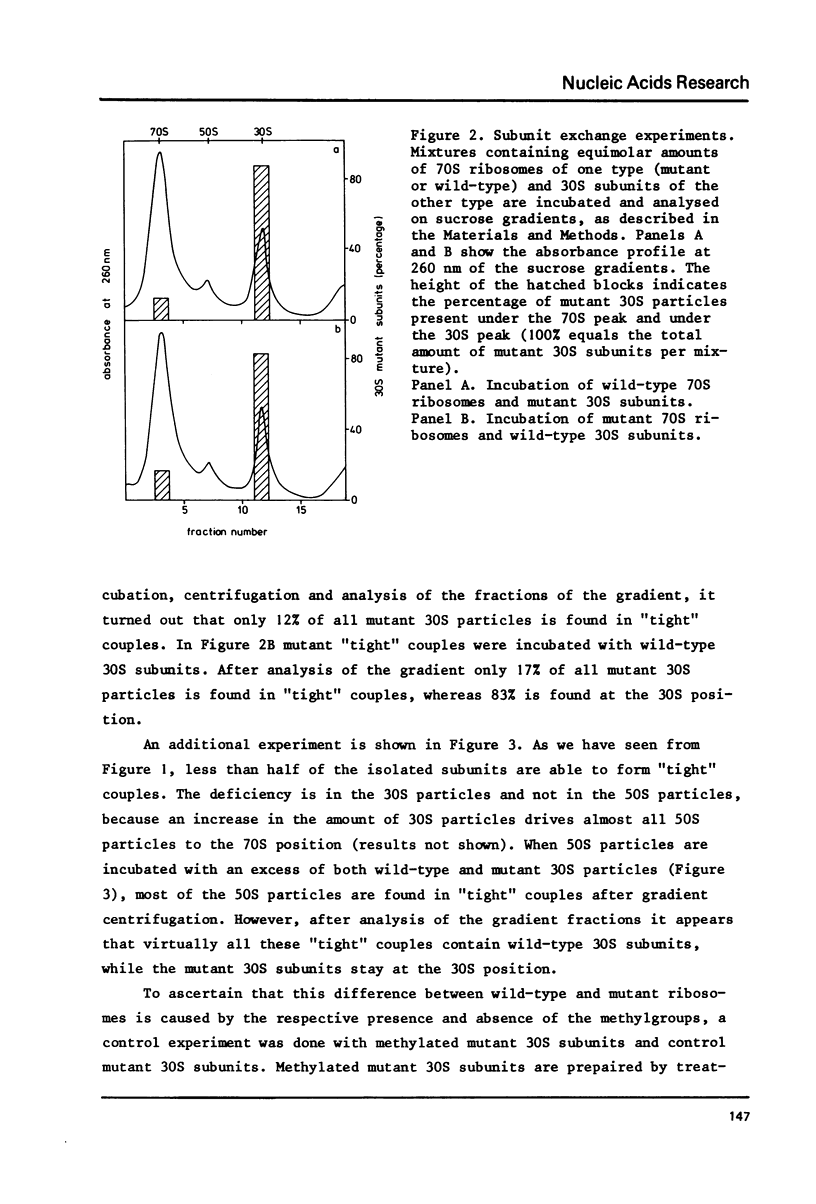
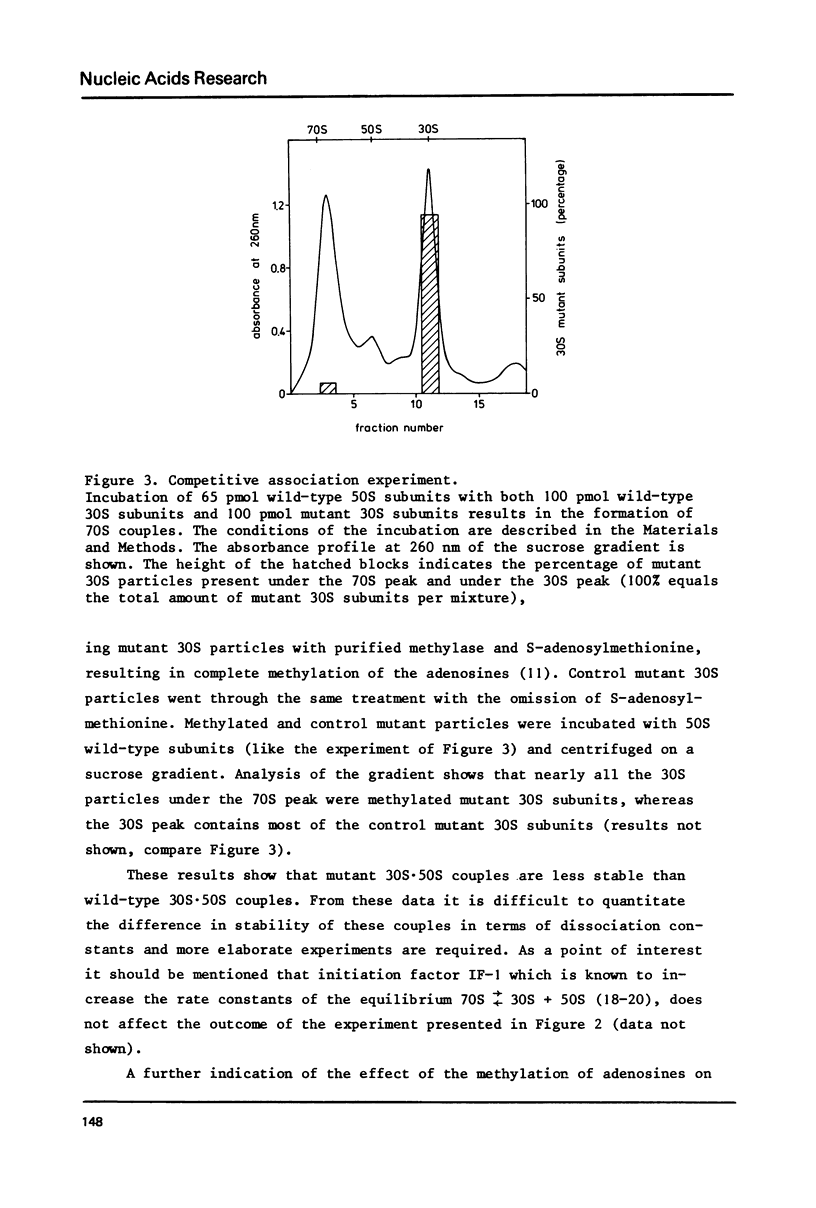
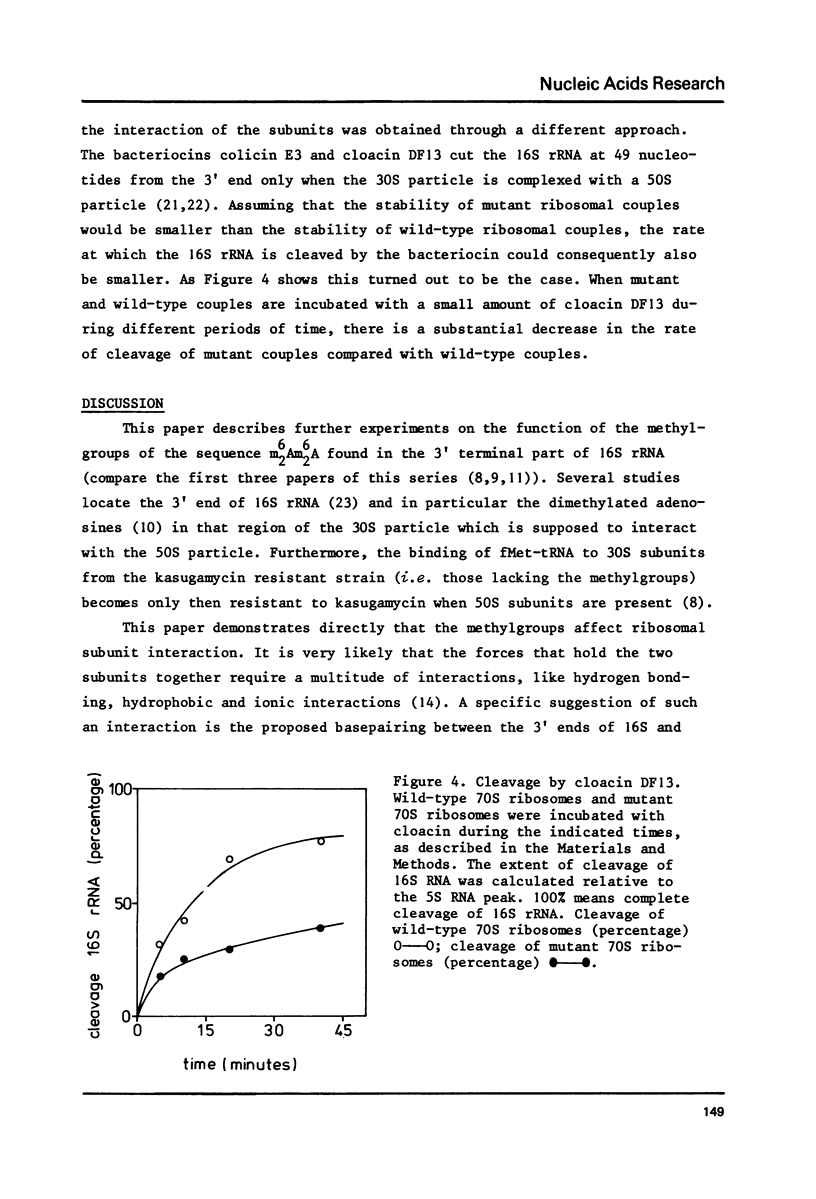
Abstract
The effect of the presence or absence of the methylgroups of the m2(6)Am2(6)A sequence near the 3' end of 16S rRNA of Escherichia coli on the interaction of the ribosomal subunits has been studied, using wild-type (methylated) and mutant (unmethylated) ribosomes. Subunit exchange experiments and competitive association experiments show a strong preference of the 50S subunit for association with methylated 30S subunits. The results indicate that the equilibrium constant of the reaction 70S in equilibrium with 30S + 50S is dependent on the methylgroups; mutant 30S.50S couples are less stable than wild-type 30S.50S couples. It is postulated that the methylgroups also stimulate the interaction between 30S subunits and initiation factor IF-3.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty H., Raba M., Gross H. J. Isolation from rat liver and sequence of a RNA fragment containing 32 nucleotides from position 5 to 36 from the 3' end of ribosomal 18S RNA. Nucleic Acids Res. 1978 Feb;5(2):425–434. doi: 10.1093/nar/5.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan R. A., Duijfjes J. J., van Leerdam E., van Knippenberg P. H., Bosch L. Specific in situ cleavage of 16S ribosomal RNA of Escherichia coli interferes with the function of initiation factor IF-1. Proc Natl Acad Sci U S A. 1976 Mar;73(3):702–706. doi: 10.1073/pnas.73.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T. Inactivation of ribosomes in vitro by colicin E 3 and its mechanism of action. Proc Natl Acad Sci U S A. 1972 Mar;69(3):549–552. doi: 10.1073/pnas.69.