Abstract
Purpose of review
Visceral pain represents a major clinical problem, yet far less is known about its mechanisms compared to somatic pains, e.g. from cutaneous and muscular structures.
Recent findings
In this review we describe the neuroanatomical bases of visceral pain signalling in the peripheral and central nervous system, comparing to somatic pains and also the channels and receptors involved in these events. We include an overview of potential new targets in the context of mechanisms of visceral pain and hypersensitivity.
Summary
This review should inform on the recognition of what occurs in patients with visceral pain, why co-morbidities are common and how analgesic treatments work.
Keywords: Visceral pain, visceral hyperalgesia, viscerosomatic convergence, descending modulation
Introduction
The recent growth in interest by researchers and clinicians in pain originating from internal organs reflects an important paradigm shift in the awareness of the magnitude and impact of visceral pain disorders. Most people have experienced pain from internal organs ranging from the mild discomfort of indigestion to the agony of a renal colic, and women are subject to many forms of visceral pain associated with reproductive life. For both men and women, pain of internal origin is a common cause for seeking medical attention. In the case of visceral cancer pain, the growth of a tumour could lead to a myriad of activating stimuli leading to the pain experience, ranging from chemicals released by cancer cells, immune cells, distension or obstruction of luminal organs, and/or neuropathic events such as denervation and/or nerve sprouting and other changes in neuronal function.
Nevertheless, much of our current understanding of pain mechanisms derives from studies of somatic, but not visceral nociception, possibly due to greater complications associated with accessing visceral structures with adequate visceral stimuli in research models. Nociceptive processing in somatic and visceral pain has both common features and important differences in neurological mechanisms and psychology. Importantly, treatment of both forms of pain is progressively becoming independent of the accompanying disease and pain itself is regarded as a syndrome, rather than a symptom or by-product of illness (1).
Functional gastrointestinal disorders (FGID) underlie the most prevalent forms of visceral pain. Irritable bowel syndrome (IBS) is one FGID characterised by abdominal pain, discomfort and altered bowel habits and creates tremendous pressure on the healthcare system affecting an estimated 10-15% of Europe and U.S. populations with consequent costs estimated to exceed US$ 40 billion (2-5). Dysmenhorrea, severe pelvic pain during menstrual cycles, underlies one of the most common gynaecologic complaints in young women (6, 7). It also contributes to economic burdens associated with lost workdays and productivity (8, 9). Although some visceral pain disorders are not life-threatening, they still contribute significantly to a large segment of healthcare resource consumption and have a considerable negative impact on lives with psychological distress, disturbance of work and sleep and sexual dysfunction (10).
Moreover, there is increasing evidence that the progression of visceral pathology and pain is substantially affected by ageing and gender. Some visceral pain syndromes are reported to be less intense in adults of advanced age than in younger individuals, e.g. appendicitis (11, 12). IBS is also reported twice as frequently in women than in men (13, 14).
The scope of this review covers knowledge of visceral pain mechanisms gained from the clinical setting to animal and in vitro studies investigating visceral nociceptive signaling. Some comparisons to somatic pain are highlighted.
Clinical features of visceral pain
Visceral pain usually has a temporal evolution and clinical features vary in different phases of pathology. ‘True visceral pain’ arises as a diffuse and poorly defined sensation usually perceived in the midline of the body, at the lower sternum or upper abdomen. In patients, pain from different visceral organs can have differing areas of presentation, e.g. bladder to perineal area, heart to left arm and neck, left ureter to left lower quadrant and loin. This diffuse nature and difficulty in locating visceral pain is due to a low density of visceral sensory innervation and extensive divergence of visceral input within the CNS. Visceral pain is therefore perceived more diffusely than noxious cutaneous stimulation with respect to location and timing (15).
Subsequent development of symptoms may entail referred pain to parietal somatic structures within the same metameric field as the affected organ. Spatial discrimination of visceral pain is thus typically referred to superficial structures to produce secondary hyperalgesia of superficial or deep body wall tissues due to viscerosomatic convergence (discussed later) (16). Referred pain with or without hyperalgesia is sharper, better localized and less likely to be accompanied by autonomic signs, and therefore difficult to differentiate from pain of somatic origin.
Visceral pain is often associated with marked autonomic phenomena, including pallor, profuse sweating, nausea, GI disturbances and changes in body temperature, blood pressure and heart rate (15). Table 1 lists the general characteristics of visceral pain in humans (17).
Table 1.
General characteristics of pain due to visceral pathology
| 1. Is poorly localized with referral to somatic structures |
| 2. Produces nonspecific regional or whole-body motor responses |
| 3. Produces strong autonomic responses |
| 4. Leads to sensitization of somatic tissues |
| 5. Produces strong affective responses |
Peripheral visceral neurotransmission
Afferent fibres innervating viscera project to the CNS through autonomic sympathetic and parasympathetic nerves - a dual sensory innervation (18, 19). Some spinal afferents travel along hypogastric, lumbar colonic and splanchnic nerves to terminate in thoracolumbar regions as part of sympathetic innervation, traversing both prevertebral and paravertebral ganglia en route to the spinal cord (20). Vagal and pelvic afferents respectively terminate in the brainstem and lumbosacral cord and contribute to parasympathetic innervation (21, 22) (see Figure 1, adapted from (23)).
Figure 1. Innervation of the rat GI tract.
Some visceral afferents innervating organs in thoracic and abdominal cavities travel either along the vagus nerve with cell bodies in the nodose ganglion (NG) and central terminals in the nucleus tractus solitarii (NTS), or along the dorsal column pathway (dotted line) with cell bodies in dorsal column nuclei (DC) in the brainstem. Other afferents innervating the same organs have terminals in the spinal cord, before passing through pre- and/or paravertebral ganglia en route with cell bodies in dorsal root ganglia (not illustrated; (23)). Straight lined pathways indicate sympathetic innervation and hyphenated pathways indicate parasympathetic innervation. (Prevertebral ganglia- CG: coeliac ganglion; SMG: superior mesenteric ganglion; IMG: inferior mesenteric ganglion; PG: pelvic ganglion. Nerves- S1, S2, S3, S4: greater, less, least and lumbar splanchnic nerves, respectively; IMN: intermesenteric nerve; HGN: hypogastric nerve; PN: pelvic nerve (adapted from (23)).
