Abstract
Redox signaling contributes to the regulation of cancer cell proliferation, survival and invasion, and participates in the adaptation of cancer cells to their microenvironment. NADPH oxidases are important mediators of redox signaling in normal and cancer cells. Redox signal specificity in normal cells is in part achieved by targeting enzymes that generate reactive oxygen species to specific subcellular microdomains such as focal adhesions, dorsal ruffles, lipid rafts or caveolae. In a similar fashion, redox signal specificity during cancer cell invasion can be regulated by targeting reactive oxygen generation to invasive microdomains such as invadopodia. Here we summarize recent advances in the understanding of the redox signaling processes that control the cancer cell pro-invasive program by modulating cell adhesion, migration and proteolysis as well as the interaction of cancer cells with the tumor microenvironment. We will focus on redox signaling events mediated by invadopodia NADPH oxidase complexes and their contribution to cancer cell invasion.
Keywords: Redox, ROS, signaling, cancer, migration, invasion, invadopodia, NADPH oxidase, Tks4, Tks5, Nox4, Nox1, p22phox, PTPs
INTRODUCTION
Reactive oxygen species (ROS) are mediators of redox signaling and oxidative stress, two distinct but related processes which contribute to neoplasia. Redox signaling is initiated by physiologically generated ROS to regulate cellular functions or decisions. ROS act as second messengers due to their ability to induce covalent modification (oxidation) of biological macromolecules which affects their functions in a similar way to phosphorylation [1]. Redox signaling pathways are used by normal and cancer cells to modulate physiological or aberrant cellular functions, respectively. Oxidative stress can be initiated by the unregulated production of ROS from either extracellular (ultraviolet irradiation, drugs, xenobiotics) or intracellular (mitochondria, peroxisomes, oncogenes) sources [2]. These ROS are usually present at high levels and can cause damage by irreversibly oxidizing cellular proteins, lipids and nucleic acids [3]. In normal cells oxidative stress elicits an anti-oxidant response that leads to either damage repair or death; however cancer cells aberrantly tolerate oxidative stress [4–7] which contributes to cancer progression by driving, for instance, genetic instability [8–10]. The physiological induction of antioxidant enzyme expression in response to oxidative stress is also considered by some investigators as a process of redox signaling [11]. Despite the differences between both processes, oxidative stress and redox signaling are far from being independent events, especially in cancer, where ROS derived from oxidative stress could potentially activate a redox signaling pathway, or a deregulated redox signaling pathway could contribute to oxidative stress by virtue of excess ROS generation. We are still far from understanding how any cell senses and integrates inputs from different ROS signals, whether dangerous or beneficial for tissue homeostasis.
Oxidative stress and redox signaling have been implicated in the initiation and/or maintenance of a pro-invasive program in cancer cells. We will focus here on recent advances in redox control of cellular signaling pathways during cancer cell invasion and, in particular, on novel redox signaling complexes which localize ROS to subcellular microdomains (invadopodia) in order to drive cell invasion.
OVERVIEW OF REDOX SIGNALING IN CANCER
Basic components of redox signaling
The generation of ROS during redox signaling is enzymatically regulated. The main sources of ROS are cellular oxidases, [12] including xanthine oxidases [13], lipoxygenases [14, 15], cyclooxygenases [16], myeloperoxidases [17] and NADPH oxidases [18]. Additionally, lysyl oxidases are amine oxidases which directly oxidize extra and intracellular substrates [19, 20]. Partial one electron reduction at mitochondrial respiratory chain complexes 1 and 3 also contributes to the generation of ROS [21], although their role in redox signaling as opposed to oxidative stress is less well understood.
NADPH oxidases are among the best-characterized enzymes that generate ROS for cellular signaling purposes [18, 22, 23]. There are seven Nox family members: Nox1, Nox2, Nox3, Nox4, Nox5, Duox1 and Duox2. All are transmembrane flavoproteins capable of generating superoxide by transferring an electron from NADPH to molecular oxygen. They contain six transmembrane alpha helical domains and an extracellular domain which can be glycosylated if the Nox subunit is localized at the plasma membrane [24]. Some NADPH oxidases localize to other cellular membranes, including endoplasmic reticulum [25] and intracellular vesicles [26].
With the possible exception of the calcium-regulated family member Nox5, Noxes require additional subunits for maximal oxidase activity. Nox1, Nox2, Nox3 and Nox4 bind to the transmembrane protein p22phox [27], which in turn recruits organizers (p47phox, p40phox or NoxO1), activators (p67phox or NoxA1) and small GTPases (Rac1 or Rac2) [28–32]. In the case of Nox2 for example, the SH3 domains of p47phox associate with the C-terminus of p22phox while the proline-rich tail of p47phox associates with p67phox, which in turn is associated with active Rac2. In over-expression experiments, Nox4-p22phox seems to behave as a constitutively active enzyme with full enzymatic activity in the absence of organizers or activators [33], although that remains to be demonstrated for the endogenous protein. In the case of Duox1 and Duox2, protein maturation and plasma membrane localization require the function of the accessory proteins DuoxA1 and DuoxA2 [34] that also seem to contribute to Duox activity at the plasma membrane [35, 36].
Superoxide generated by NADPH oxidases is converted to hydrogen peroxide in a process known as dismutation, either spontaneously at low pH, or with higher efficiency by the enzymatic activity of superoxide dismutases (SODs), which are present at different subcellular locations [37]. Three SODs have been described: SOD1 (also known as Mn-SOD) localized to mitochondria; SOD2 (also known as CuZn-SOD), localized in the nucleus and cytoplasm; and EC-SOD, which is more abundant in extracellular matrix (ECM) and fluids [38]. Hydrogen peroxide is the prominent ROS implicated in signal transduction and acts mainly through oxidation of specific cysteine and methionine residues on target molecules.
Multiple eukaryotic cellular signaling pathways use oxidation of effector molecules to transmit signals. Many of these pathways use a redox signaling module to increase the amplitude and/or duration of a tyrosine phosphorylation signal [39]. Hydrogen peroxide was first suggested to act as a signal-transducing molecule downstream of PDGF signaling in smooth muscle cells [40], but the mechanism was unknown. The discovery of transient regulation of phosphatase activity by reversible oxidation was a fundamental step in understanding the importance and extent of redox signaling [41–43]. During redox signaling, hydrogen peroxide reversibly oxidizes a conserved cysteine residue in the active site of protein tyrosine phosphatases (PTPs), inhibiting their catalytic activity [41, 44, 45]. Many studies have described the regulation of cellular processes by the transient inactivation of protein tyrosine phosphatases and dual-specificity phosphatases through redox signaling, often in the context of normal or cancer cell signaling downstream growth factor receptor or integrin activation. Examples include PTP1B [46, 47], LMW-PTP [48, 49], Shp-2 [43], PTEN [50] and PTP-PEST [51]. Amplification of tyrosine phosphorylation signals is perhaps also achieved by direct redox activation of protein kinases such as c-Src [52–54]. In addition, redox modification of many other effector molecules is relevant in normal or cancer cell signaling, including cell cycle regulators such as Cdc25C [55]; RasGTPase [56] and Ras-dependent signaling [57]; transcription factors such as c-Jun/c-Fos and Stat3 [58, 59]; matrix metalloproteases (MMPs) [60, 61]; and cytoskeletal components such as actin [62].