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M. Inactivation of ribosomes by colicin E3 in vitro: Requirement for 50 S ribosomal subunits. FEBS Lett. 1972 Apr 15;22(1):73–75. doi: 10.1016/0014-5793(72)80222-9. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Stöffler G., Wittmann H. G. Ribosome structure. Annu Rev Biochem. 1978;47:217–249. doi: 10.1146/annurev.bi.47.070178.001245. [DOI] [PubMed] [Google Scholar]
- Chaires J. B., Kegeles G. Sucrose density gradient sedimentation of E. coli ribosomes. Biophys Chem. 1977 Nov;7(3):173–178. doi: 10.1016/0301-4622(77)87019-1. [DOI] [PubMed] [Google Scholar]
- Chaires J. B., Tai M., Huang C., Kegeles G., Infante A. A., Wahba A. J. Relaxation kinetics of E. coli ribosomes. Biophys Chem. 1977 Nov;7(3):179–188. doi: 10.1016/0301-4622(77)87020-8. [DOI] [PubMed] [Google Scholar]
- De Jonge P., Klootwijk J., Planta R. J. Sequence of the 3'-terminal 21 nucleotides of yeast 17S ribosomal RNA. Nucleic Acids Res. 1977 Oct;4(10):3655–3663. doi: 10.1093/nar/4.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottavio-Martin D., Suttle D. P., Ravel J. M. The effects of initiation factors IF-1 and IF-3 on the dissociation of Escherichia coli 70 S ribosomes. FEBS Lett. 1979 Jan 1;97(1):105–110. doi: 10.1016/0014-5793(79)80062-9. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Magrum L. J., Balch W. E., Wolfe R. S., Woese C. R. Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4537–4541. doi: 10.1073/pnas.74.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg-Manago M., Gros F. Initiation mechanisms of protein syntehesis. Prog Nucleic Acid Res Mol Biol. 1977;20:209–284. doi: 10.1016/s0079-6603(08)60474-2. [DOI] [PubMed] [Google Scholar]