Visceral fibres can serve ‘sensory’ and ‘afferent’ functions; the former can evoke conscious sensations and the latter regulate autonomic flow (24). Accordingly, activation of hepatic chemoreceptors or pulmonary stretch receptors is not perceived consciously (25), whereas sensory afferents innervating the GI and urinary tracts serve regulatory functions of the gut (e.g. absorption, secretion, propulsion) and contribute to consciously evoked sensations such as pain and fullness (26).
Visceral sensory afferents are almost exclusively thinly myelinated Aδ-fibres and unmyelinated C-fibres. However, the distinction between nociceptive afferents and non-nociceptive afferents is not clear in visceral neurotransmission compared to somatic nociception, given the functional division of mechanosensitive visceral receptors into two physiological classes (20, 27, 28). ‘High-threshold receptors’ in organs such as the heart, oesophagus, colon, ureter and uterus respond only to noxious mechanical stimuli. ‘Low-threshold receptors’ are intensity-encoding and thus respond to a range of innocuous to noxious stimuli. An important contrast with somatic nociception is the role of low-threshold Aβ-fibres, which only convey innocuous mechanical sensations in normal conditions.
Viscera are also innervated by so-called ‘silent’ nociceptors, more accurately designated as mechanically insensitive afferents (MIAs) (29). These can acquire mechanosensitivity following inflammation, and have been thoroughly characterised in significant proportions in rodent pelvic and splanchnic innervations (27, 30) and in human microneurographic studies of cutaneous C-afferents (31, 32).
Viscerosomatic convergence
The neurophysiological convergence of visceral and somatic afferent inputs to the CNS is thought to underlie referred visceral pain, where noxious stimulation of viscera triggers pain referred to somatic sites (33, 34). Viscerosomatic convergence may occur as a result of the scarcity of visceral afferent fibres with spinal cord terminations; the relative contribution of visceral afferent fibres to the total spinal cord afferent input is less than 10%. Visceral afferent terminals also show extensive divergence and intraspinal distribution compared to cutaneous afferents (21).
Because of viscerosomatic convergence, somatic injury and visceral inflammation can respectively alter central processing of visceral and somatic inputs (35). Axons can send peripheral terminals to anatomically distinct segments to produce pain sensations distant to the primary site (36). Viscerosomatic convergence also accounts for altered central nociceptive processing through sensitization of primary afferent pathways, ultimately modifying neuronal input at sites of convergence in the spinal cord or higher centres (37, 38). This convergence of visceral and somatic messages may be one reason for visceral pains often accompanying somatic pain conditions or vice versa. In addition there can be viscero-visceral convergence whereby pain from one organ is referred to another.
Brain-gut axis
The “brain-gut axis” is a theoretical model depicting bidirectional neural pathways linking cognitive, emotional and autonomic centres in the brain to neuroendocrine centres, the enteric nervous system and the immune system. Bodily visceral functions (e.g. digestion, nutrient resorption, gaseous exchange, excretion) require complex regulation in which the CNS is highly integrated with the peripheral and enteric nervous systems and hormonal controls. Accordingly, altered brain-gut interactions can contribute to autonomic dysregulation of the gut and associated pain and perceptual changes in visceral disorders like IBS (39).
Vagal afferents project to the nucleus tractus solitarius (NTS) in the brainstem with cell bodies in nodose ganglion. Spinally-converging visceral afferents terminate in the dorsal horn with second order neurones projecting to higher centres through the dorsal column pathway (DC), parabrachial pathway and spinothalamic tract (see Figure 2, adapted from (40)). Studies involving DC lesions have shown suppressed inhibition of exploratory behaviour induced by noxious visceral stimulation and inhibition of potentiated visceromotor reflexes evoked by colorectal distension during inflammation (41-43). Superficial dorsal horn projections mostly form the spinoparabrachial pathway (44), associated with autonomic and affective responses to painful stimuli (45). Along with NTS projections from vagal afferents, spinoparabrachial projections are transmitted to limbic and cognitive higher centres including parts of the brain involved in affect, such as the amygdala, hypothalamus and periaqueductal grey (PAG) (40, 46, 47).
Figure 2. Ascending pathways from spinal cord neurones in visceral nociception (in rodents).
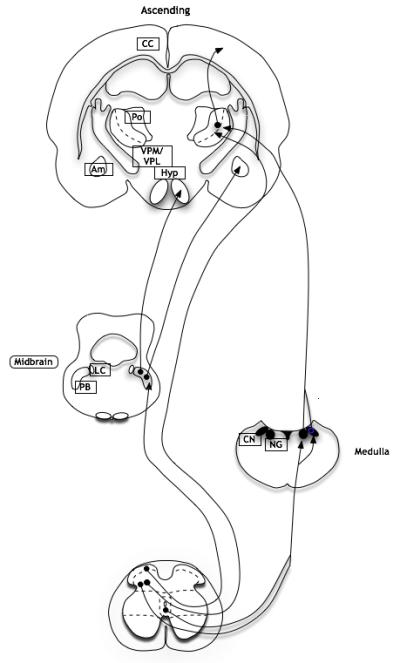
Ascending projections from lamina I neurones in the spinal cord travel along the spinoparabrachial pathway to the parabrachial nuclei (PB) with ensuing projections to the amygdala (Am) and hypothalamus (Hyp), whereas projections from deep dorsal horn neurones travel along the spinothalamic tract to thalamic nuclei (VPM and VPL), with further projections to the insula, somatosensory cortex and prefrontal cortex. Neurones from lamina III-IV and X can also travel along the post-synaptic dorsal column with medullary cell bodies, with further projections to thalamic nuclei (LC, locus coeruleus; Po, posterior thalamic nuclei; CC, cingulate cortex; CN: cuneate nucleus; GN: gracile nucleus; adapted from (40).
