Abstract
Objective
Direct revascularization surgery is regarded as the most effective method of treatment of adults with moyamoya disease. These patients, however, have a higher risk of perioperative ischemic complications than do patients with atherosclerotic stroke, and are at risk for ischemic complications in the hemisphere contralateral to the one operated on. We investigated the incidence and risk factors for ischemic stroke in the contralateral hemisphere after surgical treatment of adults with moyamoya disease.
Methods
We retrospectively reviewed the medical records and results of neuroimaging studies on 79 hemispheres of 73 consecutive patients with adult moyamoya disease (mean±SD age, 37.96±11.27 years; range, 18-62 years) who underwent direct bypass surgery over 6 years.
Results
Ischemic complications occurred in 4 of 79 (5.1%) contralateral hemispheres, one with Suzuki stage 3 and three with Suzuki stage 4. Three patients showed posterior cerebral artery (PCA) involvement by moyamoya vessels. Advanced stage of moyamoya disease (Suzuki stages 4/5/6; p=0.001), PCA involvement (p=0.001) and postoperative hypotension (mean arterial blood pressure <80% of preoperative mean arterial blood pressure) on the first (p<0.0001) and second (p=0.003) days after surgery were significantly correlated with postoperative contralateral ischemic complications.
Conclusion
In patients with advanced moyamoya disease and involvement of the PCA, intentional hypotension can result in ischemic stroke in the hemisphere contralateral to the one operated on. Careful control of perioperative blood pressure is crucial for good surgical results.
Keywords: Cerebral revascularization, Complications, Moyamoya diseases, Perioperative period
INTRODUCTION
Moyamoya disease is a chronic, occlusive cerebrovascular disease of unknown etiology, characterized by bilateral steno-occlusive changes at the terminal portion of the internal carotid artery and an abnormal vascular network at the base of the brain15). Because surgical revascularization prevents further cerebral ischemic attacks by improving cerebral blood flow (CBF), superficial temporal artery-middle cerebral artery (STA-MCA) anastomosis with or without indirect bypass is generally used as the standard surgical treatment for adults with moyamoya disease1,4,8,12). Despite the benefits of surgical treatment, however, the incidence of perioperative ischemic complications is higher in patients undergoing surgery for moyamoya disease (3-31%) than in those undergoing any other type of cerebrovascular surgery3,5,9,13). Also, in contrast to other cerebrovascular diseases, ischemic stroke in the hemisphere contralateral to the one operated on is not rare, occurring in about 3% to 14% of patients3,6,7). Despite reports of its incidence, the causes of this complication have not been described. We assessed the incidence of this complication in 79 hemispheres of 73 adult patients with moyamoya disease that underwent surgery over the past 6 years, as well as the risk factors associated with this complication.
MATERIALS AND METHODS
Patients
This study included 79 hemispheres of 73 consecutive adults with moyamoya disease that underwent direct bypass surgery, performed by a single surgeon, from April 2004 to August 2009. We reviewed their medical records and post-surgical neuroimaging results. We enrolled patients with symptomatic episodes in spite of maximal medical therapy, with compromised cerebral hemodynamic reserve as demonstrated by single photon emission computed tomography (SPECT), and lack of major hymedical comorbidity.
Interventions
The neurologic state of the patients was assessed by neurologists and their radiologic findings, including computed tomography (CT), transfemoral cerebral angiography (TFCA) and magnetic resonance image (MRI), were interpreted by neuroradiologists and a neurosurgeon.
Preoperative TFCA, including bilateral internal carotid artery (ICA), bilateral external carotid artery, and vertebral artery injection, was performed to evaluate donor vessels, collateral circulation, moyamoya vessels and recipient vessels. SPECT with and without acetazolamide challenge to assess for loss of cerebrovascular reserve capacity was performed. TFCA was performed to evaluate postoperative bypass patency, extent of revascularization, and regression of moyamoya vessels. Postoperative SPECT was used to assess improvement in baseline perfusion and hemodynamic reserve.