Novel redox signaling components: Tks family of proteins
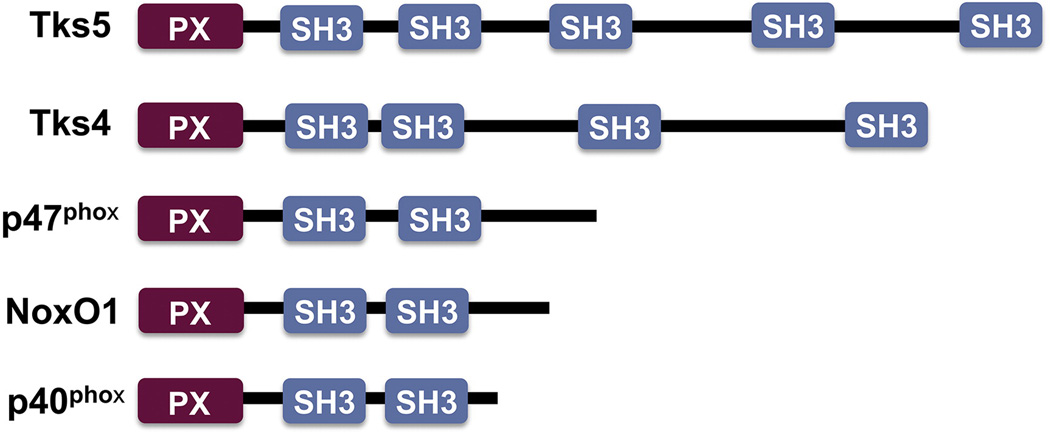
Recently, the adaptor proteins Tks4 and Tks5 have been shown to be novel components of NADPH oxidase complexes [63, 64]. Tks5 (previously known as Fish) was first described in a cDNA library screen for Src substrates [65]. The existence of a Tks5 orthologue was suspected from information on public databases [66] and confirmed by the cloning of Tks4, which has an overall identity of 43% to Tks5 [67]. Tks4 and Tks5 are large scaffolding proteins that contain an N-terminal Phox homology (PX) domain and four or five Src homology 3 (SH3) domains, respectively [65, 67]. Both Tks family members are c-Src substrates [65, 67, 68], and several binding partners have been identified, including ADAM-family metalloproteases [69], Grb2 and N-WASP [70], dystroglycan [71], and Nck [68]. Tks proteins are expressed in different organs including brain, lung, liver, kidney, skeletal muscle and heart [65, 67]. Furthermore, Tks4 has been shown to be disrupted in patients with Frank-Ter Haar syndrome, a genetic disorder characterized by skeletal, ocular and cardiac abnormalities [72]. The Tks adaptors are expressed in several human cancer cell lines and tumors [73], and have overlapping non-redundant functions in regulating the formation and activity of podosomes/invadopodia [67, 73], although the molecular basis for these differences are still unknown.
The homology between Tks adaptors and p47phox led to the hypothesis that the Tks proteins could act as organizers for Nox-dependent ROS generation [65]. Indeed, both Tks4 and Tks5 are structurally similar to p47phox, p40phox and NoxO1: all contain amino-terminal PX domains which preferentially bind PtdIns(3,4)P2 and/or PtdIns(3)P, followed by 2 or more SH3 domains [65, 67, 74]. There is a single primordial Tks/p47 gene in tunicates and echinodermata, which is likely to be the common ancestor of the vertebrate orthologues Tks4, Tks5, p40phox, p47phox and NoxO1 [75]. The demonstration that Tks4 and Tks5 support Nox1 and Nox3-dependent generation of ROS in the absence of other organizers confirmed this hypothesis [64]. Moreover, Tks5 can associate with p22phox through a mechanism involving the first two SH3 domains of Tks5 and the proline-rich region of p22phox [63], and Tks4 and Tks5 can bind NoxA1 through their SH3 domains in a Rac-independent manner [64]. More importantly, the total levels of ROS generated by cancer cells decreases considerably after silencing of Tks5 or Tks4 [63, 64], indicating that endogenous Tks proteins may be required for ROS generation in cancer cells. The existence of Tks-dependent ROS generation during normal cell biology remains to be proven, but it is likely that Tks proteins play an important role in redox signaling in normal tissues as well. Although in HEK293 cells the activity of the over-expressed Nox4-p22phox complex seems to be independent of adaptor or organizer proteins [33], and over-expressed Tks4 or Tks5 in these cells did not increase ROS generation by over-expressed Nox4 [64], the silencing of endogenous Tks5 decreased ROS generation by Nox4-p22phox over-expressing mouse melanoma cells [63], indicating a possible role for Tks5 in Nox4-dependent ROS generation in certain cancer cells, although this hypothesis awaits further investigation. Another PX domain-containing protein, Poldip2, has recently been shown to interact with p22phox and modulate Nox4 localization and activity in vascular smooth muscle cells [76].
Regulation of Redox signaling: how, when and where?
An excess of hydrogen peroxide is potentially dangerous; therefore its generation is tightly regulated, even in cancer cells that are known to have increased antioxidant defenses and tolerate higher ROS levels than normal cells. Regulation of ROS generation is performed at several levels: control of expression and activity of cellular oxidases, regulation of the antioxidant cellular machinery and spatial restriction of ROS generation. In addition, redox signaling is terminated by the reduction of reversibly oxidized targets.
NADPH oxidase components are expressed in different cancer cell lines and tumor tissues [77, 78]. Inducers of expression and activity of NADPH oxidases include growth factors, oncogene expression, hypoxia, as well as integrin-dependent stimulation [23, 79]. Hydrogen peroxide diffusion is limited by the cytoplasmic reducing environment, and ROS activity is restricted by cellular antioxidant defenses that include glutathione peroxidase, catalase, thioredoxin and peroxiredoxin [11, 80]. Redox signaling is terminated by the reduction of reversibly oxidized targets by cellular thiols. In the case PTP1B, this effect seems to be mediated in vitro at physiological pH by thioredoxin rather than by glutharedoxin or glutathione [46]. Which specific cellular thiols are implicated in redox signaling termination in different cellular contexts is still poorly understood.
Spatial regulation is key for the specificity and control of redox signaling pathways and has been shown to be achieved by targeting oxidants and/or limiting antioxidants to subcellular microdomains [39, 81, 82].
REDOX SIGNALING AND CANCER INVASION
Cell invasion is considered one of the limiting steps for the progression of primary solid tumors to metastatic lesions [83]. Without local invasion into neighboring tissues, the primary tumor might be resectable, but the initiation and maintenance of a cell invasive program allows the spreading of the cancer cells into the surrounding tissues and may promote metastatic disease [84]. The main cellular mechanisms implicated in the initiation and maintenance of an invasive program include modulation of the adhesive, migratory and proteolytic abilities of the cancer cell as well as its adaptation to tumor microenvironmental cues [84]. Redox signaling pathways play an important role in the regulation of all these processes.
Redox control of adhesion and migration
In cancer cells, cell-cell and cell-substrate adhesion properties change in response to pro-invasive stimulation. The modification of these adhesion complexes not only alters the adhesive and migratory properties of cells by inducing cytoskeletal remodeling, but also modulates survival and cell cycle progression by affecting the cross-talk between adhesion platforms and the cell signaling networks that regulate the communication between cells and their microenvironment [85, 86].
In epithelial cells, cell-cell adhesion through adherens junctions is diminished by the downregulation of the cell-cell adhesion component E-cadherin. The loss of adherens junctions facilitates cell migration and it is best studied in the context of embryonic development, where the downregulation of E-cadherin in specific subsets of cells initiates the process of epithelial to mesenchymal transition (EMT) which contributes to morphogenesis [87]. In a process similar to EMT, during cancer progression epithelial cells adopt a migratory phenotype which allows the dissemination of cancer cells from their tissues of origin. Indeed, loss of E-cadherin is enough to mediate the transition from adenoma to carcinoma in mice [88]. While different mechanisms have been described to mediate the loss of E-cadherin in normal and cancer cells [89–91], ROS are necessary for EMT induced by TGFβ in renal tubular epithelial cells through activation of MAPK and Smad [92], and they promote the MMP3-dependent activation of Snail to induce EMT in SCp2 mammary carcinoma cells [93]. Furthermore lysyl oxidase-like 2 interacts with Snail and induces EMT, possibly through Snail oxidation [94].
