Abstract
Background
Inflammation plays an instrumental role in all stages of atherosclerosis. C-reactive protein (CRP), a systemic inflammatory marker, has been gaining recognition as an independent risk factor for cardiovascular disease (CVD). Both baseline CRP levels and drug-induced CRP changes are highly variable and potentially subject to the genetic regulation.
Content
This review summarizes the current studies which have examined the effect of genetic and environmental factors on baseline plasma CRP levels with main focus on CRP genetic polymorphisms and various dietary components. We also address the association of CRP genetic variations with risk of CVD, which may provide support or refute to the causality of CRP in atherosclerotic process. Moreover, we discuss the impact of CRP genetic polymorphisms on CRP change in response to 3-week fenofibrate treatment in the genetic intervention of the GOLDN study.
Conclusions
Both genetic variants on CRP locus and other loci, and dietary and lifestyle factors are responsible for the inter-individual variability of plasma CRP levels. CRP genetic variants further differentiate plasma CRP response after 3-week fenefibrate treatment among subjects with metabolic syndrome. Future studies focusing on the influence and interaction of genetic variation on the CRP response to dietary and other behavior modification as well as drug treatment could have great implication for the development of more personalized preventive and therapeutic approaches to reduce CVD.
Introduction
C-reactive protein (CRP) is an acute phase protein and belongs to the family of protein known as pentraxins. It is a phylogenetically highly conserved plasma protein across different species, which participates in the systemic response to inflammation (1). CRP is synthesized and released primarily by hepatocytes, although local CRP synthesis and secretion have been suggested at other sites, such as macrophages (2) and smooth muscle cells (3).
Inflammation plays an instrumental role in all stages of atherosclerosis. Among a number of inflammatory markers that have been explored as a means to predict cardiovascular disease (CVD), CRP has been gaining recognition as an independent risk factor for CVD events. Recent studies using animal models suggest that CRP also directly participate in the pathogenesis of CVD (4). In addition, elevated CRP is associated with multiple risk factors for CVD, including obesity, insulin resistance and hypertension and significantly predicts metabolic syndrome (MetS) risk (5).
Plasma CRP levels in apparently healthy individuals are widely distributed, reflecting substantial inter-individual variability (6) driven by multiple environmental, sociodemographic, behavioral and lifestyle factors, as well as by obesity and type II diabetes (T2D) (7, 8). Moreover, twin and family studies have estimated that genetic factors could contribute up to 35%-50% of the phenotypic variation of plasma CRP levels (9, 10).
This review will summarize studies which have examined the effect of genetic polymorphisms as well as environmental factors, mainly dietary components, on plasma baseline CRP levels and associations with risk of CVD events. We will also discuss the influence of CRP polymorphisms on the individual difference in response to lipid-lowering drug, fenofibrate treatment.
Association between CRP polymorphisms and plasma baseline CRP levels and risk of CVD events
Data from the study of three cohorts including the Women's Health Study (WHS), the Pravastatin Inflammation/CRP Evaluation trial (PRINCE) and the Physicians’ Health Study (PHS) demonstrated that a set of CRP SNPs including two promoter SNPs, rs3093059 and rs3091244; intron 1 SNP, rs1417938; exon 2 SNP, rs1800947 and two 3'-UTR SNPs, rs1130864 and rs1205 were consistently associated with plasma CRP levels in all three cohorts (11). Carson, et al evaluated 7 haplotype-tagging SNPs (including rs309358, rs3091244, rs1417938, rs1800947, rs3093066, rs1205 and rs2808630) in 6.8kb surrounding the CRP locus and showed that CRP haplotypes were strongly associated with CRP levels in a large of cohort study of CVD risk in European American and African American young adults. Among those SNPs, a promoter triallelic SNP rs3091244 explained the greatest proportion of overall CRP variance. Functional experiment discovered that this SNP resides within the hexameric core of transcription factor binding elements. The presence of mutations alters a transcription factor (such as upstream stimulatory factor-1, USF-1) binding motif and thus, markedly affects the transcriptional activity of the CRP gene (12). Two haplotypes constructs (tagged by triallelic SNP, rs3091244) displayed high IL6-induced promoter activity, which was consistent with observed strong association of those two haplotypes with CRP levels (13). The associations between CRP genotypes and plasma CRP levels were also reported by other large scale studies including the third National Health and Nutritional Examination Survey (NHANES III) and the Framingham Heart Study (FHS) (14-17).
