Abstract
Objectives
To compare the extravascular lung water index (EVLWi) and other markers of disease severity in patients with acute lung injury (ALI) versus patients at risk to develop it and to determine their ability to predict progression to ALI in patients at risk.
Design
EVLWi, dead space fraction, PaO2/FiO2, and other markers of disease severity were measured prospectively in 29 patients daily for five days after admission to the ICU. Patients had ALI as defined by the American European Consensus Committee (AECC) criteria or had risk factors to develop it.
Setting
The intensive care units of an academic tertiary referral hospital.
Measurements and Main Results
The mean EVLWi on day one for patients who progressed to ALI was higher than for those that did not (15.5ml/kg ± 7.4 vs. 8.7ml/kg ± 2.3, p=.04. None of the other physiologic parameters tested discriminated progression to ALI – to include the mean physiologic dead space (0.61 ± 0.06 vs. 0.59 ± 0.10, p = .67), PaO2/FiO2 ratio (322 ± 35 vs. 267 ± 98, p = .15) and static lung compliance (30.9 ± 13.5 vs. 38.5 ± 11.7, p= .24). An EVLWi cutoff value on day 1 of 10ml/Kg had a 63% sensitivity; 88% spec; PPV 83%, NPV 70% to predict progression to ALI. There was no difference in EVLWi between those who progressed to ALI vs. those who had ALI. (14.3 ± 4.7 vs. 15.5 ± 7.4, p= .97).
Conclusions
Elevated EVLWi is a feature of early ALI and discriminates between those with ALI and those without. Furthermore, EVLWi predicts progression to ALI in patients with risk factors to develop it 2.6 ± 0.3 days before the patients meet AECC criteria for it. These 2.6 ± 0.3 days may then represent missed opportunity for therapeutic intervention and improved outcome.
Keywords: acute lung injury, extravascular lung water, pulmonary edema, pulmonary permeability index, physiologic dead-space fraction, transpulmonary thermodilution
INTRODUCTION
Acute lung injury (ALI) is a common cause of respiratory failure. An estimated 75,000 patients per year in the United States die from ALI and its associated morbidities (1). Current standard of care is supportive only and there remains a major need for novel therapeutic strategies to treat this illness. As a result, much attention has turned to developing better tools for diagnosis and management of lung injury.
The American-European definition of ALI is based on radiographic and oxygenation criteria both of which have been shown to be insensitive markers of disease severity and predictors of outcome (2–6). The radiographic criteria used to identify patients with ALI are known to have high inter-observer variability (5, 6). Arterial hypoxemia can result from disease processes other than pulmonary edema. Furthermore, the relationship between the ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2) and the FiO2 is nonlinear with varying degrees of intrapulmonary shunt (7, 8) and can be confounded by changes in mean airway pressure and positive end-expiratory pressure (PEEP) (9). Practically speaking, a patient may or may not meet ALI criteria when PaO2 is measured with varying amounts of PEEP and/or tidal volume, inspiratory times, ventilator mode, or FiO2. Therefore, we postulated that current ALI criteria could be insensitive in identifying patients with lung injury and pulmonary edema. As a result, some patients who may benefit from lung protective ventilation and possibly fluid restriction/depletion may have a delay in treatment and worse outcome.
Markers of severity of illness such as Acute Physiology and Chronic Health Evaluation II (APACHE II) (10) and the Simplified Acute Physiology Score II (SAPS II) (11) have been utilized in predicting mortality in ALI. Pulmonary specific markers, such as the Murray Lung Injury Score (LIS) (12) and physiologic markers such as physiologic deadspace fraction (VD/VT) (13), oxygenation index (OI) (14), and extravascular lung water (EVLWi) (4, 15, 16) have been found to be excellent markers of disease severity and predictors of outcome in ALI. However, none of these have been studied for use in predicting progression to ALI in patients at increased risk.