Cell adhesion to the ECM is also modulated during invasion. Integrins and cellular adhesion molecules, or CAMs, are both implicated in cell-ECM adhesion and mediate cancer cell invasion [95, 96]. In cancer, integrin-mediated adhesion and signaling can be modulated by increased integrin recycling [97] as well as aberrant expression or activation of integrins and integrin signaling adaptors [98, 99]. Nox1 mediates anchorage-independent proliferation in vitro and subcutaneous tumor growth of K-Ras transformed cells in athymic mice [100]. This appears to be mediated by decreased cell adhesion and stress fiber formation, through inactivation of the GTPase Rho. Indeed, stress fiber formation may be abrogated by Nox1 activity through ROS-dependent transient inactivation of LMW-PTP and the consequent activation of p190RhoGAP, which in turn suppresses Rho GTPase activity [101]. Redox regulation of stress fiber formation was originally described to occur in HeLa cells, possibly through a Nox2-dependent pathway [102]. In human colon tumors, Nox1 expression correlates with K-Ras activating mutations [103], and in colon cancer cells, Nox1 dependent signaling promotes cell migration downstream of arachidonic acid and PKCs by modulating integrin recycling [104]. In bladder cancer cells, Nox1 and Nox4 regulate invasiveness through NFκB activation downstream of the leukotriene B4 receptor [105], and the activity of Nox2 and Nox4 in transformed myeloid cells increases migratory ability, perhaps by modulating the phosphorylation of MARCKS, an actin filament crosslinking protein [106]. Furthermore, ROS-dependent signaling facilitates the survival of cancer cells in the absence of attachment to the ECM. Normal cells die by anoikis in the absence of ECM-dependent signals, and this is in part due to the induction of oxidative stress. This response is abrogated by antioxidants or oncogenes that, interestingly, by protecting from oxidative stress-induced anoikis facilitate cancer progression [6]. Furthermore, Nox1 mediates the activation of an Akt-dependent survival pathway initiated by the binding of angiopoietin-like 4 protein to integrins in the absence of ECM attachment, allowing cancer cells to escape anoikis by mimicking ECM-integrin signaling [107].
Redox control of proteolysis and ECM remodeling
In some instances, invasive cells have the ability to reach the blood or lymphatic circulation and spread to distant sites, a process largely restricted in adult organs to immune surveillance. Invasive cells travel through different ECMs -mainly interstitial matrix and basement membranes- in order to extravasate, intravasate, and colonize target organs. To date, there is no unifying hypothesis to explain the mechanism used by cancer cells to cross these barriers, and it is likely that both proteolytic and non-proteolytic mechanisms are utilized [108, 109]. Non-proteolytic mechanisms may suffice for extravasation through tumor vasculature with compromised integrity, or lymphatic vessels in which cell-cell junctions are sparser. Non-proteolytic mechanisms may also be sufficient to cross defective basement membranes (BMs) associated with decreased deposition or crosslinking of its components [110] or to travel through interstitial collagen [111]. Proteolytic mechanisms are better understood and often involve proteolysis of ECM components and their consequent irreversible remodeling. Matrix metalloproteases (MMPs) are prominent among ECM remodeling enzymes, and their activity is associated with normal physiology [112] as well as with cancer cell invasion and tumor progression [113–115]. NADPH oxidase-dependent ROS generation regulates expression or activation of MMPs in endothelial cells [116–118], cardiomyocytes [119], and alveolar macrophages [120]. In breast cancer cells, MMP-2 is activated by SOD1 through the generation of ROS [121], and in HT1080 sarcoma cells by a RTK-PI3K-NFkB pathway downstream of hydrogen peroxide [122]. MMP9 secretion in keratinocytes treated with the carcinogenic compound TPA seems to require NADPH oxidase activity [123]. Furthermore, the expression of mitochondrial SOD in fibrosarcoma cells increases the levels of MMP-1 and the incidence of experimental pulmonary metastasis [124], a n d t h e secretion and activation of MMP2 downstream of EGF signaling in pancreatic cancer cells is dependent on NADPH-generated ROS mediated by PI3K- and Src-dependent activation of Rac1 [125]. Although in some of these studies the cellular sources of ROS remain unclear, the findings suggest an important role of redox signaling in the regulation of extracellular matrix remodeling.
Redox signaling and the tumor microenvironment
Cancer cells are exposed to an abnormal microenvironment that is affected by, and in turn modulates, cancer cell biology. The tumor microenvironment contains a modified ECM and a stromal cell component that includes tumor-associated fibroblasts, adipocytes and immune cells. The tumor microenvironment evolves during tumor progression, becoming low in oxygen and nutrients and influencing and being modulated by angiogenesis, fibrosis and inflammation [126–128]. ROS participate in the regulation of these processes. For instance, secreted lysyl oxidases mediate the enzymatic oxidation of specific lysine residues on collagen to increase the formation of fibrillar collagen, the ligand for β1 integrin. Lysyl oxidase expression is induced by hypoxia and promotes cancer cell migration and invasion through FAK activation [129] as well as metastasis by generating a pre-metastatic niche [130]. Nox1 overexpression increases angiogenesis and the growth of prostate cancer cells injected subcutaneously in mice, in part through the up-regulation of VEGF [131]. Nox-dependent ROS induce angiogenesis and tumor growth of ovarian cancer cells through up-regulation of HIF1α and VEGF [132]. Furthermore, inhibition of NADPH oxidases reduces angiogenesis and tumor growth in mice [133].
LOCALIZING REDOX SIGNALING TO INVASIVE MICRODOMAINS
Invadopodia: cellular tools for invasion
In vitro, many invasive cancer cells have the ability to form invadopodia, specialized protrusions of the plasma membrane that concentrate adhesive proteins and proteolytic enzymes and contain a complex array of molecular components that coordinate cell adhesion, cell migration and pericellular proteolysis [134–136]. The cytoplasmic component of invadopodia contains cytoskeletal proteins such as microfilaments, microtubules and intermediate filaments [137]. Actin filaments serve as a scaffold for multiple actin-binding proteins and cellular signaling proteins, including the Nox organizers Tks5 and Tks4, which are required for invadopodia formation and function [64, 67, 69]. The plasma membrane at invadopodia contains a specialized subset of lipids [70] and transmembrane proteins, which include integrins [138], membrane matrix metalloproteases [139, 140] and, in Src-transformed fibroblasts, the NADPH oxidase subunit Nox4 [63]. Invadopodia formation and their dynamic turnover depend on growth factor and cell adhesion signals. For instance, EGF and TGFβ can induce invadopodia formation in breast cancer cells [141, 142], and Twist, a transcription factor that drives EMT and metastasis, has been shown to regulate invadopodia formation [143].