Although growing evidence from experimental studies supports the direct participation of CRP in the atherosclerotic process, its causality is still under debate. Genetic dissection of CRP may provide additional evidence determining its causal role by taking advantage of the “Mendelian randomization.” This method is similar to a randomized clinical trial in which the random assignment is the genotype that occurs at conception (18). So the observed association between a disease and functional genetic polymorphisms is not susceptible to residual confounding or reverse causation which may potentially bias the results of observational studies (18). The hypothesis is that if CRP is causally involved in disease pathogenesis, then alleles which influence CRP synthesis, could mediate the onset of clinical CVD events. In this regard, multiple cohort studies have examined the effect of CRP SNPs associated with baseline CRP levels on atherosclerotic disease progression or occurrence of clinical CVD events (such as myocardial infarction, MI). Results from the Cardiovascular Health Study (CHS) in older American adults with median follow-up of 13 years showed that SNPs rs1417938 was associated with increased risk of stroke and CVD mortality in white participants and SNP rs3093058 was associated with a 4-fold increased risk of MI in black participants whereas two SNPs (rs1800947 and rs1205) were associated with decreased risk of CVD mortality in white participants (17). The direction of the CVD risk estimates paralleled that of the associations with plasma CRP levels. The NHANES III also reported that the triallelic SNP rs3091244 was cross-sectional associated with the prevalence of coronary heart disease (CHD) in the non-Hispanic white population (15). However, the results have been controversial as other cohorts including the PHS, the FHS and the Rotterdam Study suggested little or no association between CRP genotype and risk of MI or stroke (11, 16, 19). A number of factors need to be considered while interpreting those results. First, the CRP locus only accounts for a small proportion of the variability of CRP levels. Therefore, some studies may be underpowered to detect the genetic impact on disease outcome, especially given the small sample size and relatively small relative risk of disease. Secondly, age may play an important role as positive associations are more commonly seen among older than among younger subjects (17). Thirdly, the influence of CRP may depend on the different stages of disease. The association between CRP genotype and clinical CVD, together with the absence of association with carotid intima-media thickness (CIMT)(20), which measures the extent of subclinical atherosclerotic disease, suggests a greater involvement of CRP in the transition from subclinical to clinical disease than in atherosclerosis progression. This observation was in agreement with the lack of association between plasma CRP levels and CIMT (21). Moreover, associations are more likely observed with fatal outcomes (11, 17, 19) which is in line with the involvement of CRP levels in the increase of infarct size (22), suggesting the possibility that through an effect on acute phase response (23), CRP genotype may be more strongly associated with more severe events. Finally, using genetic markers as an instrumental variable to assess the causality could provide misleading conclusion in the presence of linkage disequilibrium, genetic heterogeneity, pleiotropy, or population stratification (24).
In summary, current studies have provided convincing evidence of the significant impact of CRP polymorphisms on baseline plasma CRP levels; however, the genetic association with risk of CVD events is equivocal despite the fact that CRP levels are a strong independent predictor of CVD. In this regard, the recently launched international consortium, the CRP CHD Genetics Collaboration, from more than 30 relevant studies of CRP genetic variants and CHD risk should provide compelling evidence determining the casual relationship of CRP genetic variants, circulating CRP levels with CHD (25).
CRP polymorphisms affect CRP response to fenofibrate lipid-lowering therapy
Fenofibrate, a PPARα agonist, is a member of the fibrate class of lipid-lowering drugs. Both experimental studies and clinical trials have demonstrated that fibrates may additionally reduce CVD risk through anti-inflammatory effects, including attenuation of constitutive and induced CRP gene expression (26, 27). Fibrates target both the atherogenic “lipid triad” (high triglycerides and low HDL with small and dense LDL particles) and inflammation (28). Because both phenotypes are important components of diabetes and the MetS and potentially link these two metabolic disorders to CVD (29, 30), fibrates are hypothesized to be candidates for treating dyslipidemia associated with diabetes and the MetS and more effective to reduce CVD in those at high risk (31-33).
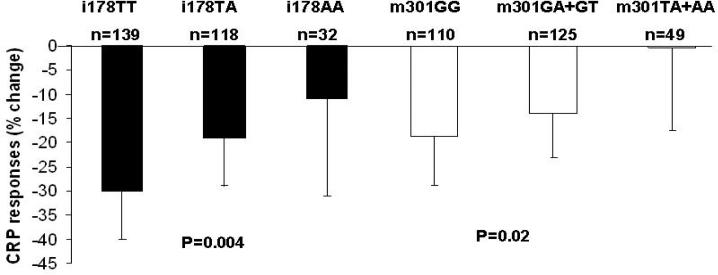
It has been documented that individual response to fibrate is highly variable and multiple genes have been identified to associate with efficiency of lipid lowering capacity (34, 35). However, the genetic impact on the anti-inflammatory effect of this drug is less well understood. In this regard, we examined the association of CRP genetic polymorphisms with CRP response to a 3-week fenofibrate treatment among US White participants in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study. The GOLDN study is a single arm, uncontrolled, non-randomized intervention funded by the NHLBI with the purpose of identifying genetic variants associated with interindividual variability of triglyceride responses to a high fat meal and fenofibrate (36, 37). The study population consisted of 539 men and 584 women, and the majority of participants were re-recruited from the ongoing NHLBI Family Heart Study (FHS) in two genetically homogeneous centers (Minneapolis and Salt Lake City) with predominantly Caucasian populations. The baseline characteristics of the study participants are provided in online supplement table 1. In this study, and consistent with previous reports, SNPs m301G>A >T (rs3091244), i178T>A (rs1417938), 3u1273C>T (rs1130864) and 3u2131C>T (rs1205) were significantly associated with baseline CRP levels. Moreover, we observed effects of the m301G>A >T and the i178T>A SNPs on CRP response to fenofibrate intervention among participants with MetS. Specifically, G allele carriers for the m301G>A>T SNP displayed the greater reduction of CRP levels than non-carriers. Similarly, TT individuals with the i178T>A SNP had greater reduction in CRP levels than TA and AA subjects (Figure 1.). It is of interest that the alleles associated with high baseline inflammatory status appear to be more resistant to the anti-inflammatory effect of fenofibrate. Conversely, in the presence of an inflammatory stimuli, these alleles, such as the minor allele of 3u1273C>T (previously reported as 1444C>T, rs1130864, in complete LD with i178T>A, rs1417938), have been shown to have a greater CRP rise in coronary artery bypass graft (CABG) patients or periodontal therapy (14, 38). The mechanism underlying the modulation of genetic variants on CRP response to fenofibrate among MetS subjects is undefined. However, as both SNPs appear to be involved in transcription factor binding motifs, it is possible that the interaction of these transcription factors, in particular USF1 with PPARα might underlie some of the genetic effect. Our findings have practical relevance as lipid-lowering drugs, such as statins and fibrates, remain critical elements in the prevention of CVD (35). In addition, although a series of large-scale intervention trials using fibrates have established the role of fibrates in normalizing lipid profiles, the results regarding their efficacy on the reduction of CVD events have been inconsistent (39). Further subgroup analyses revealed that features of the MetS modify the effect of fibrate on CVD such that cardiovascular benefits are largely confined to subjects with features of the MetS (39). Our data provide additional insight into the heterogeneity of the treatment response, suggesting that genetic difference could further differentiate individuals’ response to fenofibrate, and thus potentially may affect the disease outcome among subjects at high risk. Lastly, if the reduction of CRP could directly lead to the decreased rate of recurrent events of CVD and the progression of coronary atherosclerosis as reported from multiple randomized clinical trials using statins (27, 40), those individuals carrying certain genotypes which potentially impact on CRP response to treatment may have different trajectory of cardiovascular outcomes from non-carriers, and therefore, may require different lifestyle modification or therapeutic regime.