- Hapke B., Noll H. Structural dynamics of bacterial ribosomes. IV. Classification of ribosomes by subunit interaction. J Mol Biol. 1976 Jul 25;105(1):97–109. doi: 10.1016/0022-2836(76)90196-0. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat New Biol. 1971 Sep 1;233(35):12–14. doi: 10.1038/newbio233012a0. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Khan M. S., Salim M., Maden B. E. Extensive homologies between the methylated nucleotide sequences in several vertebrate ribosomal ribonucleic acids. Biochem J. 1978 Mar 1;169(3):531–542. doi: 10.1042/bj1690531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaktgeboren N., Roobol K., Voorma H. O. The effect of initiation factor IF-1 on the dissociation of 70-S ribosomes of Escherichia coli. Eur J Biochem. 1977 Jan 3;72(1):49–56. doi: 10.1111/j.1432-1033.1977.tb11223.x. [DOI] [PubMed] [Google Scholar]
- Oudega B., Meekel C. J., De Graaf F. K. Effects of temperature on the activity of cloacin DF13 and cloacin DF13-immunity protein. Biochim Biophys Acta. 1975 Jul 14;399(1):213–216. doi: 10.1016/0304-4165(75)90227-5. [DOI] [PubMed] [Google Scholar]
- Poldermans B., Goosen N., Van Knippenberg P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3' end of 16 S ribosomal RNA of Escherichia coli. I. The effect of kasugamycin on initiation of protein synthesis. J Biol Chem. 1979 Sep 25;254(18):9085–9089. [PubMed] [Google Scholar]
- Poldermans B., Roza L., Van Knippenberg P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3' end of 16 S ribosomal RNA of Escherichia coli. III. Purification and properties of the methylating enzyme and methylase-30 S interactions. J Biol Chem. 1979 Sep 25;254(18):9094–9100. [PubMed] [Google Scholar]