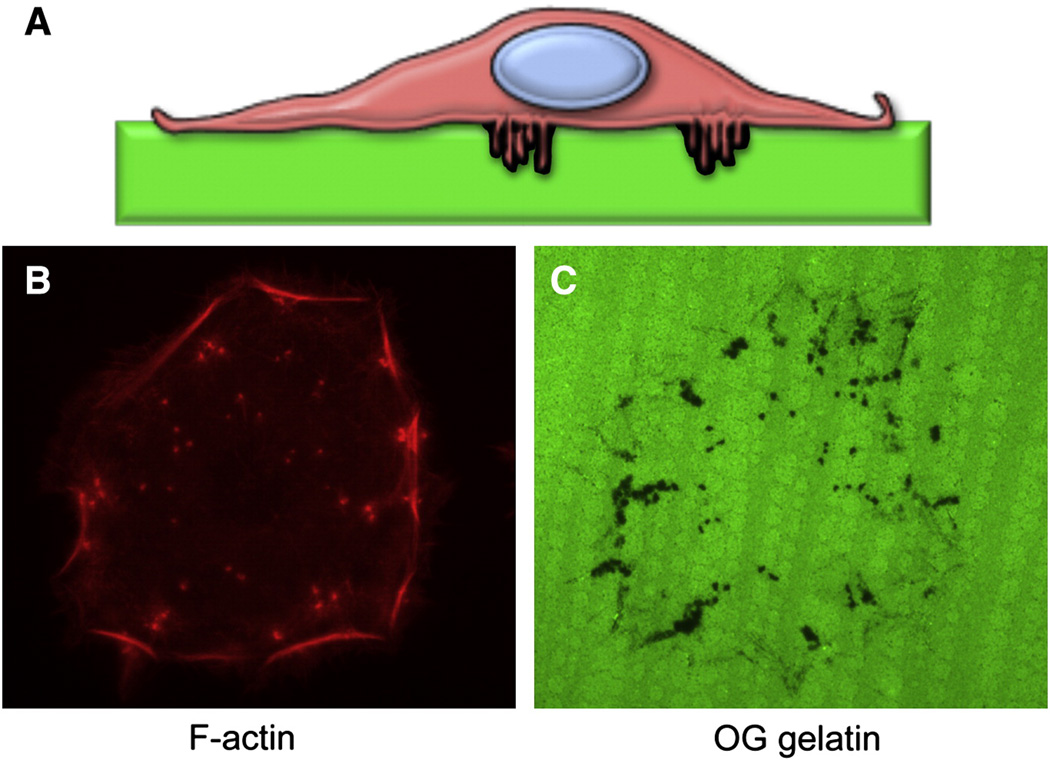
Invadopodia are usually studied in 2-dimensional cell culture, although recent studies have analyzed them in more complex environments [137, 144]. The ability of invadopodia to regulate pericellular proteolysis is commonly assessed in vitro by growing cells on top of a fluorescently labeled ECM substrate. After processing for immunofluorescence, focal proteolysis can be visualized as “dark spots” where the labeled substrate has been digested (Figure 1). The ability to form invadopodia in vitro correlates with increased invasiveness in vitro and in vivo [73, 145]. While still awaiting a definitive demonstration of the presence of these structures in vivo, invadopodial components such as Tks5 and cortactin have been shown to be necessary for efficient tumor growth in animal models [146–148], suggesting that invadopodia mediate tumor progression in vivo.
Figure 1.
Comparison between the structural domain organization of Tks proteins and their homologues p47phox, NoxO1 and p40phox.
Redox signaling and invadopodia
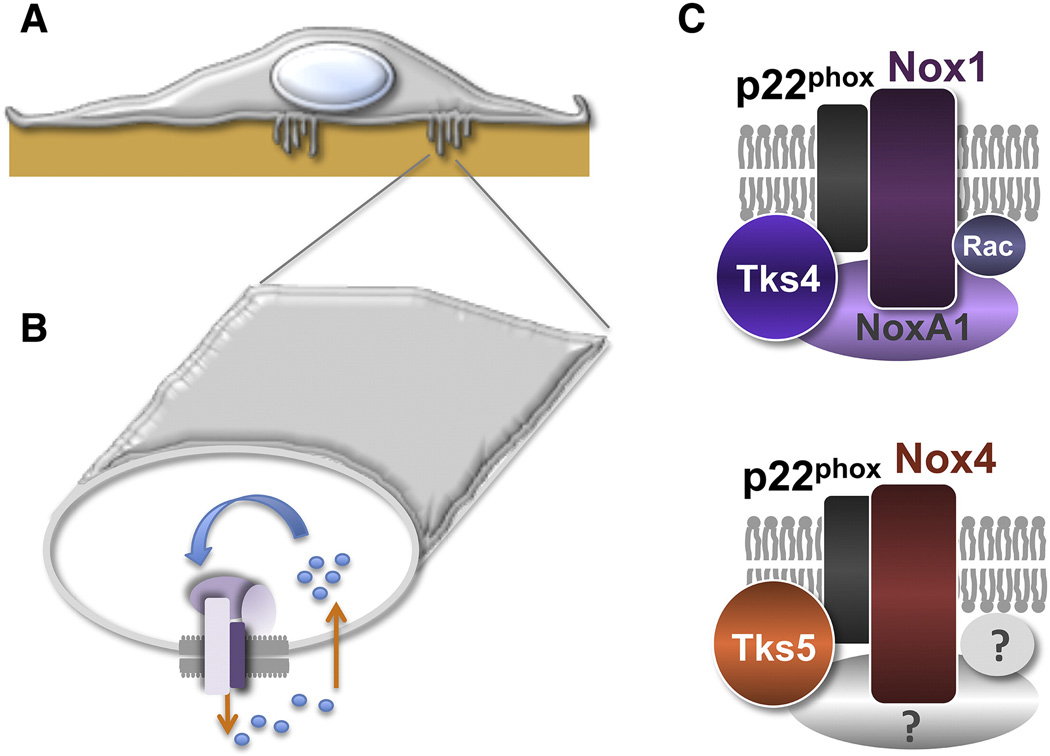
An additional mechanism by which redox signaling controls cancer cell invasion is through the regulation of invadopodia formation and activity [63, 64]. ROS can be detected at invadopodia using the ROS-sensitive fluorescent probe DCF-DA and live-cell imaging [63]. ROS are necessary for invadopodia formation in both mouse and human cancer cells, and a prominent source of ROS in this context might be novel NADPH oxidase complexes (Figure 2) containing Nox1 or Nox4 along with the novel NADPH organizers and invadopodia components Tks4 or Tks5 [63, 64]. These complexes might be exclusively formed at invadopodial membranes in cancer cells, since both Tks4 and Tks5 are invadopodia markers and localize preferentially to these structures [67, 69, 73].
Figure 2. Invadopodia are invasive microdomains.
- Diagram of a cancer cell growing on top of a fluorescent ECM substrate. Invadopodia are thin cellular protrusions from the cell-substrate interface into the substrate.
- F-actin staining of a head and neck carcinoma cell showing invadopodia (dots).
- OregonGreen-labeled gelatin from the same field as B) showing the areas of invadopodia degradation (black dots).
Invadopodia membranes, as well as membrane ruffles, focal adhesions and caveolae can be considered as subcellular microdomains, specialized regions of the plasma membrane that contain specific signaling complexes and perform unique cellular functions. These structures, unlike other subcellular compartments such as endoplasmic reticulum, mitochondria or nuclei, are not separated from the rest of the cytoplasm by a physical barrier such as a cellular membrane. Therefore, the local regulation of these structures and its activity by redox signaling represents an additional challenge to signal-specificity mechanisms. NADPH oxidase complexes in the plasma membrane generate superoxide to the outer side of the membrane. Superoxide is not membrane permeable, unlike its dismutated product H2O2, which has a longer half life and is believed to cross the plasma membrane at the site of generation and locally affect susceptible targets. The radius of action of H2O2 is believed to be limited by the cytoplasmic reducing environment. How could then H2O2 reach its targets? This is a subject of intense investigation, and some clues are starting to emerge. For instance, Src activity has recently been shown to mediate redox signaling by facilitating a local increase in H2O2 concentration through the phosphorylation and transient inactivation of membrane-bound Peroxiredoxin-1 [82]. Src kinase activity promotes invadopodia formation [149, 150], and it is tempting to speculate that redox control of invadopodia formation also requires local inactivation of Peroxiredoxin-1 locally present to modulate the redox signal. At subcellular microdomains such as invadopodia, redox signal specificity is then regulated by the activation of ROS-generating enzymes and the inactivation of ROS-scavenging proteins within the limits of the microdomain.