Figure1.
Plasma CRP response to 3-week fenofibrate treatment among subjects with MetS according to CRP i178T>A and m301G>A>T genotypes (geometric means ±95%CI). P-value obtained from additive model was adjusted for baseline CRP levels, change of triglyceride, change of IL6, age, gender, BMI, smoking status, alcohol intake, physical activity, use of aspirin and NSAID, drugs for lowering cholesterol, diabetes and hypertension, and hormone treatment in women. Subjects with CRP>10mg/L at the baseline or after the treatment were excluded from the analysis.
Association of plasma CRP levels with other inflammatory markers and genetic determinants
CRP is considered as an important downstream inflammatory marker and its activity may reflect the action of several upstream cytokines. Experimental studies indicated that hepatic expression of CRP is regulated at the transcription level by various cytokines, including IL-6, IL-1B (1) and TNF-α (41). Recent study uncovered that protein kinase C pathway is also involved in the regulation of CRP and IL-8 as a potential physiological PKC activator significantly induces hepatic CRP release (42). CRP may also exert pro-inflammatory activity through the induction of adhesion molecules expression, activation of complement system and inhibition of fibrinolysis by inducing plasminogen activator inhibitor-1 (PAI-1) (43, 44). Because of the strong biological connection between CRP and those inflammatory mediators, circulating CRP values correlates closely with other markers of inflammation such as IL-6, TNFα, ICAM-1 and Fibrinogen (45-47). Some of those inflammatory markers have been shown to predict CVD; however, the association is less significant and consistent compared to CRP (47). Nevertheless, characterizing the relationship between CRP and other markers of inflammation opens up a new perspective for the identification of improved predictors and additional therapeutic target for CVD.
Moreover, understanding the interrelation between CRP and other inflammatory mediator has laid the foundation for search for the genetic determinants of CRP which reside outside of CRP locus. To date, multiple candidate genes involving in the CRP regulatory pathway have been reported to affect CRP levels. The IL6 SNPs -174G/C and -572C>G, the TNF-α G-308A and IL1B -511C/T and 3954C/T SNPs have been all shown to associate with baseline CRP levels (48-50). In addition to the obvious inflammation candidate genes, other loci reported to be associated with CRP levels include the tyrosine hydroxylase, a rate-limiting enzyme of catecholamine biosynthesis which has been linked to CRP production; two β-adrenergic receptors; ADRB1 and ADRB2 (51) and the APOE locus (52).
In addition, a genome-wide association study among 6345 healthy women participating in the Women's Genome Health Study in which 336,108 SNPs were successfully genotyped has revealed several novel loci associated with plasma CRP levels. Those new loci included the leptin-receptor protein (LEPR), interleukin-6 receptor (IL6R), glucokinase regulatory protein (GCKR) and hepatic transcription-factor (HNF1A). Because those genes are directly involved in MetS, insulin resistance, beta-cell function and weight homeostasis, this new pathogenetic link opens a new field of the regulation of plasma CRP and the role of CRP in CVD as both a useful biomarker and an active participant (53).
The association of environmental factors with plasma CRP levels
Several environmental factors have been shown to contribute to the phenotypic variation in CRP, and dietary intake has been among the best studied (7, 8).
1) Dietary fat and CRP levels
Epidemiological studies have reported that dietary fatty acid composition could modulate inflammation (54). A cross-sectional study of 730 women from the Nurses’ Health Study I cohort found that women in the highest quintile of trans fat intake had a 73% higher level of CRP compared with the lowest quintile (55). Similarly, data from both a subsample of the Health Professionals Follow-up Study (n=446 men) and the NHANES participants (1999-2000, n=4,900) suggested that high fat intake, particularly saturated and trans fatty acids was associated with elevated CRP concentrations (56, 57). Intervention trials further provide evidence in support of the relation of increase in inflammatory with the intake of saturated and trans fatty acids. Pirro, et al found that the 8-week consumption of a low-cholesterol/low-saturated fat diet significantly decreased CRP levels in 35 hypercholesterolemic patients (58). Similar increases were reported in healthy individuals when trans fatty acid was used as a replacement fat within the context of a high-fat diet (39% fat) (59).