For a least 1 week preoperatively, patients were discontinued from aspirin and other antiplatelet medications. All patients were placed under general anesthesia using a combination of isoflurane and intravenous agents. Before middle cerebral artery (MCA) occlusion for bypass graft, patients were administered an intravenous bolus of 250-300 mg thiopental. After surgery, patients were kept overnight in the intensive care unit with strict blood pressure control, usually a mean arterial blood pressure (MABP) between 80 and 100 mm Hg. Patients were administered a loading dose of 300 mg aspirin on the day after surgery, followed by a maintenance dose of 100 mg. Patients were clinically evaluated every day after surgery and were examined by a neurologist 1, 6, and 18 months after discharge. Cerebral angiography was performed 1 week after surgery and cerebral perfusion after 6 months. Surgical morbidity was defined as newly developed neurological deficits lasting 7 days or longer after revascularization surgery, correlated with findings on MRI or CT imaging.
Analysis of risk factors
To investigate the risk factors for contralateral ischemic complications after revascularization surgery, we analyzed the perioperative factors of each revascularization procedure. Patients were divided into two groups, one with and one without postoperative contralateral ischemic complications. All statistical analyses were performed using the SPSS program (version 12.0; SPSS, Inc., Chicago, IL, USA). Categorical variables were compared using Pearson's chi-square tests or Fisher exact tests, where appropriate. Continuous variables were reported as means±standard deviations and compared using independent Student t-tests or Mann-Whitney U-tests. A probability less than 0.05 was considered statistically significant.
RESULTS
Over the past six years, 79 hemispheres of 76 patients underwent direct anastomosis (STA-MCA bypass) with or without indirect methods. Of these 79 hemispheres, 9 (11.4%) showed postoperative ischemic complications, 5 in the ipsilateral hemisphere and four in the contralateral hemisphere.
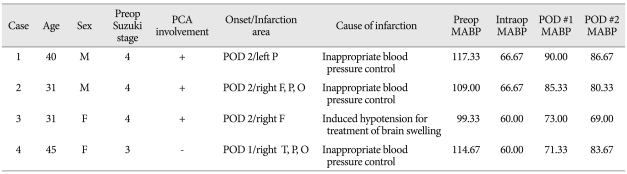
Table 1 summarize the clinical characteristics of the four patients with postoperative contralateral ischemic complications. All four had ischemic symptoms, with low density areas observed on preoperative CT scans. Preoperative angiography showed that one patient had Suzuki stage 3 and three had Suzuki stage 4. SPECT showed moderately decreased perfusion of the MCA territory with or without perfusion defects, and mild to moderately decreased perfusion reserve of the anterior cerebral artery (ACA), in all patients. All four patients underwent STA-MCA bypass surgery with or without indirect surgery. Postoperative ischemic complication in the contralateral hemisphere occurred from the 1 day after surgery to 2 days after surgery.
Table 1.
Characteristics of patients with postoperative contralateral ischemic complications
MABP : mean arterial blood pressure, PCA : posterior cerebral artery, POD : postoperative day, F : frontal, T : temporal, P : parietal, O : occipital
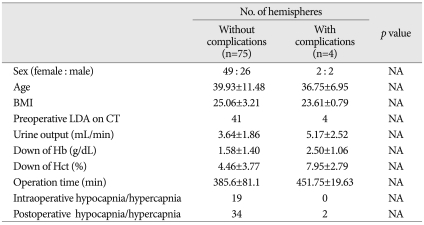
The occurrence of these ischemic complications did not correlate with sex, age, body mass index, preoperative low density on CT, urine output during the operation, hematocrit reduction, hemoglobin reduction or perioperative hypocapnia or hypercapnia (Table 2).
Table 2.