Local accumulation of ROS at membrane sites has also been observed at membrane ruffles of endothelial cells induced to migrate using the scratch assay [39, 151, 152]. The local generation of ROS by targeting Nox to discrete subcellular membrane locations through protein-specific associations contributes to redox signaling specificity and regulates directed-cell migration in endothelial cells (reviewed in [81]). For example, after VEGF stimulation of endothelial cells, p47phox is targeted to membrane ruffles by association with the cytoskeletal protein WAVE1, and forms a complex containing Rac1 and PAK1 that seems to modulate actin assembly and JNK activation through a ROS-dependent pathway [153]. An NADPH complex containing Nox2 and the scaffold protein IQGAP1 localizes to the lamellipodia of migrating endothelial cells and is necessary for localized ROS generation, cytoskeletal reorganization and migration [152]. The orphan adaptor TRAF4 recruits p47phox to focal complexes along with Hic-5 to activate PAK1 and drive endothelial cell migration [51]. All these complexes contain the canonical Nox organizer p47phox, which is cytosolic and targeted to membranes after phosphorylation [154, 155]. In contrast, the NADPH oxidase complexes described at invadopodia seem to contain the Tks proteins as organizers. Tks5 localizes to mature invadopodia and it is recruited to nascent invadopodia by binding of the PX domain to discrete membrane domains enriched in PtdIns(3,4)P2 and PtdIns(3,4,5)P3 [70]. Whether Nox1 or Nox4 are localized to invadopodial membranes before Tks adaptor proteins are recruited to these sites is unknown. The recruitment of Nox1-p22phox or Nox4-p22phox complexes to Tks-enriched membrane domains has been suggested to involve the targeted fusion of Nox-containing vesicles [156]. Indeed, NADPH oxidases are present in internal vesicles [26], and invadopodia are sites of active vesicle trafficking for delivering of transmembrane components as well as exocytosis of proteases [140, 157].
Could NADPH complexes containing other members of the p47phox organizer superfamily (p47phox, p40phox, NoxO1) also be present at invadopodia or at podosomes (the invadopodia counterparts in non-cancer cells)? The PX domain of NoxO1 binds preferentially PtdIns(3,5)P2, PtdIns(5)P and PtdIns(4)P [158], which are not present at invadopodia [70], making it an unlikely candidate for organizing invadopodial or podosomal NADPH complexes. Furthermore, overexpression of NoxO1 in colon cancer cells reduces invadopodia formation, likely by competing with Tks4 to bind NoxA1 [64]. The PX domain of p40phox binds preferentially to PtdIns(3)P but has some affinity for PtdIns(3,4,5)P3 [74, 159], which is also present at invadopodia [70], making it a potential component of invadopodia NADPH complexes. p47phox is another potential candidate, since its PX domain has affinity for PtdIns(3,4)P2 [74, 159], which is enriched at invadopodia [70], and p47phox mRNA has been detected in colon cancer samples [78]. Furthermore, p47phox has been described to bind cortactin [160], IQGAP1 [152], and the paxillin paralogue Hic-5 [51]. Cortactin, IQGAP1 and paxillin are also present at invadopodia [161, 162]. Conversely, one could also speculate that p47phox binding proteins such as WAVE1 [153], moesin [163], coronin [164] or TRAF4 [51] could bind to Tks adaptors at invadopodia or podosomes. ROS are also necessary for podosome formation in normal macrophages [63], indicating that a similar mechanism is at play in controlling invasive cellular microdomains in normal cells and cell invasion in non-diseased tissues.
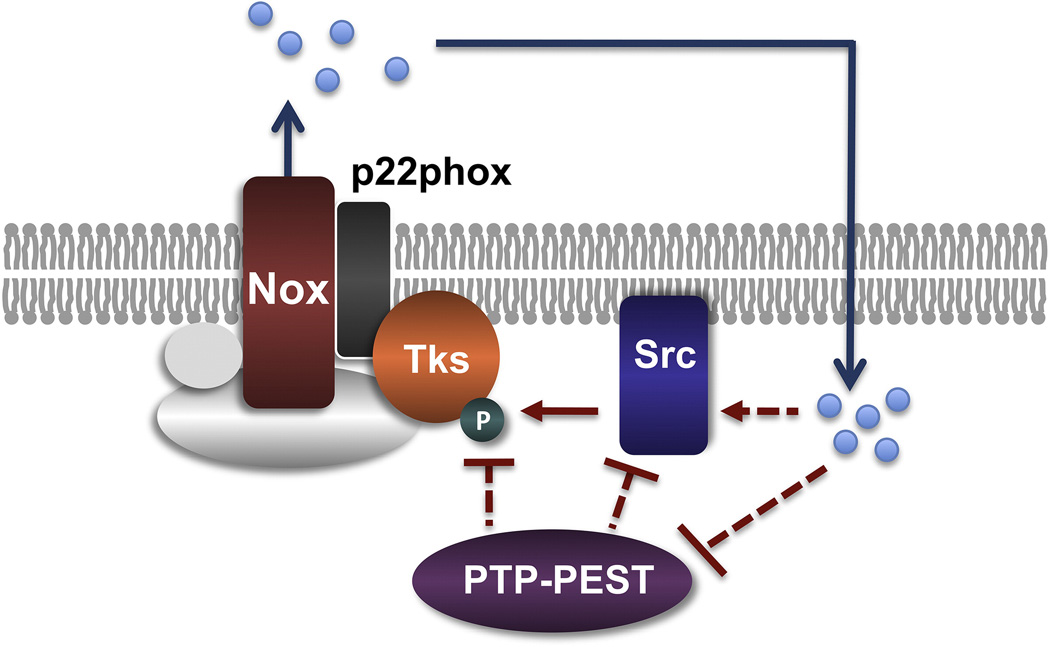
Inhibition of Nox activity in cancer cells leads to a specific decrease in the levels of tyrosine phosphorylation of Tks5 and Tks4 [63], indicating that redox signaling may regulate invadopodia formation by locally inactivating a tyrosine phosphatase that has Tks proteins as its main targets. The silencing of PTP-PEST in transformed cells increases invadopodia number [63], suggesting that the correct timing of invadopodia turnover requires the inactivation of phosphatases, which is achieved by localization of redox signaling to discrete microdomains. A similar mechanism controls focal adhesion turnover [165]. Correct assembly and disassembly of adhesive structures is necessary for cellular migration [165–167] as is the coordination of migration and pericellular proteolysis for local invasion. Consistent with a function of PTP-PEST in the regulation of invadopodia formation, other phosphatases have been shown to regulate the formation of these structures, including PTP1B [168], synaptojanin2 [148] and PTEN [169] in different cell types. Alternatively, redox activation of Src could increase Tks5 phosphorylation directly, although that possibility remains to be proven. The proposed model for redox signaling control at invasive microdomains by novel invadopodial NADPH complexes containing Tks proteins in cancer cells is summarized in Figure 3.
Figure 3. Invadopodia NADPH oxidase complexes regulate redox signaling at invasive microdomains.
- Diagram of a cancer cell with invadopodia extending into the ECM substrate.
- Diagram showing the section of an invadopodium and the localization of NADPH oxidase complexes that mediate local generation of ROS (dots) to regulate invadopodia formation and activity.
- Specific NADPH oxidase complexes containing Tks4 and Tks5 proteins might be locally present at invadopodia. Question marks denote possible additional components of the invadopodia complex containing Nox4.
Implications for cancer therapy
Redox chemotherapeutics are drugs that target either the antioxidant system of cancer cells (taking advantage of the fact that cancer cells are less tolerant to a decrease in antioxidants than normal cells), or the pro-oxidant system of cancer cells, to allow them to proliferative and survive under oxidative stress. These type of drugs are currently in clinical trials [170]. NADPH oxidases are considered as targets for cancer therapy [22, 171, 172], and several NADPH oxidase inhibitors have been developed [173–175]. Some of them decrease angiogenesis or tumor growth in animal models [133, 176]. The implication of Nox1 and Nox4 in the redox control of invasive cellular structures further supports the use of Nox1 or Nox4 inhibitors as anti-invasive drugs. The levels of transcript expression for Nox1 and/or Nox4 are often higher in tumor samples when compared with normal tissue [78], and both Nox1 and Nox4 deficient mice are viable [177, 178], supporting the possible therapeutic use of Nox1/4 inhibitors in humans. Recent findings in transgenic mice suggest that Nox4 inhibitors could be also useful in the treatment of ischemic stroke and hypertension, although cardiotoxicity may be a limitation [178, 179]. Nevertheless, it is worth testing whether Nox-isoform specific inhibitors would make promising anti-cancer drugs.