The relationship between dietary n-3 PUFAs (alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA)) and chronic inflammation has been also examined. The Nurses’ Health Study I cohort (n=727 women) showed that lower concentrations of markers of inflammation and endothelial activation, including CRP, IL-6, and E-selectin, among those in the highest quintile of n-3 PUFA compared with those in the lowest quintile (60). Moreover, results from a study of 3042 Greek healthy adults showed that those who consumed at least 300 g of fish per week had 33% lower CRP compared with non-fish consumers (61). Several clinical trials also confirmed the anti-inflammatory effect of ALA. Thus, Rallidis, et al found a significant reduction of CRP and IL-6 levels after ALA supplementation, whereas LA ( linoleic acid) supplementation decreased cholesterol levels with no significant effects on inflammation in 90 male dyslipidemic patients (The ratio of n-6:n-3 was 1.3:1 in ALA supplemented group and 13.2:1 in LA supplemented group) (62). Additionally, Bemelmans, et al reported a lowering of CRP levels in 103 moderately hypercholesterolemic men and women after consumption of an ALA-enriched margarine compared to those consuming a LA-enriched margarine (63). Although epidemiological studies have shown an inverse correlation between dietary fish or fish oil (EPA and DHA) consumption and biomarkers of inflammation (60, 64), results from clinical trials are inconclusive (65, 66).
As for monounsaturated fatty acids (MUFA), a cross-sectional study of Japanese population consisting of 1,556 men and 1,461 women suggested that intake of oleic acid was inversely associated with CRP levels (67). Another intervention in 180 patients with MetS but free of CVD, demonstrated that patients consuming a Mediterranean-style diet high in oleic acid as well as fiber and antioxidants had lower serum concentrations of CRP and cytokines (IL-6, IL-7, and IL-18) compared with a control group consuming their usual diet (68).
Taken together, the current evidence supports the notion that the overall quantity of fat intake, the sources and type of dietary fat, with special emphasis on saturated and trans fatty acids, ALA and oleic acid, and the ratio of n-6:n-3 PUFA play a significant role in modulating CRP levels and other markers of inflammation.
2) Carbohydrates, dietary fiber, micronutrients and CRP levels
The quantity and quality of carbohydrate intake affects the risk of CVD through altering blood lipid concentrations and inflammation. High intake of refined carbohydrates, such as starch and sugar, results in the increase of postprandial hyperglycemia which may lead to the increased circulating levels of free radicals and pro-inflammatory cytokines and CRP levels (69). Additionally, among 18,137 healthy women participating in the WHS, dietary glycemic index (GI) and glycemic load (GL) was significantly associated with CRP such that the highest quintile of GL and GI were associated with high CRP compared with lowest quintiles (70).
Regarding the relation between dietary fiber and inflammation, data from the NHANES indicated that dietary fiber intake was inversely associated with serum CRP concentration among 3920 adult participants (71). Moreover, a longitudinal study involving 524 subjects demonstrated that the likelihood of elevated CRP concentrations was 63% lower (OR: 0.37; 95% CI: 0.16, 0.87) in participants in the highest quartile of total fiber intake than in participants in the lowest quartile (72). The anti-inflammatory effect of dietary fiber may be mediated through the reduction of lipid oxidation, normalizing bowel flora and the inhibition of hyperglycemia (73).
The anti-inflammatory effect of micronutrients (i.e., vitamins and minerals) has also been explored and several studies have demonstrated an inverse association between vitamin E, vitamin B6, carotenoids and magnesium and plasma CRP levels (74).
3) Fruits and vegetables, nuts intake and inflammation
Esmaillzadeh, et al reported that among 486 Tehrani women, higher intake of fruits and vegetables were associated with a lower risk of MetS and lower CRP levels, and after adjusting for age, BMI and waist circumference, mean plasma CRP levels across increasing quintile categories of fruit intakes were 1.94, 1.79, 1.65, 1.61, and 1.56 mg/L and of vegetable intakes were 2.03, 1.82, 1.58, 1.52, and 1.47 mg/L (75). A randomized controlled 4-week trial in 64 nonsmoking men reported that subjects assigned to consume 8 servings /d of carotenoid-rich vegetables and fruit had significantly reduced CRP levels compared with those who consumed 2 servings /day (76). The anti-inflammatory effect appears to be mainly attributed to the antioxidant components of fruit and vegetables (77).
High consumption of nuts which are rich in MUFA, PUFA and arginine contents has been inversely associated with risk of CVD. The beneficial effect is partially due to their anti-inflammatory effect (78).
4) Dietary pattern and CRP levels
In addition to individual nutrient and food item, research has also focused on dietary pattern which potentially may reveal more details of nutrient-nutrient synergy and interaction with respect to the modulation of inflammation.