Risk factors for postoperative contralateral ischemic complications
BMI : body mass index, LDA : low density area, CT : computed tomography, Hb : Hemoglobin, Hct : hematocrit, hypocapnia : PCO2 <35 mm Hg, hypercapnia : PCO2 >45 mm Hg
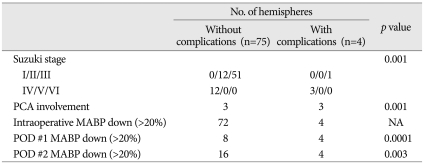
When we compared patients with and without postoperative contralateral ischemic complications, we found that these ischemic complications were related with advanced stage of moyamoya disease (Suzuki stages 4 and 5; p=0.001), involvement of the posterior cerebral artery (PCA) (p=0.001) and postoperative hypotension (mean arterial blood pressure <80% of preoperative MABP) on the first (p<0.0001) and second (p=0.003) days after surgery (Table 3).
Table 3.
Risk factors for postoperative contralateral ischemic complications
PCA : posterior cerebral artery, POD : postoperative day, MABP : mean arterial blood pressure
Case illustration
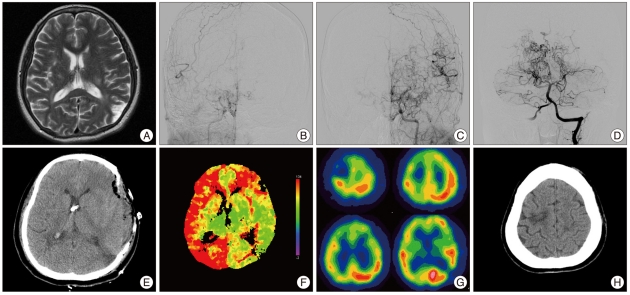
This patient was admitted to our department due to right leg weakness (grade 4). MRI showed chronic infarction in the frontal white matter, corona radiata, and left parietal lobe (Fig. 1A). Magnetic resonance angiography and conventional angiography showed occlusion at the distal ICA on both sides and occlusion of both PCAs at the level of the P2/P3 junction (Fig. 1B, C and D). SPECT and MRI showed decreased perfusion of the right MCA and PCA territory and a perfusion defect in the left temporo-occipital cortex due to previous infarction. The patient underwent STA-MCA bypass with encephaloduroarteriomyosinangiosis (EDAMS) operation in the left hemisphere. Release of the vessel clamp used for cross clamping of the M4 segment after successful STA-MCA anastomosis resulted in sudden brain swelling with cortical subarachnoid hemorrhage. There was no visible bleeding site on the exposed cerebral cortex, and brain swelling was controlled by placing extraventricular drainage at the frontal cortex, draining the subarachnoid hemorrhage through multiple arachnoid incisions over the cerebral cortex, and reducing blood pressure to below the normal range. Even with copious saline irrigation through the arachnoid incision, however, we could not identify a visible bleeding site and the operation was completed by EDAMS operation with temporalis muscle flap. Immediate postoperative CT scan showed no evidence of focal hematoma or acute infarction on the operated hemisphere (Fig. 1E), and time-to-peak image of the perfusion CT after direct bypass showed improvement of the delayed time on the left compared with the right side (Fig. 1F). Since sudden brain swelling with subarachnoid hemorrhage after direct bypass surgery is indicate of hyperperfusion syndrome, the patient was treated with strict blood pressure control. Two days after the operation, the patient worsened neurologically to a slightly stuporous level of consciousness and left motor power decreased to grade 2. Follow-up SPECT showed a perfusion defect on the right frontal cortex and a watershed zone on the right ACA-MCA due to a newly developed infarction (Fig. 1G). A CT scan performed 1 month later showed infarction on the right ACA territory (Fig. 1H). The patient recovered after treatment but experienced weakness in the left leg.
Fig. 1.