CONCLUSIONS
ROS are important mediators of normal and pathological cellular processes by acting as second messengers in the process of redox signaling. Cancer cells have evolved to use redox signaling pathways to drive aberrant proliferation, survival and invasion, and to adapt to the tumor microenvironment. By targeting redox signaling to invasive microdomains, such as invadopodia, cancer cells maintain a pro-invasive program that contributes to tumor cell invasion and metastasis. The invadopodia NADPH oxidase complexes that drive redox signaling at invasive microdomains represent a new mechanism by which redox signaling controls cancer progression and suggest the possibility of targeting invadopodia-specific NADPH oxidase components for therapeutic purposes.
Figure 4. Model for a redox signaling pathway at invadopodia.
An active invadopodia NADPH oxidase complex containing a Tks protein family as an organizer generates ROS (dots) that locally amplify the tyrosine-phosphorylation signal initiated by Src-dependent phosphorylation of Tks proteins. The intensity and/or duration of the Tks tyrosine phosphorylation could be modulated by ROS through direct activation of Src or by inactivation of the phosphatase PTP-PEST, which may in turn dephosphorylate Src or Tks organizers. Dashed lines with question marks denote putative interactions.
ACKNOWLEDGEMENTS
We would like to thank all members of the Courtneidge laboratory for useful discussions, and Pilar Cejudo-Martin, Christine Gould and Elisabeth Rico-Bautista for comments on the manuscript. Research was supported by the National Cancer Institute and the Mathers Foundation to SAC. B.D. would like to dedicate this work to the memory of Fernando Pérez.
ABBREVIATIONS
- ADAM
a disintegrin and metalloprotease
- Cdc25C
cell division cycle 25 homologue C
- DCF-DA
5-(and-6)-carboxy-2',7' -dichlorofluorescein
- Duox
Dual oxidase
- E-Cadherin
epithelial cadherin
- EC-SOD
extracellular superoxide dismutase
- ECM
extracellular matrix
- EGF
epithelial growth factor
- EMT
epithelial to mesenchymal transition
- FAK
focal adhesion kinase
- Grb2
growth factor receptor bound protein 2
- Hic-5
hydrogen peroxide-inducible clone 5
- HIF1α
hypoxia inducible factor 1 alpha
- IQGAP1
IQ motif containing GTPase activating protein 1
- JNK
Jun N-terminal kinase
- k-Ras
V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- LMW-PTP
low molecular weight protein tyrosine phosphatase
- MARCKS
myristoylated alanine-rich protein kinase C substrate
- MMP
matrix metalloproteinase
- MT1-MMP
membrane type 1 metalloprotease
- N-WASP
neuronal Wiskott-Aldrich syndrome protein
- NADPH
reduced form of nicotinamide adenine dinucleotide phosphate
- Nck
non-catalytic region of tyrosine kinase adaptor protein 1
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nox
NADPH oxidase
- NoxA1
NADPH oxidase adaptor 1
- NoxO1
NADPH oxidase organizer 1
- p190RhoGAP
p190 Rho GTPase activating protein
- p22phox
p22 phagocytic oxidase
- p40phox
p40 phagocytic oxidase
- p47phox
p47 phagocytic oxidase
- p67phox
p67 phagocytic oxidase
- PAK1
p21 protein (Cdc42/Rac)-activated kinase 1
- PDGF
platelet-derived growth factor
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- Poldip2
polymerase (DNA-directed), delta interacting protein 2
- PtdIns(3,4,5)P3
phosphatidylinositol (3,4,5)-trisphosphate
- PtdIns(3,4)P2
phosphatidylinositol (3,4)-bisphosphate
- PtdIns(3,5)P2
phosphatidylinositol (3,5)-bisphosphate
- PtdIns(3)P
phosphatidylinositol 3-phosphate
- PtdIns(4)P
phosphatidylinositol 4-phosphate
- PtdIns(5)P
phosphatidylinositol 5-phosphate
- PTEN
phosphatase and tensin homolog
- PTP-PEST
Protein tyrosine phosphatase with a C-terminal PEST motif
- PTP1B
protein tyrosine phosphatase 1B
- PTP
protein tyrosine phosphatase
- PX
phox homology
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- SH3
Src homology 3 domain
- SOD1
superoxide dismutase 1
- Stat3
signal transducer and activator of transcription 3
- TGFβ
transforming growth factor beta
- Tks4
tyrosine kinase substrate with four SH3 domains
- Tks5
tyrosine kinase substrate with five SH3 domains
- TPA
12-O-Tetradecanoylphorbol-13-acetate
- VEGF
vascular endothelial growth factor
- WASP
family verprolin-homologous protein
REFERENCES
- 1.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 2.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Bjelland S, Seeberg E. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat Res. 2003;531:37–80. doi: 10.1016/j.mrfmmm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer research. 1991;51:794–798. [PubMed] [Google Scholar]
- 5.Dolado I, Swat A, Ajenjo N, De Vita G, Cuadrado A, Nebreda AR. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Indran IR, Hande MP, Pervaiz S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Research. 2011;71:266–276. doi: 10.1158/0008-5472.CAN-10-1588. [DOI] [PubMed] [Google Scholar]
- 8.Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477:7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- 9.Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031–1044. doi: 10.1016/s1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 10.Burhans WC, Weinberger M. DNA replication stress, genome instability and aging. Nucleic Acids Res. 2007;35:7545–7556. doi: 10.1093/nar/gkm1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free radical biology & medicine. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Droge W. Free radicals in the physiological control of cell function. Physiological reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 13.Hille R, Nishino T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. Faseb J. 1995;9:995–1003. [PubMed] [Google Scholar]
- 14.Roy P, Roy SK, Mitra A, Kulkarni AP. Superoxide generation by lipoxygenase in the presence of NADH and NADPH. Biochimica et biophysica acta. 1994;1214:171–179. doi: 10.1016/0005-2760(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 15.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends in biochemical sciences. 2007;32:332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 17.Winterbourn CC, Vissers MC, Kettle AJ. Myeloperoxidase. Curr Opin Hematol. 2000;7:53–58. doi: 10.1097/00062752-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 19.Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- 20.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. Journal of cellular biochemistry. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 21.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–645. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 23.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free radical biology & medicine. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallach TM, Segal AW. Analysis of glycosylation sites on gp91phox, the flavocytochrome of the NADPH oxidase, by site-directed mutagenesis and translation in vitro. The Biochemical journal. 1997;321(Pt 3):583–585. doi: 10.1042/bj3210583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. The Journal of cell biology. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2009;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinauer MC, Orkin SH, Brown R, Jesaitis AJ, Parkos CA. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature. 1987;327:717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- 28.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 29.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 30.Nunoi H, Rotrosen D, Gallin JI, Malech HL. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988;242:1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- 31.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. The Journal of biological chemistry. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 32.Volpp BD, Nauseef WM, Clark RA. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science. 1988;242:1295–1297. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- 33.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. The Journal of biological chemistry. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 35.Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. Faseb J. 2009;23:1205–1218. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luxen S, Noack D, Frausto M, Davanture S, Torbett BE, Knaus UG. Heterodimerization controls localization of Duox-DuoxA NADPH oxidases in airway cells. Journal of cell science. 2009;122:1238–1247. doi: 10.1242/jcs.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free radical biology & medicine. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 38.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free radical biology & medicine. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 39.Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. The Journal of cell biology. 2006;174:615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 41.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 42.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 43.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 44.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 45.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. The Journal of biological chemistry. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 47.Chen YY, Chu HM, Pan KT, Teng CH, Wang DL, Wang AH, Khoo KH, Meng TC. Cysteine S-nitrosylation protects protein-tyrosine phosphatase 1B against oxidation-induced permanent inactivation. The Journal of biological chemistry. 2008;283:35265–35272. doi: 10.1074/jbc.M805287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. The Journal of biological chemistry. 2001;276:33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- 49.Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. The Journal of biological chemistry. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 51.Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Jr, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171:893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakashima I, Kato M, Akhand AA, Suzuki H, Takeda K, Hossain K, Kawamoto Y. Redox-linked signal transduction pathways for protein tyrosine kinase activation. Antioxid Redox Signal. 2002;4:517–531. doi: 10.1089/15230860260196326. [DOI] [PubMed] [Google Scholar]