Spinothalamic projections travel contralaterally from the deep dorsal horn in sub-primates, along with a proportion of the lamina I population in primates (40, 48). The main thalamic projections sites are located in the ventroposterior lateral thalamus and ventroposterior medial thalamus. Ensuing projections to the insular and somatosensory cortices enable sensory discrimination. The medial thalamic nuclei are thought play greater role in the affective and motivational aspects of pain processing (49), and accordingly project to the various areas of the prefrontal cortex that are significantly correlated with visceral pain responses in imaging studies, including the anterior cingulate cortex (ACC) (50-53).
Descending modulation from higher centres and limbic structures is a dynamic system producing both facilitatory and inhibitory influences on spinal cord excitability (see Figure 3, adapted from (40)). The RVM in the brain stem is a principal component of this supraspinal modulatory system and recruitment of different RVM neurones in different conditions can potentiate or suppress central sensory transmission through descending pathways to the spinal cord (54-56). Importantly, in animals, RVM neurones do not respond in the same direction to visceral and cutaneous stimulation – i.e. neurones excited by somatic stimuli can be inhibited by visceral activity and vice versa (57-59). Electrical stimulation of the RVM produces biphasic modulation of spinal neuronal responses to CRD (54, 60) and selective ablation of RVM cells prevents the maintenance of pancreatitis abdominal hypersensitivity (55). RVM neurones also show reflex-related activity to colorectal distension that is altered by systemic analgesics (57).
Figure 3. Descending pathways in nociception (in rodents).
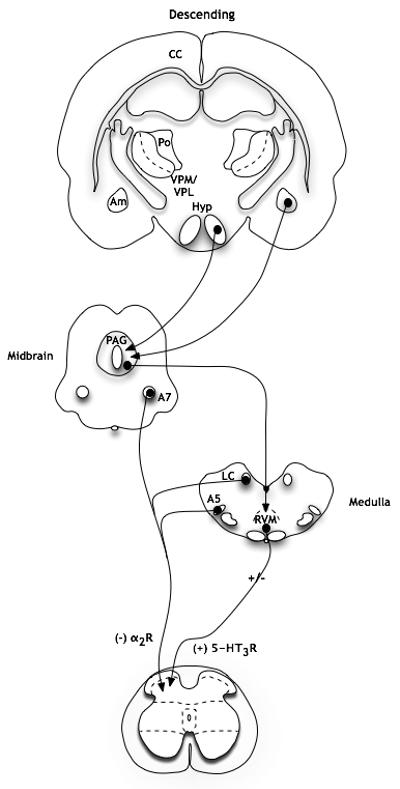
Descending projections from higher centres are integrated in the midbrain and brainstem. Inhibitory controls from the locus coeruleus complex (A5, locus coeruleus, A7) are mediated by pre- and postsynaptic α2-adrenergic receptors in the dorsal horn. Descending controls from the RVM can be both inhibitory and facilitatory, although one facilitatory pathway is mediated by presynaptic 5-HT3 receptors (Am: amygdala; Hyp: hypothalamus; VPM and VPL: thalamic nuclei; Po: posterior thalamic nuclei; CC: cingulate cortex; LC: locus coeruleus; PAG: periaqueductal grey; RVM: rostral ventromedial medulla; adapted from (40).
Functional MRI studies in humans show cortical activation following subliminal visceral stimulation (61). Cortical and subcortical circuitry can modulate brainstem pain processing, e.g. the RVM and PAG, in a top-down pain fashion, underlying possible mechanisms of distraction to decreasing both the intensity and affective components of the pain experience (62, 63). Key brainstem structures that are activated upon visceral stimulation, including the PAG and RVM, are also activated upon evoked somatic pain in humans, as has been seen in preclinical studies (64).
Visceral hyperalgesia
In peripheral sensitisation of viscera, persistent noxious stimulation of visceral nociceptors through inflammatory mediators, ectopic activity and/or noxious stimuli can produce hyperalgesia. Inflammatory mediators released at sites of injury or tissue damage can sensitise nociceptors by reducing thresholds for activation and enhancing responsiveness to stimuli. Ensuing disruption of cells, degranulation of mast cells, secretion by inflammatory cells and induction of enzymes changes the chemical milieu at the site of injury, contributing to peripheral hyperalgesia (65, 66).
Voltage-gated sodium channels are essential for the propagation of action potentials along axons, the control of membrane excitability and may contribute to sensitisation of visceral nociceptors. TTX-resistant currents are present on a high proportion of nociceptive afferents (67) and have been identified in colon DRG neurones (68, 69) and studies using Nav1.8 knockout mice demonstrate an important role for TTX-resistant currents in visceral nociceptor sensitisation (70-73). These may provide novel drug targets in the future.
TRPV1 is a non-selective cation channel gated by noxious heat (42-53°C), low pH and endogenous lipids (74) and is preferentially expressed in visceral afferents compared to somatic in the lower lumbar cord of rats (75). The importance of TRPV1 in visceral innervation is supported by the painful effects of capsaicin application to viscera in several experimental and therapeutic paradigms (76-80). Since viscera (or the spinal cord) are not normally exposed to noxious heat or capsaicin, the presence of TRPV1 at peripheral axons of visceral afferents renders the afferents sensitive to mediators of inflammation, thus functionally serving as nociceptors (1, 75, 81). Moreover, TRPV1 receptor expression is upregulated in tissue samples from patients with IBS, and this increase correlates significantly with visceral pain (82, 83). A possible coupling between the cooling TRPM8 channels with TRPV1 and TRPA1 on colonic high threshold sensory neurones may also underlie the desensitizing actions of TRPM8 (84).
Prolonged noxious stimulation of viscera and peripheral sensitisation of visceral nociceptors can promote excitability of the spinal cord and higher centre neurones that mediate nociceptive processing, otherwise known as central sensitisation (85). This is characterised by increased spontaneous activity of central neurones, enlarged receptive fields and an increase in stimulus-evoked responses (86). In humans with acute and chronic visceral pain states, secondary hyperalgesia in relevant dermatomes has been demonstrated (87-89). Central sensitisation has also been shown in humans following oesophageal electrical stimulation, where altered A-fibre activity produced somatic allodynia of the chest wall (90). Further evidence of central mechanisms contributing to referred visceral hyperalgesia have been shown using acid and capsaicin perfusion of the distal oesophagus to induce rectal hyperalgesia to both heat and mechanical stimulation (77).