A : T2 weighted MRI showing an old infarction in the left temporo-occipital cortex. B, C and D : Preoperative angiography showing findings typical of moyamoya disease with involvement of bilateral PCA. E : Immediate postoperative CT scan showing neither focal hematoma nor acute infarction on the operated hemisphere. An extraventricular drainage catheter was placed on the frontal horn of the lateral ventricle. F : TTP image of the perfusion CT showing improvements in delayed time on the left compared with the right hemisphere after STA-MCA bypass with encephalomyoarteriosynangiosis. G : Follow-up SPECT 9 days after surgery showing perfusion defects in the right frontal cortex and right ACA-MCA watershed zone due to a newly developed infarction. H : Non-enhanced CT scan showing focal low density on the left frontal area, the same area of the left frontal cortex. MRI : magnetic resonance image, PCA : posterior cerebral artery, CT : computed tomography, STA : superficial temporal artery, SPECT : single photon emission computed tomography, ACA : anterior cerebral artery, MCA : middle cerebral artery.
DISCUSSION
Perioperative ischemic complications are more common in patients with moyamoya disease than in patients with other cerebrovascular occlusive diseases5,9), with ischemic complications in the hemisphere contralateral to that operated on regarded as unique to moyamoya disease.
In general, the risk factors for perioperative ischemic complications in patients with moyamoya disease include hypotension, hypocapnia, preoperative low density areas on neuroimaging, perioperative hypovolemia, reduction in the hematocrit, and history of cerebrovascular disorder10,14). The relationship between these risk factors and ischemic complications is likely due to reduced cerebral blood flow and lack of cerebral autoregulation2,16). Less is known, however, about ischemic complications occurring in the contralateral hemisphere.
We found that advanced stages of moyamoya disease (Suzuki stage 4/5), involvement of the PCA and postoperative hypotension on the first and second days after surgery were significant risk factors for the postoperative development of contralateral ischemic complications.
Our finding that PCA involvement was a risk factor for postoperative contralateral ischemic complications was not surprising because, in patients with advanced stage moyamoya disease, the PCA is one component of collateral blood flow source, along with the leptomeningeal collateral circulation. In these patients, cerebral hemodynamics is impaired, with stress, such as surgery, inducing ischemic complications. Although direct bypass graft can maintain operative-side CBF, contralateral CBF could not keep up with the demand, resulting in ischemic complications.
We found that intraoperative hypotension was common, occurring in 73 of 79 (92.4%) hemispheres, but it did not independently affect the occurrence of postoperative ischemic complications. In contrast, postoperative hypotension was a significant risk factor for postoperative ischemic complications. While the absolute blood pressure was not important, the relative blood pressure, compared with preoperative level, was. Patient 1 had a preoperative blood pressure of 156/98 mm Hg and an MABP of 117.33 mm Hg. After revascularization, however, this patient's systolic blood pressure was maintained at 120-130 mm Hg to prevent hyperperfusion, resulting in MABPs on the first and second days after surgery of 90.00 mm Hg and 86.67 mm Hg, respectively. Although these MABP values were lower than this patient's preoperative MABP, it was maintained at a normal or slightly subnormal value. In the other three patients with postoperative ischemic complications, their systolic blood pressure was maintained at 107-130 mm Hg and their MABPs at 69.0-85.3 mm Hg. Thus, despite their blood pressures being normal to subnormal, they experienced postoperative contralateral ischemic complications. These findings indicate the importance of maintaining a patient's postoperative blood pressure as near as possible to that patient's preoperative blood pressure. Although MABP reduction was more common during than after surgery, it was not associated with postoperative complications. This may be due to protective effects of anesthesia on the brain and the lower demand for cerebral metabolism during surgery11,12). Upon awakening, cerebral blood flow returns to normal, making the patients more prone to hypotension. Thus, a small postoperative reduction in MABP may lead to postoperative complications.
CONCLUSION
Postoperative hypotension in patients with advanced stage moyamoya disease and PCA involvement may result in ischemic complications to the contralateral hemisphere. Careful preoperative management of blood pressure is important in preventing these ischemic complications.