- Poldermans B., Van Buul C. P., Van Knippenberg P. H. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3' end of 16 S ribosomal RNA of Escherichia coli. II. The effect of the absence of the methyl groups on initiation of protein biosynthesis. J Biol Chem. 1979 Sep 25;254(18):9090–9093. [PubMed] [Google Scholar]
- Politz S. M., Glitz D. G. Ribosome structure: localization of N6,N6-dimethyladenosine by electron microscopy of a ribosome-antibody complex. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1468–1472. doi: 10.1073/pnas.74.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin J., Kurland C. G., Dondon J., Grunberg-Mangago M., Branlant C., Ebel J. P. New aspects of the IF3-ribosome interaction. FEBS Lett. 1976 Feb 15;62(2):111–114. doi: 10.1016/0014-5793(76)80030-0. [DOI] [PubMed] [Google Scholar]
- Vass J. K., Maden B. E. Studies on the conformation of the 3' terminus of 18-S rRNA. Eur J Biochem. 1978 Apr;85(1):241–247. doi: 10.1111/j.1432-1033.1978.tb12232.x. [DOI] [PubMed] [Google Scholar]
- Wishnia A., Boussert A., Graffe M., Dessen P. H., Grunberg-Manago M. Kinetics of the reversible association of ribosomal subunits: stopped-flow studies of the rate law and of the effect of Mg2+. J Mol Biol. 1975 Apr 25;93(4):499–415. doi: 10.1016/0022-2836(75)90242-9. [DOI] [PubMed] [Google Scholar]
- van der Hofstad G. A., Buitenhek A., van den Elsen P. J., Voorma H. O., Bosch L. Binding of labeled initiation factor IF-1 to ribosomal particles and the relationship to the mode of IF-1 action in ribosome dissociation. Eur J Biochem. 1978 Aug 15;89(1):221–228. doi: 10.1111/j.1432-1033.1978.tb20916.x. [DOI] [PubMed] [Google Scholar]