The bottom-up hypothesis of development of visceral hyperalgesia maintains that peripheral sensitisation of visceral afferents and recruitment of MIAs increases the afferent barrage into the CNS, ultimately producing central sensitisation (29, 91). For example, colorectal distension in normal human subjects evokes increasing pain scores progressively with the application of repetitive stimuli of constant amplitude (92). Yet even when peripheral neuroplastic changes reverse to their normal state, central hyperexcitability may still persist (93).
However, the association between peripheral insult and visceral pain is not consistent; there is no detectable colonic abnormality in some IBS patients that have colonic hypersensitivity (94) and acute pain behaviours are evoked by distension of hollow organs that does not produce tissue injury in rodents (1, 95, 96). Thus, peripheral insult cannot always be implicated as a primary etiological factor in the development of chronic visceral hyperalgesia (97, 98). Alternatively, a top-down hypothesis can describe how initial central changes in higher centres may increase central excitability to produce visceral hyperalgesia. Psychological stress and trauma have been heavily linked to development of IBS, and dysfunction of the noradrenergic cluster, locus coeruleus, has been implicated in IBS aetiology (99-101). Anxiety and fear are also common aggravators of the pain experience (102, 103). Nevertheless, the two models are not mutually exclusive and differ only with respect to the primary site of abnormality. Both models share a central component with a positive feedback loop involving visceral afferents, changes in spinal cord excitability and altered descending modulation of nociceptive processing.
Interoception- a role of the gut in how we feel
Regardless of differences in voluntary motor control between the gut and skin, sensory inputs of our bodies relate to an evolutionarily primal need for homeostasis - signals pertaining to physiological conditions that the brain can integrate to maintain integrity of the body by optimizing energy use. In healthy humans, unique feelings and sensation of the physiological condition of the body – interoception – are perceived and contribute to background emotions that can drive behaviours that promote survival (typical visceral sensations e.g. hunger and satiation, and non-visceral sensations e.g. temperature and itch) (104).
Patients affected by FGIDs suffer from an alteration in the attentional, perceptual and affective responses to stimuli from internal sources (105). Symptoms are not only limited to visceral pain and discomfort, but often embrace a range of other feelings of physical and emotional distress. Ongoing pain that outlasts its homeostatic role is pathological and reflects a chronic dysfunction of nociceptive processing in the nervous system (106).
Projections from lamina I are involved in homeostatic signalling, terminating in autonomic (spinal cord sympathetic cell column) and homeostatic centres (PB nucleus, RVM, PAG and locus coeruleus). Together with parasympathetic afferent activity from the NTS, which has neural connections with the hypothalamus and amygdala, the spinal and bulbar projections from lamina I generate a thalamocortical representation of the physiological state of the body (48).
Treating visceral pain
Opioids form the core of pain management for a range of acute to chronic visceral pain conditions and cancer pain, yet are not always optimal due to their analgesic actions being accompanied by side-effects of constipation and sedation (107). Moreover, the paradoxical development of analgesic tolerance and nociceptive sensitisation with prolonged opioid use, opioid-induced hyperalgesia, has also proved an unfortunate obstacle in the clinic for patients with prolonged exposure to opioid analgesia (108).
NSAIDS, paracetemol and serotonergic compounds form other treatment options for a range of visceral pain conditions with minimal controlled studies, but generally none of these compounds are selective for visceral conditions and are also used to treat other forms of chronic pain (109).
This review has covered some other potential targets such as sodium channels and heat and cold sensors of the TRP channel family that could be used to treat visceral pain syndromes. Recently there has also been a convergence of preclinical and human data showing analgesic efficacy of pregabalin in acute and chronic visceral pain conditions, acting through subcortical mechanisms that likely include the spinal cord and RVM (57, 102, 110-113).
Major challenges still exist in developing analgesics in visceral pain disorders, largely due to a lack in understanding of the aetiopathogenesis and mechanisms of chronic pain development in FGIDs and other visceral pain disorders (114, 115). It is often difficult to identify whether the primary abnormality leading to the observed enhanced perception of visceral signals is due to increased peripheral encoding and transduction of stimuli, due to central pain amplification or due to both.
Conclusion
Research into the mechanisms mediating visceral nociception and the role of higher centres in modulating excitability through changes in biphasic descending modulation can provide a better understanding of the differences between visceral neurotransmission and the more thoroughly explored signalling mechanisms of somatic stimuli. Ultimately, appreciating these contrasts and similarities between the development and maintenance of somatic and visceral pain states and the means by which central excitability occurs in visceral disorders, in its own right, is also crucial for providing a better understanding of therapeutic treatments for visceral pain syndromes.
Key Points.
Visceral pain can manifest using some mechanisms also seen in somatic pain syndromes, although there are also crucial differences in central nociceptive processing of pain from these different origins.
Changes in the balance of facilitation and inhibition of central excitability along the brain-gut axis can contribute to prolonged chronic pain seen in some visceral pain disorders.
Visceral pain can negatively affect the general physiological state of how we feel along with changes in autonomic controls.
Visceral pain typically has a strong affective component, and therefore can be reinforced by anxiety and depression.