A cross-sectional study of 732 healthy women suggested that the prudent dietary pattern characterized by higher intake of fruit, vegetables, legumes, fish, poultry, and whole grains was inversely associated with plasma CRP, whereas a western-type diet with high intake in red and processed meats, sweets, desserts, french fries and refined grains was positively associated with CRP. Those categorized as having the most prudent diet had average CRP of 1.3mg/L compared with an average of 1.7 mg/L in those classified as having the most Western diet (55). Interestingly, a randomized trial conducted among 180 patients with MetS found that patients following the 2-year Mediterranean diet rich in fruit and vegetable, nuts (274 g/d), whole grain (103 g/d) and olive oil (8 g/d) had significantly reduced series of inflammatory markers such as CRP, increased insulin sensitivity and decreased risk of MetS compared with patients following a prudent diet (68).
5) Other lifestyle factors and CRP levels
Results from a perspective study of 27,055 healthy women participating in WHS clearly demonstrated that the beneficial effects of physical activity on a reduced risk of CVD could be mediated in part by improved chronic inflammation in addition to other known risk factors (79). The inverse association between high level of physical activity or exercise training and markers of chronic inflammation such as CRP has been consistently reported (80-82). Physical activity and cardiorespiratory fitness have been estimated to reduce CRP levels by 6-35% (83). As examples of that, data from the NHANES III which included 13,748 participants demonstrated that the odds ratios for elevated CRP levels were 0.98 (95% CI 0.78-1.23), 0.85 (0.70-1.02), and 0.53 (0.40-0.71) for participants who engaged in light, moderate, and vigorous physical activity, respectively, compared with participants who did not engage in any leisure-time physical activity(80). Moreover, physically active subjects displayed low levels of lipopolysaccharide-stimulated production of IL-6, IL-1ß, TNF-α , TLR4 and CRP compared with inactive subjects (81). Moreover, the 12 -wk aerobic and resistance exercises intervention significantly reduced CRP levels among physically inactive young and old adults down to the similar levels observed among physically active young and old adults (82). In addition to healthy subjects, the exercise training also significantly improved CRP among patients with chronic diseases, such as CHD and T2D (84, 85).
The mechanism by which increasing levels of physical activity reduce plasma CRP appears to be beyond its effect on body weight as evidenced by the study showing that both low levels of physical activity and high levels of BMI were independently associated with increased inflammatory markers(86). It has been postulated the potential favorable effects of physical activity on proatherogenic adipokines, insulin metabolism and endothelial function (87). Other mechanisms have also been suggested, including exercise-induced release of heat shock protein, altering immune function or reduced tissue hypoxia (88).
Contrary to physical activity, cigarette smoking is well-established risk factor for CVD. Smoking triggers an immunologic response and vascular injury, which is associated with increased levels of inflammatory markers, such as CRP (89). Several studies have described the strong link between smoking and elevated levels of CRP and other markers of inflammation (90, 91). For example, a study in 2,920 British older men showed that compared with never smokers, current cigarette smokers had significantly higher levels of CRP (2.53 vs. 1.35 mg/L). However, most inflammatory levels improved within 5 years of smoking cessation, but took over 20 years to revert to levels of never smokers (91). The NHANES III with 15,489 participants (1988-1994) data suggested that after adjustment for traditional CVD risk factors, cigarette smoking was related to elevated levels of CRP and fibrinogen with dose-dependent and temporal relationship (89).
Collectively, dietary habits and other lifestyle factors that influence CRP provide the tools for CVD risk prevention. Furthermore, preventive and therapeutic recommendations could be even more successful if they were tailored using genetic information at the CRP gene. However, the current knowledge is not solid enough to provide clinically relevant advice using this approach.
Conclusions
The CRP locus, together with other loci which are involved in its regulatory pathway, significantly influences plasma CRP levels. The reported impact of CRP genetic variants on CVD risk and events provides some support to the notion that CRP may play a causal role in the pathogenesis of atherosclerotic disease; however, the evidence is not yet conclusive. Moreover, CRP genetic variants could affect CRP response to lipid-lowering drug, such as fenofibrate, and thus, may potentially define the success of the intervention. Environmental exposures, such as diet and other lifestyle factors are also responsible for the inter-individual variation of CRP phenotypes, and there is support for the notion that genetic and environmental factors may interact to define this complex trait. Therefore, further characterization of the influence and interaction of genetic variation on the CRP response to dietary and other behavior modification as well as drug treatment could have great implication for the development of more personalized preventive and therapeutic approaches to reduce CVD.
Acknowledgments
Grant/Funding Support: This study was supported by contract 58-1950-9-001 from the US Department of Agriculture (Agriculture Research Service) and by NIH Heart, Lung and Blood Institute grant U 01 HL72524, Genetic and Environmental Determinants of Triglycerides and grant NIH-NHLBI R 01 HL54776. We acknowledge Abbott Laboratories (Abbott Park, Ill) for their supply of study medication for this project.