It has been established that ventilator associated lung injury significantly worsens lung injury and outcome in those with (17) and without pre-existing lung injury (18). Furthermore early initiation of a low tidal volume ventilator strategy has been found to reduce the frequency of ALI in acutely ill patients with evidence of systemic inflammatory response syndrome (SIRS) (19–21). We theorize this is due to ventilator effects on undetected lung injury not meeting AECC criteria for ALI in these patients with SIRS and/or sepsis. Earlier identification of patients with lung injury who do not yet meet AECC criteria for ALI but who will progress to it may then alter therapy and improve outcome. Since elevated EVLWi has been shown to reflect the degree of lung injury on post mortem examination (22) and is a sensitive marker of disease severity in ALI, we asked if EVLWi, and other markers of disease severity better predict progression to ALI in patients at risk (15, 23, 24). By comparing these metrics in patients with ALI vs. those at risk to develop we further we test the idea that pulmonary endothelial and epithelial injury is present in those patients that go on to develop ALI and that EVLWi is more sensitive than the current AECC criteria to detect it.
MATERIALS AND METHODS
This was a prospective, observational, single center study in the adult intensive care units (ICU) at Oregon Health & Science University conducted over a 20 month period. The protocol was approved by the Institutional Review Board. Intubated patients, at least 18 years of age, receiving positive-pressure ventilation were eligible if they had acute lung injury or were at risk to develop it - severe sepsis, massive aspiration, massive transfusion (receiving 10 units of blood in 24 hour time frame), and/or trauma with long bone fracture and/or pulmonary contusion. Informed consent was obtained from all participants and/or their surrogates. If subjects lacked capacity to give informed consent, consent was obtained from the subjects if and when they regained capacity. ALI was defined by the American-European Consensus Committee (AECC) definition (25): acute onset of bilateral opacities on the chest radiograph, a PaO2/FiO2 ratio < 300 mm Hg, and absence of clinical evidence of left atrial hypertension. Patients who were less than 18 years of age, had a positive pregnancy test, had moderate to severe chronic obstructive lung disease (using home oxygen, on chronic oral glucocorticoid therapy, or with an FEV1 < 50 % of predicted), had interstitial lung disease, had undergone lung transplantation, had a contraindication to femoral artery cannulation, had a pre-existing non-PiCCO femoral artery cannulation, or were moribund (not expected to survive > 24 hours) were excluded. Patients were enrolled within 48 hours of meeting the inclusion criteria. Measurements of EVLWi, PaO2/FiO2, CVP, and VD/VT were performed twice daily over the following seven days. APACHE II and SAPS II were recorded on day 1. Murray Lung Injury Scores were calculated for the first five days of the study. Fluid balance was recorded daily until hospital discharge.
Measurement of Extravascular Lung Water and the Pulmonary Vascular Permeability index
The transpulmonary thermodilution technique used for the assessment of extravascular lung water has been validated by postmortem gravimetric technique and with the double dilution (thermo-dye) technique (26–28) and is being used clinically in the care of critically ill patients (29–34). With sensitivity to detect clinically relevant changes in EVLWi, it has been used in both prospective and retrospective studies to discriminate between survivors and non-survivors of ALI (15, 16, 23, 35).
EVLWi was determined using the single-indicator transpulmonary thermodilution technique (TTT) (PiCCO, Pulsion Medical Systems, Munich, Germany). A 15-mL bolus of 0.9% saline at 5°C was injected via a central venous catheter. The thermodilution curve was recorded with a femoral artery thermistor and used to calculate the EVLWi as previously described (15). The boluses were performed in triplicate and an average of the three values was recorded. EVLWi was calculated by dividing the measured lung water by predicted body weight (PBW). PBW was calculated as 50 + 0.91 × (centimeters of height - 152.4) for males and 45.5 + 0.91 (centimeters of height - 152.4) for females (36). Additionally this technique allows for the determination of the intrathoracic blood volume (ITBV) and the global end-diastolic volume (GEDV). The GEDV index (GEDVi) and the ITBV index (ITBVi) were determined by dividing these volumes by the body surface area. Pulmonary vascular permeability index (PVPI) was calculated as the EVLW divided by the pulmonary blood volume (PBV) defined as the ITBV - GEDV (PVPI = EVLW/PBV) as a means to normalize EVLW for differences in central blood volumes.