Acknowledgements
This work was supported by IMI Europain and the Wellcome Trust London Pain Consortium. There are no conflicts of interest.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the past 18 months of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Cervero F, Laird JM. Visceral pain. Lancet. 1999 Jun 19;353(9170):2145–8. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Williams DE. Economic burden of irritable bowel syndrome. Proposed strategies to control expenditures. Pharmacoeconomics. 2000 Apr;17(4):331–8. doi: 10.2165/00019053-200017040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Farmer AD, Aziz Q. Visceral pain hypersensitivity in functional gastrointestinal disorders. Br Med Bull. 2009;91:123–36. doi: 10.1093/bmb/ldp026. [DOI] [PubMed] [Google Scholar]
- 4.Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24(1):21–37. doi: 10.2165/00019053-200624010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Quigley EM, Bytzer P, Jones R, Mearin F. Irritable bowel syndrome: the burden and unmet needs in Europe. Dig Liver Dis. 2006 Oct;38(10):717–23. doi: 10.1016/j.dld.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Wang X, Wang W, Chen C, Ronnennberg AG, Guang W, et al. Stress and dysmenorrhoea: a population based prospective study. Occup Environ Med. 2004 Dec;61(12):1021–6. doi: 10.1136/oem.2003.012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol. 1996 Jan;87(1):55–8. doi: 10.1016/0029-7844(95)00360-6. [DOI] [PubMed] [Google Scholar]
- 8.Ohde S, Tokuda Y, Takahashi O, Yanai H, Hinohara S, Fukui T. Dysmenorrhea among Japanese women. Int J Gynaecol Obstet. 2008 Jan;100(1):13–7. doi: 10.1016/j.ijgo.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 9.Patel V, Tanksale V, Sahasrabhojanee M, Gupte S, Nevrekar P. The burden and determinants of dysmenorrhoea: a population-based survey of 2262 women in Goa, India. BJOG. 2006 Apr;113(4):453–63. doi: 10.1111/j.1471-0528.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 10.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003 Mar 1;17(5):643–50. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 11.Giamberardino MA, Affaitati G, Costantini R. Referred pain from internal organs. In: Cervero F, Jensen TS, editors. Handbook of Clinical Neurology. Elsevier; Amsterdam: 2006. pp. 343–60. [DOI] [PubMed] [Google Scholar]
- 12.Wroblewski M, Mikulowski P. Peritonitis in geriatric inpatients. Age Ageing. 1991 Mar;20(2):90–4. doi: 10.1093/ageing/20.2.90. [DOI] [PubMed] [Google Scholar]
- 13.Olden KW. The challenge of diagnosing irritable bowel syndrome. Rev Gastroenterol Disord. 2003;3(Suppl 3):S3–11. [PubMed] [Google Scholar]
- 14.Wilson S, Roberts L, Roalfe A, Bridge P, Singh S. Prevalence of irritable bowel syndrome: a community survey. Br J Gen Pract. 2004 Jul;54(504):495–502. [PMC free article] [PubMed] [Google Scholar]
- 15.Procacci P, Zoppi M, Maresca M. Visceral Sensation. In: Cervero F, Morrison JFB, editors. Progress in Pain Research. Elsevier; Amsterdam: 1986. pp. 21–8.pp. 39 [DOI] [PubMed] [Google Scholar]
- 16.Hardy JD, Wolff HG, Goodell H. Experimental evidence on the nature of cutaneous hyperalgesia. J Clin Invest. 1950 Jan;29(1):115–40. doi: 10.1172/JCI102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ness TJ. Historical and Clinical Perspectives. In: Gebhart GF, editor. Visceral Pain, Progress in Pain Research and Management. IASP Press; Seattle: 1995. pp. 3–23. [Google Scholar]
- 18.McSwiney BA. Visceral functions of the nervous system. Annu Rev Physiol. 1944;(6):365–90. [Google Scholar]
- 19.Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994 Jan;74(1):95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- 20.Sengupta JN, Gebhart GF. Mechanosensitive Afferent Fibres in the Gastrointestinal and Lower Urinary Tracts. In: Gebhart GF, editor. Visceral Pain. IASP Press; Seattle: 1995. pp. 75–98. [Google Scholar]
- 21.Gebhart GF. Visceral pain-peripheral sensitisation. Gut. 2000 Dec;47(Suppl 4):iv54–5. doi: 10.1136/gut.47.suppl_4.iv54. discussion iv8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989 May 8;283(2):248–68. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 23.Sengupta JN, Gebhart GF. Gastrointestinal afferent fibers and sensation. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 3 ed Raven Press; New York: 1994. pp. 483–519. [Google Scholar]
- 24.Langley JN. The Autonomic Nervous System. Brain. 1903;(26):1–16. [Google Scholar]
- 25.Cervero F. Visceral nociception: peripheral and central aspects of visceral nociceptive systems. Philos Trans R Soc Lond B Biol Sci. 1985 Feb 19;308(1136):325–37. doi: 10.1098/rstb.1985.0033. [DOI] [PubMed] [Google Scholar]
- 26.Janig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996 Jan 5;42(1-2):29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- 27.Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 1994 Jun;71(6):2046–60. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- 28.Al-Chaer ED, Traub RJ. Biological basis of visceral pain: recent developments. Pain. 2002 Apr;96(3):221–5. doi: 10.1016/S0304-3959(02)00046-5. [DOI] [PubMed] [Google Scholar]
- 29.McMahon SB, Koltzenburg M. Silent afferents and visceral pain. In: Fields HL, Liebeskind JC, editors. Pharmacological Approaches to the Treatment of Chronic Pain: New Concepts and Critical Issues. Seattle: 1994. pp. 11–30. [* This study is the first to adequately characterise the proportion and attributes of colorectal MIAs in rodents.] [Google Scholar]
- 30.Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol. Jan;300(1):G170–80. doi: 10.1152/ajpgi.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995 Jan;15(1 Pt 1):333–41. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999 Nov 15;19(22):10184–90. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cervero F. Somatic and visceral inputs to the thoracic spinal cord of the cat: effects of noxious stimulation of the biliary system. J Physiol. 1983 Apr;337:51–67. doi: 10.1113/jphysiol.1983.sp014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995 Jul;109(1):40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 35.Cameron DM, Brennan TJ, Gebhart GF. Hind paw incision in the rat produces long-lasting colon hypersensitivity. J Pain. 2008 Mar;9(3):246–53. doi: 10.1016/j.jpain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993 Mar;52(3):259–85. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 37.Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. J Neurophysiol. 1991 Jul;66(1):20–8. doi: 10.1152/jn.1991.66.1.20. [DOI] [PubMed] [Google Scholar]