Footnotes
Financial Disclosures: None declared
Reference
- 1.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279:48487–90. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 2.Dong Q, Wright JR. Expression of C-reactive protein by alveolar macrophages. J Immunol. 1996;156:4815–20. [PubMed] [Google Scholar]
- 3.Kobayashi S, Inoue N, Ohashi Y, Terashima M, Matsui K, Mori T, et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler Thromb Vasc Biol. 2003;23:1398–404. doi: 10.1161/01.ATV.0000081637.36475.BC. [DOI] [PubMed] [Google Scholar]
- 4.Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, Chan L. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–55. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97:3A–11A. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–8. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 7.de Maat MP, Kluft C. Determinants of C-reactive protein concentration in blood. Ital Heart J. 2001;2:189–95. [PubMed] [Google Scholar]
- 8.Ford ES, Giles WH, Mokdad AH, Myers GL. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin Chem. 2004;50:574–81. doi: 10.1373/clinchem.2003.027359. [DOI] [PubMed] [Google Scholar]
- 9.MacGregor AJ, Gallimore JR, Spector TD, Pepys MB. Genetic effects on baseline values of C-reactive protein and serum amyloid a protein: a comparison of monozygotic and dizygotic twins. Clin Chem. 2004;50:130–4. doi: 10.1373/clinchem.2003.028258. [DOI] [PubMed] [Google Scholar]
- 10.Pankow JS, Folsom AR, Cushman M, Borecki IB, Hopkins PN, Eckfeldt JH, Tracy RP. Familial and genetic determinants of systemic markers of inflammation: the NHLBI family heart study. Atherosclerosis. 2001;154:681–9. doi: 10.1016/s0021-9150(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 11.Miller DT, Zee RY, Suk Danik J, Kozlowski P, Chasman DI, Lazarus R, et al. Association of common CRP gene variants with CRP levels and cardiovascular events. Ann Hum Genet. 2005;69:623–38. doi: 10.1111/j.1529-8817.2005.00210.x. [DOI] [PubMed] [Google Scholar]
- 12.Szalai AJ, Wu J, Lange EM, McCrory MA, Langefeld CD, Williams A, et al. Single-nucleotide polymorphisms in the C-reactive protein (CRP) gene promoter that affect transcription factor binding, alter transcriptional activity, and associate with differences in baseline serum CRP level. J Mol Med. 2005;83:440–7. doi: 10.1007/s00109-005-0658-0. [DOI] [PubMed] [Google Scholar]
- 13.Carlson CS, Aldred SF, Lee PK, Tracy RP, Schwartz SM, Rieder M, et al. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet. 2005;77:64–77. doi: 10.1086/431366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brull DJ, Serrano N, Zito F, Jones L, Montgomery HE, Rumley A, et al. Human CRP gene polymorphism influences CRP levels: implications for the prediction and pathogenesis of coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:2063–9. doi: 10.1161/01.ATV.0000084640.21712.9C. [DOI] [PubMed] [Google Scholar]
- 15.Crawford DC, Sanders CL, Qin X, Smith JD, Shephard C, Wong M, et al. Genetic variation is associated with C-reactive protein levels in the Third National Health and Nutrition Examination Survey. Circulation. 2006;114:2458–65. doi: 10.1161/CIRCULATIONAHA.106.615740. [DOI] [PubMed] [Google Scholar]
- 16.Kathiresan S, Larson MG, Vasan RS, Guo CY, Gona P, Keaney JF, Jr., et al. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation. 2006;113:1415–23. doi: 10.1161/CIRCULATIONAHA.105.591271. [DOI] [PubMed] [Google Scholar]
- 17.Lange LA, Carlson CS, Hindorff LA, Lange EM, Walston J, Durda JP, et al. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. Jama. 2006;296:2703–11. doi: 10.1001/jama.296.22.2703. [DOI] [PubMed] [Google Scholar]
- 18.Davey Smith G, Lawlor DA, Harbord R, Timpson N, Rumley A, Lowe GD, et al. Association of C-reactive protein with blood pressure and hypertension: life course confounding and mendelian randomization tests of causality. Arterioscler Thromb Vasc Biol. 2005;25:1051–6. doi: 10.1161/01.ATV.0000160351.95181.d0. [DOI] [PubMed] [Google Scholar]
- 19.Kardys I, de Maat MP, Uitterlinden AG, Hofman A, Witteman JC. C-reactive protein gene haplotypes and risk of coronary heart disease: the Rotterdam Study. Eur Heart J. 2006;27:1331–7. doi: 10.1093/eurheartj/ehl018. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Hunt SC, Xu Q, Chen YE, Province MA, Eckfeldt JH, et al. Association study of CRP gene polymorphisms with serum CRP level and cardiovascular risk in the NHLBI Family Heart Study. Am J Physiol Heart Circ Physiol. 2006;291:H2752–7. doi: 10.1152/ajpheart.01164.2005. [DOI] [PubMed] [Google Scholar]
- 21.Juonala M, Viikari JS, Ronnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2006;26:1883–8. doi: 10.1161/01.ATV.0000228818.11968.7a. [DOI] [PubMed] [Google Scholar]
- 22.Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–21. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 23.Suk Danik J, Chasman DI, Cannon CP, Miller DT, Zee RY, Kozlowski P, et al. Influence of genetic variation in the C-reactive protein gene on the inflammatory response during and after acute coronary ischemia. Ann Hum Genet. 2006;70:705–16. doi: 10.1111/j.1469-1809.2006.00272.x. [DOI] [PubMed] [Google Scholar]
- 24.Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 25.Collaborative pooled analysis of data on C-reactive protein gene variants and coronary disease: judging causality by Mendelian randomisation. Eur J Epidemiol. 2008;23:531–40. doi: 10.1007/s10654-008-9249-z. [DOI] [PubMed] [Google Scholar]