Measurement of Pulmonary Dead-Space Fraction
Physiologic dead space fraction (VD/VT) measurements were performed using a bedside metabolic monitor to determine mean expired carbon dioxide fraction (PECO2) (NiCO, Respironics, Murrysville, Pennsylvania) after each EVLWi determination. Since VD/VT is dependent on tidal volume (37), each patient was ventilated at 10 ml/kg PBW for 10 minutes, with no bias flow at the time. After ensuring the monitor was appropriately zeroed, the monitor was attached in line with the endotracheal tube. After the PECO2 reading was stable for 5 minutes, an arterial blood gas analysis was done to obtain PaCO2, and the physiologic dead space fraction was calculated using the Bohr-Enghoff equation.
Determination of the Extravascular Lung Water- Physiologic Deadspace Index (EDI)
In an attempt to better quantify total lung injury – both vascular and alveolar - the product of EVLWi * VD/VT (EDI) was calculated and analyzed. We theorized that an index that incorporated markers of primarily endothelial injury as reflected by VD/VT and a marker of endothelial and epithelial injury – EVLWi may have enhanced sensitivity as marker of disease severity. This is a previously untested index developed for this study.
APACHE II and SAPS II scores were calculated using the website http://www.sfar.org. Murray Lung Injury Scores were calculated using the website http://www.medal.org.
Evaluation of chest radiographs
Two critical care attending physicians independently evaluated the chest radiographs in a blinded fashion. They reviewed all the portable antero-posterior chest radiographs for all days that the patient was enrolled in the study. Differences in opinion were settled by discussion between the two physicians while blinded or by the decision of a third blinded physician.
Statistical Analysis
Data are expressed as mean ± standard deviation, frequency (%), or mean (range), unless otherwise noted. Histograms were drawn to determine whether continuous data was normally distributed. Continuous data that was normally distributed was compared using Student’s t-test and non-normally distributed data was compared with the Mann-Whitney U test. Categorical comparisons were made using the chi-square test and Fisher's exact test with SPSS 15.0 for Windows (SPSS, Chicago, IL.) A p < .05 was considered statistically significant. To determine receiver-operating characteristics (ROC) curves and comparison between different ROC curves, SPSS software was used. All graphs were created with IgorPro version 5.0.4.8 (WaveMetrics, Inc., Lake Oswego, OR.)
RESULTS
Thirty one patients were enrolled in this study. Two patients withdrew prior to the collection of data as the attending physicians removed the femoral artery catheter. Of the remaining 29 patients, 19 had ALI at some point in the study - 11 had ALI and 18 patients did not at the time of enrollment. Of these 18, forty four percent (n=8) progressed to ALI within 5 days of enrollment. There was no significant difference in 28-day mortality between the three groups.
Table 1 demonstrates patient characteristics at the time of enrollment. The mean age of the patients was 64 ± 15 years (range, 35 – 86 yrs) and 11 of 18 (61 %) were male. The mean APACHE II score was 25 ± 9 (range, 10 – 41) and the mean SAPS II was 54 ± 15 (range, 23 – 92). Those with ALI at presentation had higher actual body weight, had higher APACHE II and SAPS II scores, lower PaO2/FiO2, and greater VD/VT than those that progressed to ALI. Actual body weight, APACHE II, SAPS, and static lung compliance did not discriminate between those that progressed to ALI and those that never developed it. Only EVLWi, the EDI, and the PVPI discriminated between those that developed ALI and those that did not as measured on day 1 of the study (Table 1).
Table 1.