- 38.Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol. 1991 Jul;66(1):29–39. doi: 10.1152/jn.1991.66.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Mayer EA. Emerging disease model for functional gastrointestinal disorders. Am J Med. 1999 Nov 8;107(5A):12S–9S. doi: 10.1016/s0002-9343(99)00277-6. [DOI] [PubMed] [Google Scholar]
- 40.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001 Feb;2(2):83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- 41.Al-Chaer ED, Feng Y, Willis WD. A role for the dorsal column in nociceptive visceral input into the thalamus of primates. J Neurophysiol. 1998 Jun;79(6):3143–50. doi: 10.1152/jn.1998.79.6.3143. [DOI] [PubMed] [Google Scholar]
- 42.Palecek J, Paleckova V, Willis WD. The roles of pathways in the spinal cord lateral and dorsal funiculi in signaling nociceptive somatic and visceral stimuli in rats. Pain. 2002 Apr;96(3):297–307. doi: 10.1016/S0304-3959(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 43.Palecek J, Willis WD. The dorsal column pathway facilitates visceromotor responses to colorectal distention after colon inflammation in rats. Pain. 2003 Aug;104(3):501–7. doi: 10.1016/S0304-3959(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 44.Todd AJ. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp Physiol. 2002 Mar;87(2):245–9. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- 45.Bester H, Menendez L, Besson JM, Bernard JF. Spino (trigemino) parabrachiohypothalamic pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1995 Feb;73(2):568–85. doi: 10.1152/jn.1995.73.2.568. [DOI] [PubMed] [Google Scholar]
- 46.Blomqvist A, Ma W, Berkley KJ. Spinal input to the parabrachial nucleus in the cat. Brain Res. 1989 Feb 20;480(1-2):29–36. doi: 10.1016/0006-8993(89)91563-1. [DOI] [PubMed] [Google Scholar]
- 47.Bester H, Matsumoto N, Besson JM, Bernard JF. Further evidence for the involvement of the spinoparabrachial pathway in nociceptive processes: a c-Fos study in the rat. J Comp Neurol. 1997 Jul 14;383(4):439–58. [PubMed] [Google Scholar]
- 48.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002 Aug;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 49.Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78(2):415–8. doi: 10.1007/BF00228914. [DOI] [PubMed] [Google Scholar]
- 50.Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988 Feb;24(2):379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- 51.Cao Z, Wu X, Chen S, Fan J, Zhang R, Owyang C, et al. Anterior cingulate cortex modulates visceral pain as measured by visceromotor responses in viscerally hypersensitive rats. Gastroenterology. 2008 Feb;134(2):535–43. doi: 10.1053/j.gastro.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 52.Moisset X, Bouhassira D, Denis D, Dominique G, Benoit C, Sabate JM. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: A visceral pain network. Eur J Pain. 2009 May 25; doi: 10.1016/j.ejpain.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000 May;118(5):842–8. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 54.Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol. 2002 May;87(5):2225–36. doi: 10.1152/jn.2002.87.5.2225. [DOI] [PubMed] [Google Scholar]
- 55.Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006 Jun;130(7):2155–64. doi: 10.1053/j.gastro.2006.03.025. [* This review discusses the role of endogenous pain modulation in the development and treatment of visceral pain disorders. Altered balances bottom-up and top-down facilitation and inhibition can contribute to the hypersensitivities seen in visceral pain disorders.] [DOI] [PubMed] [Google Scholar]
- 56.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut. 2011 Jul 18;60(11):1589–99. doi: 10.1136/gutjnl-2011-300253. [* This preclinical study shows differential role of RVM neurones in different pain states provides a plausible mechanism for the analgesic effects for the results seen in humans in study 109.] [DOI] [PubMed] [Google Scholar]
- 57.Sikandar S, Dickenson AH. Pregabalin modulation of spinal and brainstem visceral nociceptive processing. Pain. 2011 Jul 19;152(10):2312–22. doi: 10.1016/j.pain.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chandler MJ, Oh UT, Hobbs SF, Foreman RD. Responses of feline raphespinal neurons to urinary bladder distension. J Auton Nerv Syst. 1994 May;47(3):213–24. doi: 10.1016/0165-1838(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 59.Brink TS, Mason P. Raphe magnus neurons respond to noxious colorectal distension. J Neurophysiol. 2003 May;89(5):2506–15. doi: 10.1152/jn.00825.2002. [DOI] [PubMed] [Google Scholar]
- 60.Zhuo M, Gebhart GF. Facilitation and attenuation of a visceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterology. 2002 Apr;122(4):1007–19. doi: 10.1053/gast.2002.32389. [DOI] [PubMed] [Google Scholar]
- 61.Kern MK, Shaker R. Cerebral cortical registration of subliminal visceral stimulation. Gastroenterology. 2002 Feb;122(2):290–8. doi: 10.1053/gast.2002.30989. [DOI] [PubMed] [Google Scholar]
- 62.Miron D, Duncan GH, Bushnell MC. Effects of attention on the intensity and unpleasantness of thermal pain. Pain. 1989 Dec;39(3):345–52. doi: 10.1016/0304-3959(89)90048-1. [DOI] [PubMed] [Google Scholar]
- 63.Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002 Feb;125(Pt 2):310–9. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 64.Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005 Aug 10;25(32):7333–41. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007 Aug 2;55(3):353–64. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 66.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005 Jul;6(7):521–32. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 67.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996 Jan 18;379(6562):257–62. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 68.Laird JMA, Fernand C, Parnham MJ, Coward K, Baker MD. Sodium Channels, Pain, and Analgesia. Birkhäuser Basel; Basel: 2005. Voltage-gated sodium channels and visceral pain; pp. 63–70. [Google Scholar]