- 53.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Molecular and cellular biology. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kemble DJ, Sun G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc Natl Acad Sci U S A. 2009;106:5070–5075. doi: 10.1073/pnas.0806117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savitsky PA, Finkel T. Redox regulation of Cdc25C. The Journal of biological chemistry. 2002;277:20535–20540. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- 56.Lander HM, Ogiste JS, Teng KK, Novogrodsky A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. The Journal of biological chemistry. 1995;270:21195–21198. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- 57.Deora AA, Win T, Vanhaesebroeck B, Lander HM. A redox-triggered ras-effector interaction. Recruitment of phosphatidylinositol 3'-kinase to Ras by redox stress. The Journal of biological chemistry. 1998;273:29923–29928. doi: 10.1074/jbc.273.45.29923. [DOI] [PubMed] [Google Scholar]
- 58.Abate C, Patel L, Rauscher FJ, 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 59.Li B, Gordon GM, Du CH, Xu J, Du W. Specific killing of Rb mutant cancer cells by inactivating TSC2. Cancer Cell. 2010;17:469–480. doi: 10.1016/j.ccr.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free radical biology & medicine. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem. 2006;281:22983–22991. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- 63.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal. 2009;2:ra54. doi: 10.1126/scisignal.2000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lock P, Abram CL, Gibson T, Courtneidge SA. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. Embo J. 1998;17:4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Courtneidge SA, Azucena EF, Pass I, Seals DF, Tesfay L. The SRC substrate Tks5, podosomes (invadopodia), and cancer cell invasion. Cold Spring Harbor symposia on quantitative biology. 2005;70:167–171. doi: 10.1101/sqb.2005.70.014. [DOI] [PubMed] [Google Scholar]
- 67.Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Courtneidge SA. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stylli SS, Stacey TT, Verhagen AM, Xu SS, Pass I, Courtneidge SA, Lock P. Nck adaptor proteins link Tks5 to invadopodia actin regulation and ECM degradation. Journal of cell science. 2009;122:2727–2740. doi: 10.1242/jcs.046680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, Courtneidge SA. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. The Journal of Biological Chemistry. 2003;278:16844–16851. [Google Scholar]
- 70.Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson O, Kleino I, Crimaldi L, Gimona M, Saksela K, Winder SJ. Dystroglycan, Tks5 and Src mediated assembly of podosomes in myoblasts. PLoS One. 2008;3:e3638. doi: 10.1371/journal.pone.0003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iqbal Z, Cejudo-Martin P, de Brouwer A, van der Zwaag B, Ruiz-Lozano P, Scimia MC, Lindsey JD, Weinreb R, Albrecht B, Megarbane A, Alanay Y, Ben-Neriah Z, Amenduni M, Artuso R, Veltman JA, van Beusekom E, Oudakker A, Millan JL, Hennekam R, Hamel B, Courtneidge SA, van Bokhoven H. Disruption of the podosome adaptor protein TKS4 (SH3PXD2B) causes the skeletal dysplasia, eye, and cardiac abnormalities of Frank-Ter Haar Syndrome. Am J Hum Genet. 2010;86:254–261. doi: 10.1016/j.ajhg.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 74.Ago T, Takeya R, Hiroaki H, Kuribayashi F, Ito T, Kohda D, Sumimoto H. The PX domain as a novel phosphoinositide- binding module. Biochemical and biophysical research communications. 2001;287:733–738. doi: 10.1006/bbrc.2001.5629. [DOI] [PubMed] [Google Scholar]
- 75.Kawahara T, Lambeth JD. Molecular evolution of Phox-related regulatory subunits for NADPH oxidase enzymes. BMC Evol Biol. 2007;7:178. doi: 10.1186/1471-2148-7-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circulation research. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 78.Juhasz A, Ge Y, Markel S, Chiu A, Matsumoto L, van Balgooy J, Roy K, Doroshow JH. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic Res. 2009;43:523–532. doi: 10.1080/10715760902918683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown DI, Griendling KK. Nox proteins in signal transduction. Free radical biology & medicine. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 81.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Science's STKE : signal transduction knowledge environment. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 82.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 84.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 85.Manz BN, Groves JT. Spatial organization and signal transduction at intercellular junctions. Nat Rev Mol Cell Biol. 2010;11:342–352. doi: 10.1038/nrm2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabodi S, Di Stefano P, Leal Mdel P, Tinnirello A, Bisaro B, Morello V, Damiano L, Aramu S, Repetto D, Tornillo G, Defilippi P. Integrins and signal transduction. Adv Exp Med Biol. 2010;674:43–54. doi: 10.1007/978-1-4419-6066-5_5. [DOI] [PubMed] [Google Scholar]
- 87.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 88.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 89.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 90.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, Lee HB. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 93.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. Embo J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 96.Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T, Ben-Ze'ev A. L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168:633–642. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, Cromer A, Brugge JS, Sansom OJ, Norman JC, Vousden KH. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 98.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 2010;10:858–870. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- 100.Mitsushita J, Lambeth JD, Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004;64:3580–3585. doi: 10.1158/0008-5472.CAN-03-3909. [DOI] [PubMed] [Google Scholar]
- 101.Shinohara M, Shang WH, Kubodera M, Harada S, Mitsushita J, Kato M, Miyazaki H, Sumimoto H, Kamata T. Nox1 redox signaling mediates oncogenic Ras-induced disruption of stress fibers and focal adhesions by down-regulating Rho. J Biol Chem. 2007;282:17640–17648. doi: 10.1074/jbc.M609450200. [DOI] [PubMed] [Google Scholar]
- 102.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nature cell biology. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 103.Laurent E, McCoy JW, 3rd, Macina RA, Liu W, Cheng G, Robine S, Papkoff J, Lambeth JD. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer. 2008;123:100–107. doi: 10.1002/ijc.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sadok A, Bourgarel-Rey V, Gattacceca F, Penel C, Lehmann M, Kovacic H. Nox1-dependent superoxide production controls colon adenocarcinoma cell migration. Biochim Biophys Acta. 2008;1783:23–33. doi: 10.1016/j.bbamcr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 105.Kim EY, Seo JM, Kim C, Lee JE, Lee KM, Kim JH. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radic Biol Med. 2010;49:1072–1081. doi: 10.1016/j.freeradbiomed.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 106.Reddy MM, Fernandes MS, Salgia R, Levine RL, Griffin JD, Sattler M. NADPH oxidases regulate cell growth and migration in myeloid cells transformed by oncogenic tyrosine kinases. Leukemia. 2011;25:281–289. doi: 10.1038/leu.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC, Pal M, Lam CR, Boukamp P, Pan JY, Tan SH, Kersten S, Li HY, Ding JL, Tan NS. Angiopoietin-like 4 Protein Elevates the Prosurvival Intracellular O(2)(−):H(2)O(2) Ratio and Confers Anoikis Resistance to Tumors. Cancer Cell. 2011;19:401–415. doi: 10.1016/j.ccr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 108.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Madsen CD, Sahai E. Cancer dissemination--lessons from leukocytes. Dev Cell. 2010;19:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 110.Martins VL, Vyas JJ, Chen M, Purdie K, Mein CA, South AP, Storey A, McGrath JA, O'Toole EA. Increased invasive behaviour in cutaneous squamous cell carcinoma with loss of basement-membrane type VII collagen. J Cell Sci. 2009;122:1788–1799. doi: 10.1242/jcs.042895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 112.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 114.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Inoue N, Takeshita S, Gao D, Ishida T, Kawashima S, Akita H, Tawa R, Sakurai H, Yokoyama M. Lysophosphatidylcholine increases the secretion of matrix metalloproteinase 2 through the activation of NADH/NADPH oxidase in cultured aortic endothelial cells. Atherosclerosis. 2001;155:45–52. doi: 10.1016/s0021-9150(00)00530-x. [DOI] [PubMed] [Google Scholar]