Patient characteristics
| Variable | All (n = 29) |
ALI at enroll (n = 11) |
Developed ALI (n = 8) |
No ALI (n = 10) |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, yrs | 63 ± 13 | 62 ± 10 | 61 ± 15 | 66 ± 16 |
| Male, n | 16 | 5 | 3 | 8 |
| Actual body weight , kga | 92.3 ± 28.4 | 102.8 ± 24.1 | 71.4 ± 14.6 | 97.4 ± 33.8 |
| Predicted body weight, kg | 63.6 ± 11.5 | 63.0 ± 11.0 | 62.1 ± 12.8 | 65.5 ± 11.8 |
| APACHE IIa | 28 ± 10 | 33 ± 9 | 23 ± 9 | 27 ± 9 |
| SAPS IIa | 60 ± 19 | 69 ± 20 | 51 ± 15 | 56 ± 16 |
| PEEP, cm H20c | 8 ± 3 | 10 ± 4 | 8 ± 2 | 6 ± 3 |
| Plateau pressure, cm H20 | 24 ± 8 | 25 ± 7 | 27 ± 9 | 22 ± 6 |
| Fluid balance, L | 12.1 ± 7.8 | 14.8 ± 10.6 | 12.1 ± 5.4 | 8.7 ± 2.3 |
| GEDI, ml/m2 | 786 ± 173 | 740 ± 177 | 813 ± 196 | 815 ± 155 |
| EVLWI, ml/kgb, c | 12.7 ± 5.7 | 14.3 ± 4.7 | 15.5 ± 7.4 | 8.7 ± 2.3 |
| PVPI b, c | 2.48 ± 1.51 | 2.72 ± 1.13 | 3.31 ± 2.19 | 1.57 ± 0.10 |
| VD/VT a, c | 0.63 ± 0.10 | 0.70 ± 0.08 | 0.61 ± 0.06 | 0.58 ± 0.10 |
| PaO2/FiO2 a,c | 244 ± 94 | 167 ± 59 | 322 ± 35 | 267 ± 98 |
| Static lung compliance/cm H2O | 37.6 ± 13.1 | 41.5 ± 13.6 | 30.9 ± 13.5 | 38.5 ± 11.7 |
| Diagnosis | ||||
| Sepsis, n (%) | 21 (72%) | 8 (73%) | 5 (63%) | 8 (80%) |
| Trauma, n (%) | 3 (10%) | 0 (0%) | 1 (13%) | 2 (20%) |
| Massive transfusion, n (%) | 4 (14%) | 2 (18%) | 1 (13%) | 1 (10%) |
| Massive aspiration, n (%) | 3 (10%) | 1 (9%) | 1 (13%) | 1 (10%) |
| Outcome | ||||
| ICU length of stay, days | 13 ± 7 | 14 ± 10 | 14 ± 5 | 10 ± 6 |
| Hospital length of stay, days | 21 ± 16 | 22 ± 19 | 27 ± 18 | 16 ± 8 |
| 28 day mortality, days | 10/29 (34 %) | 6/11 (55 %) | 1/8 (13 %) | 3/10 (30 %) |
Values are expressed as mean ± standard deviation or frequency (%) unless otherwise noted.
APACHE, Acute Physiology and Chronic Health Evaluation; SAPS, Simplified Acute Physiology Score; ALI, acute lung injury; GEDI, global end diastolic volume index; EVLWi, extravascular lung water indexed for predicted body weight; PVPI, pulmonary vascular permeability index; VD/VT, physiologic deadspace fraction; PaO2/FiO2, ratio of arterial partial pressure of oxygen to delivered oxygen fraction; ICU, intensive care unit.
p < .05 for those who had ALI at enrollment vs. those who developed ALI,
p < .05 for those who developed ALI vs. those who did not,
p < .05 for those had had ALI at enrollment vs. those who did not develop ALI.
Baseline characteristics represent the values obtained on study day 1.
Values are expressed as mean ± standard deviation or frequency (%) unless otherwise noted.