- 26.Kleemann R, Verschuren L, de Rooij BJ, Lindeman J, de Maat MM, Szalai AJ, et al. Evidence for anti-inflammatory activity of statins and PPARalpha activators in human C-reactive protein transgenic mice in vivo and in cultured human hepatocytes in vitro. Blood. 2004;103:4188–94. doi: 10.1182/blood-2003-11-3791. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 28.Libby P, Plutzky J. Inflammation in diabetes mellitus: role of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma agonists. Am J Cardiol. 2007;99:27B–40B. doi: 10.1016/j.amjcard.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Roberts AW, Evans M. The metabolic syndrome, inflammation and cardiovascular disease in type 2 diabetes. Curr Opin Lipidol. 2004;15:89–91. doi: 10.1097/00041433-200402000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). Bmj. 1998;316:823–8. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 32.Rubins HB, Robins SJ, Collins D, Nelson DB, Elam MB, Schaefer EJ, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT). Arch Intern Med. 2002;162:2597–604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- 33.Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med. 2005;165:1154–60. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 34.Brisson D, Ledoux K, Bosse Y, St-Pierre J, Julien P, Perron P, et al. Effect of apolipoprotein E, peroxisome proliferator-activated receptor alpha and lipoprotein lipase gene mutations on the ability of fenofibrate to improve lipid profiles and reach clinical guideline targets among hypertriglyceridemic patients. Pharmacogenetics. 2002;12:313–20. doi: 10.1097/00008571-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz G, Schmitz-Madry A, Ugocsai P. Pharmacogenetics and pharmacogenomics of cholesterol-lowering therapy. Curr Opin Lipidol. 2007;18:164–73. doi: 10.1097/MOL.0b013e3280555083. [DOI] [PubMed] [Google Scholar]
- 36.Corella D, Arnett DK, Tsai MY, Kabagambe EK, Peacock JM, Hixson JE, et al. The -256T>C Polymorphism in the Apolipoprotein A-II Gene Promoter Is Associated with Body Mass Index and Food Intake in the Genetics of Lipid Lowering Drugs and Diet Network Study. Clin Chem. 2007;53:1144–52. doi: 10.1373/clinchem.2006.084863. [DOI] [PubMed] [Google Scholar]
- 37.Shen J, Arnett DK, Parnell LD, Peacock JM, Lai CQ, Hixson JE, et al. Association of common C-reactive protein (CRP) gene polymorphisms with baseline plasma CRP levels and fenofibrate response: the GOLDN study. Diabetes Care. 2008;31:910–5. doi: 10.2337/dc07-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D'Aiuto F, Casas JP, Shah T, Humphries SE, Hingorani AD, Tonetti MS. C-reactive protein (+1444C>T) polymorphism influences CRP response following a moderate inflammatory stimulus. Atherosclerosis. 2005;179:413–7. doi: 10.1016/j.atherosclerosis.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Barter PJ, Rye KA. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler Thromb Vasc Biol. 2008;28:39–46. doi: 10.1161/ATVBAHA.107.148817. [DOI] [PubMed] [Google Scholar]
- 40.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 41.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivashchenko Y, Kramer F, Schafer S, Bucher A, Veit K, Hombach V, et al. Protein kinase C pathway is involved in transcriptional regulation of C-reactive protein synthesis in human hepatocytes. Arterioscler Thromb Vasc Biol. 2005;25:186–92. doi: 10.1161/01.ATV.0000150041.81963.68. [DOI] [PubMed] [Google Scholar]
- 43.Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 44.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation. 2003;108:1917–23. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 45.Berrahmoune H, Herbeth B, Lamont JV, Lambert D, Blankenberg S, Tiret L, et al. Association of classical and related inflammatory markers with high-sensitivity C-reactive protein in healthy individuals: results from the Stanislas cohort. Clin Chem Lab Med. 2007;45:1339–46. doi: 10.1515/CCLM.2007.279. [DOI] [PubMed] [Google Scholar]
- 46.Piche ME, Lemieux S, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–7. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 48.Eklund C, Jahan F, Pessi T, Lehtimaki T, Hurme M. Interleukin 1B gene polymorphism is associated with baseline C-reactive protein levels in healthy individuals. Eur Cytokine Netw. 2003;14:168–71. [PubMed] [Google Scholar]
- 49.Lakka HM, Lakka TA, Rankinen T, Rice T, Rao DC, Leon AS, et al. The TNF-alpha G-308A polymorphism is associated with C-reactive protein levels: the HERITAGE Family Study. Vascul Pharmacol. 2006;44:377–83. doi: 10.1016/j.vph.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Vickers MA, Green FR, Terry C, Mayosi BM, Julier C, Lathrop M, et al. Genotype at a promoter polymorphism of the interleukin-6 gene is associated with baseline levels of plasma C-reactive protein. Cardiovasc Res. 2002;53:1029–34. doi: 10.1016/s0008-6363(01)00534-x. [DOI] [PubMed] [Google Scholar]
- 51.Wessel J, Moratorio G, Rao F, Mahata M, Zhang L, Greene W, et al. C-reactive protein, an ‘intermediate phenotype’ for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/beta-adrenergic pathway loci. J Hypertens. 2007;25:329–43. doi: 10.1097/HJH.0b013e328011753e. [DOI] [PubMed] [Google Scholar]
- 52.Marz W, Scharnagl H, Hoffmann MM, Boehm BO, Winkelmann BR. The apolipoprotein E polymorphism is associated with circulating C-reactive protein (the Ludwigshafen risk and cardiovascular health study). Eur Heart J. 2004;25:2109–19. doi: 10.1016/j.ehj.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE, et al. Loci related to metabolic-syndrome pathways including LEPR,HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women's Genome Health Study. Am J Hum Genet. 2008;82:1185–92. doi: 10.1016/j.ajhg.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phinney SD. Fatty acids, inflammation, and the metabolic syndrome. Am J Clin Nutr. 2005;82:1151–2. doi: 10.1093/ajcn/82.6.1151. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–35. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 56.Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61–7. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 57.King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am J Cardiol. 2003;92:1335–9. doi: 10.1016/j.amjcard.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 58.Pirro M, Schillaci G, Savarese G, Gemelli F, Mannarino MR, Siepi D, et al. Attenuation of inflammation with short-term dietary intervention is associated with a reduction of arterial stiffness in subjects with hypercholesterolaemia. Eur J Cardiovasc Prev Rehabil. 2004;11:497–502. doi: 10.1097/01.hjr.0000152243.51327.2a. [DOI] [PubMed] [Google Scholar]