References
- 1.Fujimura M, Kaneta T, Shimizu H, Tominaga T. Symptomatic hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in a child with moyamoya disease. Childs Nerv Syst. 2007;23:1195–1198. doi: 10.1007/s00381-007-0361-2. [DOI] [PubMed] [Google Scholar]
- 2.Furuya K, Kawahara N, Morita A, Momose T, Aoki S, Kirino T. Focal hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease. Case report. J Neurosurg. 2004;100:128–132. doi: 10.3171/jns.2004.100.1.0128. [DOI] [PubMed] [Google Scholar]
- 3.Guzman R, Lee M, Achrol A, Bell-Stephens T, Kelly M, Do HM, et al. Clinical outcome after 450 revascularization procedures for moyamoya disease. Clinical article. J Neurosurg. 2009;111:927–935. doi: 10.3171/2009.4.JNS081649. [DOI] [PubMed] [Google Scholar]
- 4.Houkin K, Ishikawa T, Yoshimoto T, Abe H. Direct and indirect revascularization for moyamoya disease surgical techniques and peri-operative complications. Clin Neurol Neurosurg. 1997;99(Suppl 2):S142–S145. doi: 10.1016/s0303-8467(97)00075-9. [DOI] [PubMed] [Google Scholar]
- 5.Iwama T, Hashimoto N, Tsukahara T, Murai B. Peri-operative complications in adult moyamoya disease. Acta Neurochir (Wien) 1995;132:26–31. doi: 10.1007/BF01404844. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Choi JU, Yang KH, Kim TG, Kim DS. Risk factors for postoperative ischemic complications in patients with moyamoya disease. J Neurosurg. 2005;103:433–438. doi: 10.3171/ped.2005.103.5.0433. [DOI] [PubMed] [Google Scholar]
- 7.Ohue S, Kumon Y, Kohno K, Watanabe H, Iwata S, Ohnishi T. Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol. 2008;69:281–286. doi: 10.1016/j.surneu.2007.01.047. discussion 286-287. [DOI] [PubMed] [Google Scholar]
- 8.Okada Y, Shima T, Nishida M, Yamane K, Yamada T, Yamanaka C. Effectiveness of superficial temporal artery-middle cerebral artery anastomosis in adult moyamoya disease : cerebral hemodynamics and clinical course in ischemic and hemorrhagic varieties. Stroke. 1998;29:625–630. doi: 10.1161/01.str.29.3.625. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto T, Kawaguchi M, Kurehara K, Kitaguchi K, Furuya H, Karasawa J. Risk factors for neurologic deterioration after revascularization surgery in patients with moyamoya disease. Anesth Analg. 1997;85:1060–1065. doi: 10.1097/00000539-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Shirane R, Yoshimoto T. Perioperative factors related to the development of ischemic complications in patients with moyamoya disease. Childs Nerv Syst. 1997;13:68–72. doi: 10.1007/s003810050044. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro HM. Barbiturates in brain ischaemia. Br J Anaesth. 1985;57:82–95. doi: 10.1093/bja/57.1.82. [DOI] [PubMed] [Google Scholar]
- 12.Spetzler RF, Hadley MN, Rigamonti D, Carter LP, Raudzens PA, Shedd SA, et al. Aneurysms of the basilar artery treated with circulatory arrest, hypothermia, and barbiturate cerebral protection. J Neurosurg. 1988;68:868–879. doi: 10.3171/jns.1988.68.6.0868. [DOI] [PubMed] [Google Scholar]
- 13.Starke RM, Komotar RJ, Connolly ES. Optimal surgical treatment for moyamoya disease in adults : direct versus indirect bypass. Neurosurg Focus. 2009;26:E8. doi: 10.3171/2009.01.FOCUS08309. [DOI] [PubMed] [Google Scholar]
- 14.Sumikawa K, Nagai H. Moyamoya disease and anesthesia. Anesthesiology. 1983;58:204–205. doi: 10.1097/00000542-198302000-00033. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 16.Uno M, Nakajima N, Nishi K, Shinno K, Nagahiro S. Hyperperfusion syndrome after extracranial-intracranial bypass in a patient with moyamoya disease--case report. Neurol Med Chir (Tokyo) 1998;38:420–424. doi: 10.2176/nmc.38.420. [DOI] [PubMed] [Google Scholar]