- 117.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2) via NAD(P)H oxidase-derived reactive oxygen species. Circulation research. 2003;92:e80–e86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 118.Deem TL, Cook-Mills JM. Vascular cell adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood. 2004;104:2385–2393. doi: 10.1182/blood-2004-02-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rude MK, Duhaney TA, Kuster GM, Judge S, Heo J, Colucci WS, Siwik DA, Sam F. Aldosterone stimulates matrix metalloproteinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension. 2005;46:555–561. doi: 10.1161/01.HYP.0000176236.55322.18. [DOI] [PubMed] [Google Scholar]
- 120.Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein D regulates NF-kappa B and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. Journal of immunology. 2001;166:7514–7519. doi: 10.4049/jimmunol.166.12.7514. [DOI] [PubMed] [Google Scholar]
- 121.Zhang H, Joseph J, Gurney M, Becker D, Kalyanaraman B. Bicarbonate enhances peroxidase activity of Cu,Zn-superoxide dismutase. Role of carbonate anion radical and scavenging of carbonate anion radical by metalloporphyrin antioxidant enzyme mimetics. The Journal of biological chemistry. 2002;277:1013–1020. doi: 10.1074/jbc.M108585200. [DOI] [PubMed] [Google Scholar]
- 122.Yoon SO, Park SJ, Yoon SY, Yun CH, Chung AS. Sustained production of H(2)O(2) activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappa B pathway. J Biol Chem. 2002;277:30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
- 123.Steinbrenner H, Ramos MC, Stuhlmann D, Mitic D, Sies H, Brenneisen P. Tumor promoter TPA stimulates MMP-9 secretion from human keratinocytes by activation of superoxide-producing NADPH oxidase. Free Radic Res. 2005;39:245–253. doi: 10.1080/10715760500053487. [DOI] [PubMed] [Google Scholar]
- 124.Connor KM, Hempel N, Nelson KK, Dabiri G, Gamarra A, Belarmino J, Van De Water L, Mian BM, Melendez JA. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res. 2007;67:10260–10267. doi: 10.1158/0008-5472.CAN-07-1204. [DOI] [PubMed] [Google Scholar]
- 125.Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochemical and biophysical research communications. 2009;379:445–450. doi: 10.1016/j.bbrc.2008.12.080. [DOI] [PubMed] [Google Scholar]
- 126.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 127.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 130.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer research. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 133.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH, Imhof B. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS One. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 135.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 136.Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nature reviews. Molecular cell biology. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. The Journal of cell biology. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. Journal of cell science. 1991;99(Pt 2):213–225. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- 139.Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Annals of the New York Academy of Sciences. 1999;878:361–371. doi: 10.1111/j.1749-6632.1999.tb07695.x. [DOI] [PubMed] [Google Scholar]
- 140.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 141.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. The Journal of cell biology. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mandal S, Johnson KR, Wheelock MJ. TGF-beta induces formation of F-actin cores and matrix degradation in human breast cancer cells via distinct signaling pathways. Exp Cell Res. 2008;314:3478–3493. doi: 10.1016/j.yexcr.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 143.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tolde O, Rosel D, Vesely P, Folk P, Brabek J. The structure of invadopodia in a complex 3D environment. European journal of cell biology. 2010;89:674–680. doi: 10.1016/j.ejcb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 145.Kelly T, Yan Y, Osborne RL, Athota AB, Rozypal TL, Colclasure JC, Chu WS. Proteolysis of extracellular matrix by invadopodia facilitates human breast cancer cell invasion and is mediated by matrix metalloproteinases. Clin Exp Metastasis. 1998;16:501–512. doi: 10.1023/a:1006538200886. [DOI] [PubMed] [Google Scholar]
- 146.Blouw B, Seals DF, Pass I, Diaz B, Courtneidge SA. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur J Cell Biol. 2008;87:555–567. doi: 10.1016/j.ejcb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Clark ES, Brown B, Whigham AS, Kochaishvili A, Yarbrough WG, Weaver AM. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene. 2009;28:431–444. doi: 10.1038/onc.2008.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chuang YY, Tran NL, Rusk N, Nakada M, Berens ME, Symons M. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer research. 2004;64:8271–8275. doi: 10.1158/0008-5472.CAN-04-2097. [DOI] [PubMed] [Google Scholar]
- 149.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 150.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Experimental cell research. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 151.Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–557. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 152.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 153.Wu RF, Gu Y, Xu YC, Nwariaku FE, Terada LS. Vascular endothelial growth factor causes translocation of p47phox to membrane ruffles through WAVE1. J Biol Chem. 2003;278:36830–36840. doi: 10.1074/jbc.M302251200. [DOI] [PubMed] [Google Scholar]
- 154.el Benna J, Faust LP, Babior BM. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. The Journal of biological chemistry. 1994;269:23431–23436. [PubMed] [Google Scholar]
- 155.Inanami O, Johnson JL, McAdara JK, Benna JE, Faust LR, Newburger PE, Babior BM. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47PHOX on serine 303 or 304. The Journal of biological chemistry. 1998;273:9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- 156.Weaver AM. Regulation of cancer invasion by reactive oxygen species and Tks family scaffold proteins. Sci Signal. 2009;2:pe56. doi: 10.1126/scisignal.288pe56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer research. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 158.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 159.Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nature cell biology. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 160.Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:512–518. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- 161.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 162.Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D'Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. The Journal of cell biology. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wientjes FB, Reeves EP, Soskic V, Furthmayr H, Segal AW. The NADPH oxidase components p47(phox) and p40(phox) bind to moesin through their PX domain. Biochem Biophys Res Commun. 2001;289:382–388. doi: 10.1006/bbrc.2001.5982. [DOI] [PubMed] [Google Scholar]
- 164.Grogan A, Reeves E, Keep N, Wientjes F, Totty NF, Burlingame AL, Hsuan JJ, Segal AW. Cytosolic phox proteins interact with and regulate the assembly of coronin in neutrophils. Journal of cell science. 1997;110(Pt 24):3071–3081. doi: 10.1242/jcs.110.24.3071. [DOI] [PubMed] [Google Scholar]
- 165.Angers-Loustau A, Cote JF, Charest A, Dowbenko D, Spencer S, Lasky LA, Tremblay ML. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. The Journal of cell biology. 1999;144:1019–1031. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 167.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 168.Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang ZY, Huttenlocher A. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. The Journal of cell biology. 2008;180:957–971. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Poon JS, Eves R, Mak AS. Both lipid- and protein-phosphatase activities of PTEN contribute to the p53-PTEN anti-invasion pathway. Cell Cycle. 2010;9:4450–4454. doi: 10.4161/cc.9.22.13936. [DOI] [PubMed] [Google Scholar]
- 170.Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 173.Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Boussard MF, Wierzbicki M, Verbeuren TJ, Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets nadph oxidase. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1577–1584. doi: 10.1161/hq1001.096723. [DOI] [PubMed] [Google Scholar]
- 174.ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovascular research. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 175.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem. 2010;53:7715–7730. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- 176.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, Arbiser JL. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119:2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 178.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]