- 69.Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E(2) modulates TTX-R I(Na) in rat colonic sensory neurons. J Neurophysiol. 2002 Sep;88(3):1512–22. doi: 10.1152/jn.2002.88.3.1512. [DOI] [PubMed] [Google Scholar]
- 70.Hillsley K, Lin JH, Stanisz A, Grundy D, Aerssens J, Peeters PJ, et al. Dissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null mice. J Physiol. 2006 Oct 1;576(Pt 1):257–67. doi: 10.1113/jphysiol.2006.113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laird JM, Souslova V, Wood JN, Cervero F. Deficits in visceral pain and referred hyperalgesia in Nav1.8 (SNS/PN3)-null mice. J Neurosci. 2002 Oct 1;22(19):8352–6. doi: 10.1523/JNEUROSCI.22-19-08352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matthews EA, Wood JN, Dickenson AH. Na(v) 1.8-null mice show stimulus-dependent deficits in spinal neuronal activity. Mol Pain. 2006;2:5. doi: 10.1186/1744-8069-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leo S, D’Hooge R, Meert T. Exploring the role of nociceptor-specific sodium channels in pain transmission using Nav1.8 and Nav1.9 knockout mice. Behav Brain Res. Mar 17;208(1):149–57. doi: 10.1016/j.bbr.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 74.Hwang SW, Oh U. Hot channels in airways: pharmacology of the vanilloid receptor. Curr Opin Pharmacol. 2002 Jun;2(3):235–42. doi: 10.1016/s1471-4892(02)00149-2. [DOI] [PubMed] [Google Scholar]
- 75.Hwang SJ, Valtschanoff JG. Vanilloid receptor VR1-positive afferents are distributed differently at different levels of the rat lumbar spinal cord. Neurosci Lett. 2003 Sep 25;349(1):41–4. doi: 10.1016/s0304-3940(03)00750-x. [DOI] [PubMed] [Google Scholar]
- 76.Bortolotti M, Porta S. Effect of Red Pepper on Symptoms of Irritable Bowel Syndrome: Preliminary Study. Dig Dis Sci. May 15; doi: 10.1007/s10620-011-1740-9. [** This study identifies the development of referred viscero-visceral hyperalgesia following acid and capsaicin perfusion of the oesophagus in humans. The authors found a hypersensitivity of the sigmoid colon to mechanical and heat stimulation, but not electrical, likely mediated by central neuronal hypersensitivity. This widespread visceral hyperalgesia with concomitant modality-specific reductions in pain seen in this volunteer study effectively mimics the complex clinical picture of visceral pain syndromes.] [DOI] [PubMed] [Google Scholar]
- 77.Brock C, Andresen T, Frokjaer JB, Gale J, Olesen AE, Arendt-Nielsen L, et al. Central pain mechanisms following combined acid and capsaicin perfusion of the human oesophagus. Eur J Pain. 2010 Mar;14(3):273–81. doi: 10.1016/j.ejpain.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 78.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001 Jun;92(3):335–42. doi: 10.1016/S0304-3959(01)00275-5. [** This study demonstrates a change in the activity of brainstem neurones following intracolonic capsaicin. The authors observe greater spontaneous and evoked activity of RVM ON-cells to colorectal distension and innocuous heat stimulation, as well as referred pain behaviours to the hindpaw. Thus noxious visceral stimuli can cause referred hypersensitivity, partly mediated by long-lasting sensitisation of brainstem neurones.] [DOI] [PubMed] [Google Scholar]
- 79.Sanoja R, Tortorici V, Fernandez C, Price TJ, Cervero F. Role of RVM neurons in capsaicin-evoked visceral nociception and referred hyperalgesia. Eur J Pain. 2010 Feb;14(2):120, e1–9. doi: 10.1016/j.ejpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gonlachanvit S, Mahayosnond A, Kullavanijaya P. Effects of chili on postprandial gastrointestinal symptoms in diarrhoea predominant irritable bowel syndrome: evidence for capsaicin-sensitive visceral nociception hypersensitivity. Neurogastroenterol Motil. 2009 Jan;21(1):23–32. doi: 10.1111/j.1365-2982.2008.01167.x. [DOI] [PubMed] [Google Scholar]
- 81.Ravnefjord A, Brusberg M, Kang D, Bauer U, Larsson H, Lindstrom E, et al. Involvement of the transient receptor potential vanilloid 1 (TRPV1) in the development of acute visceral hyperalgesia during colorectal distension in rats. Eur J Pharmacol. 2009 Jun 2;611(1-3):85–91. doi: 10.1016/j.ejphar.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 82.Yiangou Y, Facer P, Dyer NH, Chan CL, Knowles C, Williams NS, et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001 Apr 28;357(9265):1338–9. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- 83.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008 Jul;57(7):923–9. doi: 10.1136/gut.2007.138982. [* This study investigates the anti-nociceptive role of the cold-sensing TRPM8 channel in visceral colonic sensory pathways. It may prove a new therapeutic target given its inhibitory coupling to other TRP channels that have sensitising functions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrington AM, Hughes PA, Martin CM, Yang J, Castro J, Isaacs NJ, et al. A novel role for TRPM8 in visceral afferent function. Pain. 2011 Apr 11; doi: 10.1016/j.pain.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 85.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009 Sep;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suzuki R, Rahman W, Rygh LJ, Webber M, Hunt SP, Dickenson AH. Spinal-supraspinal serotonergic circuits regulating neuropathic pain and its treatment with gabapentin. Pain. 2005;117(3):292–303. doi: 10.1016/j.pain.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Stawowy M, Drewes AM, Arendt-Nielsen L, Funch-Jensen P. Somatosensory changes in the referred pain area before and after cholecystectomy in patients with uncomplicated gallstone disease. Scand J Gastroenterol. 2006 Jul;41(7):833–7. doi: 10.1080/00365520500463332. [DOI] [PubMed] [Google Scholar]
- 88.Drewes AM, Pedersen J, Reddy H, Rasmussen K, Funch-Jensen P, Arendt-Nielsen L, et al. Central sensitization in patients with non-cardiac chest pain: a clinical experimental study. Scand J Gastroenterol. 2006 Jun;41(6):640–9. doi: 10.1080/00365520500442559. [DOI] [PubMed] [Google Scholar]