APACHE, Acute Physiology and Chronic Health Evaluation; SAPS, Simplified Acute Physiology Score; ALI, acute lung injury; GEDI, global end diastolic volume index; EVLWi, extravascular lung water indexed for predicted body weight; PVPI, pulmonary vascular permeability index; VD/VT, physiologic deadspace fraction; PaO2/FiO2, ratio of arterial partial pressure of oxygen to delivered oxygen fraction; ICU, intensive care unit.
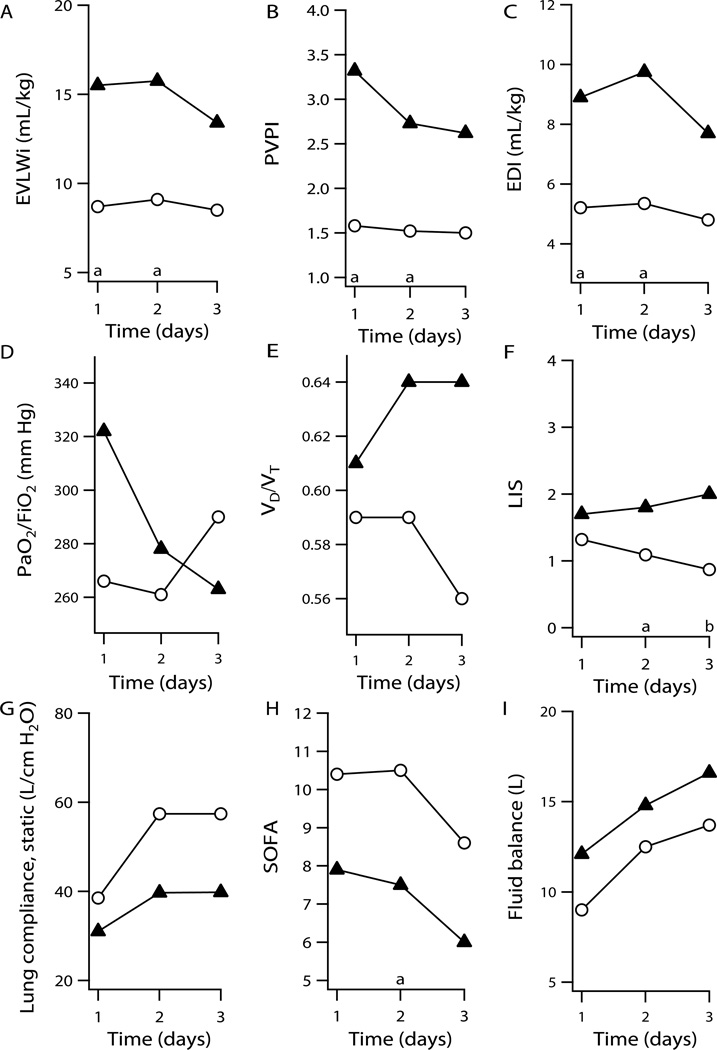
The mean EVLWi for patients who progressed to ALI was higher than for those that did not for all five days (Fig. 1), reaching statistical significance on days one (15.5 ± 7.4 vs. 8.7 ± 2.3, p=.04) and two (15.8 ± 8.0 vs. 9.1 ± 2.4, p=.03). Furthermore it was as high in these patients as it was in those with ALI on the first day of the study 14.3 ± 4.7 vs. 15.5 ± 7.4, p= .97.
Figure 1.
Plots show mean values versus time over the first three days for patients who developed ALI (▲) and did not develop ALI (○). Plots show means versus time for A, EVLWi, B, PVPI, C, EDI, D, PaO2/FiO2, E, VD/VT, F, Murray lung injury score, G, static lung compliance, H, Sequential organ failure score, I, daily fluid balance, respectively. Statistically significant differences between those who developed ALI and those who did not are noted. Probabilities of <0.05 and <0.01 are designated by a and b respectively at foot of each graph.
Other physiologic parameters commonly used to gauge lung injury did not predict which patients would go on to ALI. Neither the mean physiologic dead space (0.61 ± 0.06 vs. 0.59 ± 0.10, p = .67 on day 1) nor the mean PaO2/FiO2 ratio (315 ± 35 vs. 265 ± 92, p = .15) differentiated the two groups.