- 59.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79:969–73. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 60.Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134:1806–11. doi: 10.1093/jn/134.7.1806. [DOI] [PubMed] [Google Scholar]
- 61.Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y, Stefanadis C. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005;46:120–4. doi: 10.1016/j.jacc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 62.Rallidis LS, Paschos G, Papaioannou ML, Liakos GK, Panagiotakos DB, Anastasiadis G, Zampelas A. The effect of diet enriched with alpha-linolenic acid on soluble cellular adhesion molecules in dyslipidaemic patients. Atherosclerosis. 2004;174:127–32. doi: 10.1016/j.atherosclerosis.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Bemelmans WJ, Lefrandt JD, Feskens EJ, van Haelst PL, Broer J, Meyboom-de Jong B, et al. Increased alpha-linolenic acid intake lowers C-reactive protein, but has no effect on markers of atherosclerosis. Eur J Clin Nutr. 2004;58:1083–9. doi: 10.1038/sj.ejcn.1601938. [DOI] [PubMed] [Google Scholar]
- 64.Madsen T, Skou HA, Hansen VE, Fog L, Christensen JH, Toft E, Schmidt EB. C-reactive protein, dietary n-3 fatty acids, and the extent of coronary artery disease. Am J Cardiol. 2001;88:1139–42. doi: 10.1016/s0002-9149(01)02049-5. [DOI] [PubMed] [Google Scholar]
- 65.Geelen A, Brouwer IA, Schouten EG, Kluft C, Katan MB, Zock PL. Intake of n-3 fatty acids from fish does not lower serum concentrations of C-reactive protein in healthy subjects. Eur J Clin Nutr. 2004;58:1440–2. doi: 10.1038/sj.ejcn.1601986. [DOI] [PubMed] [Google Scholar]
- 66.Vega-Lopez S, Kaul N, Devaraj S, Cai RY, German B, Jialal I. Supplementation with omega3 polyunsaturated fatty acids and all-rac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism. 2004;53:236–40. doi: 10.1016/j.metabol.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Yoneyama S, Miura K, Sasaki S, Yoshita K, Morikawa Y, Ishizaki M, et al. Dietary intake of fatty acids and serum C-reactive protein in Japanese. J Epidemiol. 2007;17:86–92. doi: 10.2188/jea.17.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. Jama. 2004;292:1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 69.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 70.Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, et al. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism. 2008;57:437–43. doi: 10.1016/j.metabol.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ajani UA, Ford ES, Mokdad AH. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J Nutr. 2004;134:1181–5. doi: 10.1093/jn/134.5.1181. [DOI] [PubMed] [Google Scholar]
- 72.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ, 3rd, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–6. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King DE. Dietary fiber, inflammation, and cardiovascular disease. Mol Nutr Food Res. 2005;49:594–600. doi: 10.1002/mnfr.200400112. [DOI] [PubMed] [Google Scholar]
- 74.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol. 2006;26:995–1001. doi: 10.1161/01.ATV.0000214295.86079.d1. [DOI] [PubMed] [Google Scholar]
- 75.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. 2006;84:1489–97. doi: 10.1093/ajcn/84.6.1489. [DOI] [PubMed] [Google Scholar]
- 76.Watzl B, Kulling SE, Moseneder J, Barth SW, Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr. 2005;82:1052–8. doi: 10.1093/ajcn/82.5.1052. [DOI] [PubMed] [Google Scholar]
- 77.Maron DJ. Flavonoids for reduction of atherosclerotic risk. Curr Atheroscler Rep. 2004;6:73–8. doi: 10.1007/s11883-004-0119-1. [DOI] [PubMed] [Google Scholar]
- 78.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–85. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 79.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–8. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13:561–8. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 81.McFarlin BK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Stewart LK, et al. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci. 2006;61:388–93. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- 82.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Timmerman KL, et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39:1714–9. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- 83.Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–9. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- 84.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–61. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 85.Oberbach A, Tonjes A, Kloting N, Fasshauer M, Kratzsch J, Busse MW, et al. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol. 2006;154:577–85. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- 86.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. Jama. 2006;295:1412–9. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 87.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 88.Flynn MG. The Anti-Inflammatory Actions of Exercise Training. American Journal of Lifestyle Medicine. 2007;1:220–35. doi: 10.1177/1559827607300283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bakhru A, Erlinger TP. Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Examination Survey. PLoS Med. 2005;2:e160. doi: 10.1371/journal.pmed.0020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003;138:891–7. doi: 10.7326/0003-4819-138-11-200306030-00010. [DOI] [PubMed] [Google Scholar]
- 91.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26:1765–73. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]