- 89.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003 Sep;105(1-2):223–30. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 90.Willert RP, Delaney C, Kelly K, Sharma A, Aziz Q, Hobson AR. Exploring the neurophysiological basis of chest wall allodynia induced by experimental oesophageal acidification - evidence of central sensitization. Neurogastroenterol Motil. 2007 Apr;19(4):270–8. doi: 10.1111/j.1365-2982.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 91.Mayer EA, Munakata J, Mertz H, Lembo T, Bernstein RN. Visceral Hyperalgesia and Irritable Bowel Syndrome. In: Gebhart GF, editor. Visceral Pain. IASP Press; Seattle: 1995. pp. 429–68. [Google Scholar]
- 92.Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain. 1990 Dec;43(3):377–86. doi: 10.1016/0304-3959(90)90035-C. [DOI] [PubMed] [Google Scholar]
- 93.Mayer EA. Visceral hyperalgesia and IBS. In: Gebhart GF, editor. Visceral Pain, Progress in Research and Management. IASP Press; Seattle: 2005. pp. 429–68. [Google Scholar]
- 94.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002 Dec;123(6):2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 95.Ness TJ, Lewis-Sides A, Castroman P. Characterization of pressor and visceromotor reflex responses to bladder distention in rats: sources of variability and effect of analgesics. J Urol. 2001 Mar;165(3):968–74. [PubMed] [Google Scholar]
- 96.Randich A, Uzzell T, Cannon R, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urol. 2006;6:2. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barbara G, Stanghellini V. Biomarkers in IBS: when will they replace symptoms for diagnosis and management? Gut. 2009 Dec;58(12):1571–5. doi: 10.1136/gut.2008.169672. [DOI] [PubMed] [Google Scholar]
- 98.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009 Feb;58(2):196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 99.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992 Jun;33(6):825–30. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drossman DA, Leserman J, Nachman G, Li ZM, Gluck H, Toomey TC, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990 Dec 1;113(11):828–33. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 101.Elam M, Thoren P, Svensson TH. Locus coeruleus neurons and sympathetic nerves: activation by visceral afferents. Brain Res. 1986 Jun 4;375(1):117–25. doi: 10.1016/0006-8993(86)90964-9. [* This study uses Wistar Kyoto rats and rat subjected to neonatally stressed maternal separation to demonstrate the analgesic efficacy of gabapentin in both models, but only anti-anxiety effects in the latter. Importantly, this data suggests that gabapentin may be a useful treatment for disorders that encompass co-morbid visceral pain and anxiety stemming from an overactive stress system, and so may relate to IBS.] [DOI] [PubMed] [Google Scholar]
- 102.O’ Mahony S, Coelho AM, Fitzgerald P, Lee K, Winchester W, Dinan TG, et al. The effects of gabapentin in two animal models of co-morbid anxiety and visceral hypersensitivity. Eur J Pharmacol. 2011 Sep 30;667(1-3):169–74. doi: 10.1016/j.ejphar.2011.05.055. [** Acid infusion of the human esophagus produces hyperalgesia due to central sensitisation, and relative pain ratings are increased in the anxious state. This study illustrates how anxiety can exacerbate visceral pain, suggesting that the affective components of visceral hyperalgesia are also important targets in analgesic treatments.] [DOI] [PubMed] [Google Scholar]
- 103.Sharma A, Van Oudenhove L, Paine P, Gregory L, Aziz Q. Anxiety increases acid-induced esophageal hyperalgesia. Psychosom Med. 2010 Oct;72(8):802–9. doi: 10.1097/PSY.0b013e3181f5c021. [DOI] [PubMed] [Google Scholar]
- 104.Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. 1 ed Harcourt Brace; New York: 1999. [Google Scholar]
- 105.Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet. 1999 Sep 11;354(9182):936–9. doi: 10.1016/S0140-6736(98)08320-2. [DOI] [PubMed] [Google Scholar]
- 106.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006 Dec;131(6):1925–42. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 107.Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000 Apr 5;283(13):1710–4. doi: 10.1001/jama.283.13.1710. [DOI] [PubMed] [Google Scholar]
- 108.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008 Jul-Aug;24(6):479–96. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- 109.Drewes AM, Wilder-Smith CH, Staahl C. Chronic abdominal pain: evaluation and management of common gastrointestinal and urogenital diseases. In: Castro-Lopes J, Raja SN, Schmelz M, editors. Pain 2008 Refresher Course Syllabus. IASP Press; Seattle: 2008. pp. 381–91. [Google Scholar]
- 110.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007 Sep;56(9):1218–25. doi: 10.1136/gut.2006.110858. [* This study demonstrates an analgesic efficacy of pregabalin on experimental gut pain in patients with visceral hyperalgesia due to chronic pancreatitis without affecting cortical activity. The anti-nociceptive mechanisms of pregabalin are sub-cortically mediated.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olesen SS, Graversen C, Olesen AE, Frokjaer JB, Wilder-Smith O, van Goor H, et al. Randomised clinical trial: pregabalin attenuates experimental visceral pain through sub-cortical mechanisms in patients with painful chronic pancreatitis. Aliment Pharmacol Ther. 2011 Aug 16;34(8):878–87. doi: 10.1111/j.1365-2036.2011.04802.x. [DOI] [PubMed] [Google Scholar]
- 112.Ohashi-Doi K, Gale JD, Kurebayashi Y. Pregabalin inhibits accelerated defecation and decreased colonic nociceptive threshold in sensitized rats. Eur J Pharmacol. 2010 Sep 15;643(1):107–12. doi: 10.1016/j.ejphar.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 113.Bannister K, Sikandar S, Bauer CS, Dolphin AC, Porreca F, Dickenson AH. Pregabalin Suppresses Spinal Neuronal Hyperexcitability and Visceral Hypersensitivity in the Absence of Peripheral Pathophysiology. Anesthesiology. 2011 May 19;115(1):144–52. doi: 10.1097/ALN.0b013e31821f6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Knowles CH. New horizons in the pathogenesis of gastrointestinal neuromuscular disease. J Pediatr Gastroenterol Nutr. 2007 Dec;45(Suppl 2):S97–102. doi: 10.1097/MPG.0b013e31812e6569. [DOI] [PubMed] [Google Scholar]
- 115.Knowles CH, Aziz Q. Basic and clinical aspects of gastrointestinal pain. Pain. 2009 Feb;141(3):191–209. doi: 10.1016/j.pain.2008.12.011. [DOI] [PubMed] [Google Scholar]