Murray Lung Injury scores were statistically significantly higher in patients who developed ALI versus those who did not on days 2–4 (day 2, 1.81 ± 0.64 vs. 1.09 ± 0.40, p=.01; day 3, 1.96 ± 0.44 vs. 0.87 ± 0.43, p=.002; and day 4, 1.79 ± 0.43 vs. 0.95 ± 0.41, p=.009).
PVPI was calculated as the pulmonary blood volume (PBV = ITBV - GEDV) divided by the EVLW as a means to normalize EVLW for differences in central blood volumes. PVPI was significantly higher for those who developed ALI versus those who did not for the first three days (day 1, 3.32 ± 2.19 vs. 1.58 ± 0.47, p=.03; day 2, 2.95 ± 1.39 vs. 1.52 ± 0.42, p=.03; and day 3, 2.62 ± 1.27 vs. 1.50 ± 0.58, p=.04). Among the 11 patients who had acute lung injury at the time of enrollment, seven had EVLW > 7, but only two had PVPI > 3. Of the 19 patients who had ALI at some point in the study only 2 of the 19 had PVPI≥ 3 at the time they first met AECCC criteria for ALI. There were no significant differences in GEDI between the groups.
In an attempt to better quantify total lung injury – both vascular and alveolar - the product of EVLWi × VD/VT (EDI) was calculated and analyzed. Although VD/VT alone did not discriminate between individuals who would progress to ALI, EDI did. The mean EDI for patients who progressed to ALI was higher than for those that did not for all five days (Fig. 1), reaching statistical significance on days one (8.9 ± 4.7 vs. 5.2 ± 1.9, p=.04) and two (9.7 ± 4.2 vs. 5.3 ± 2.2, p=.02).
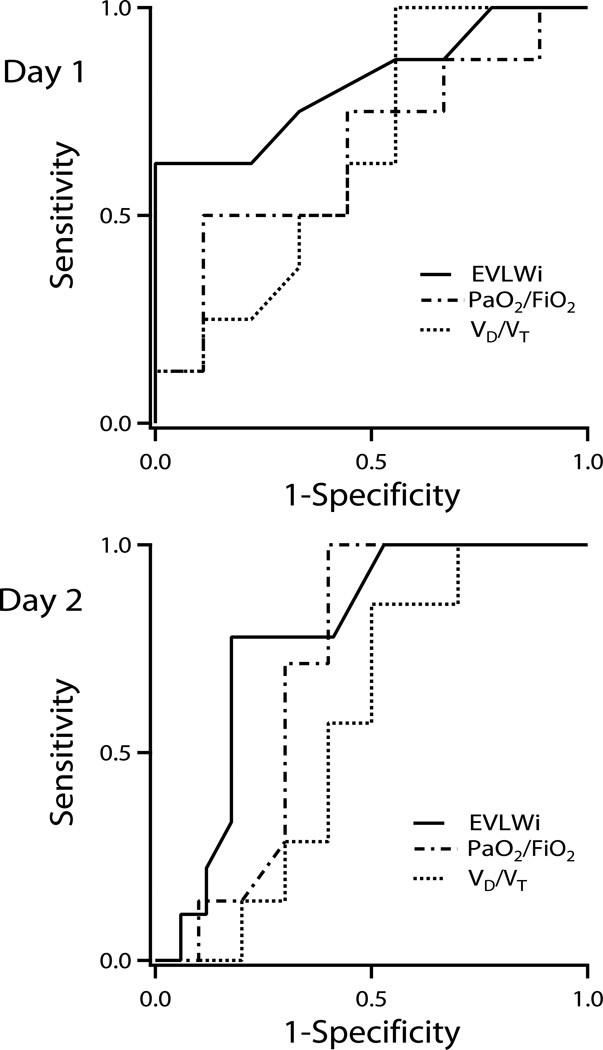
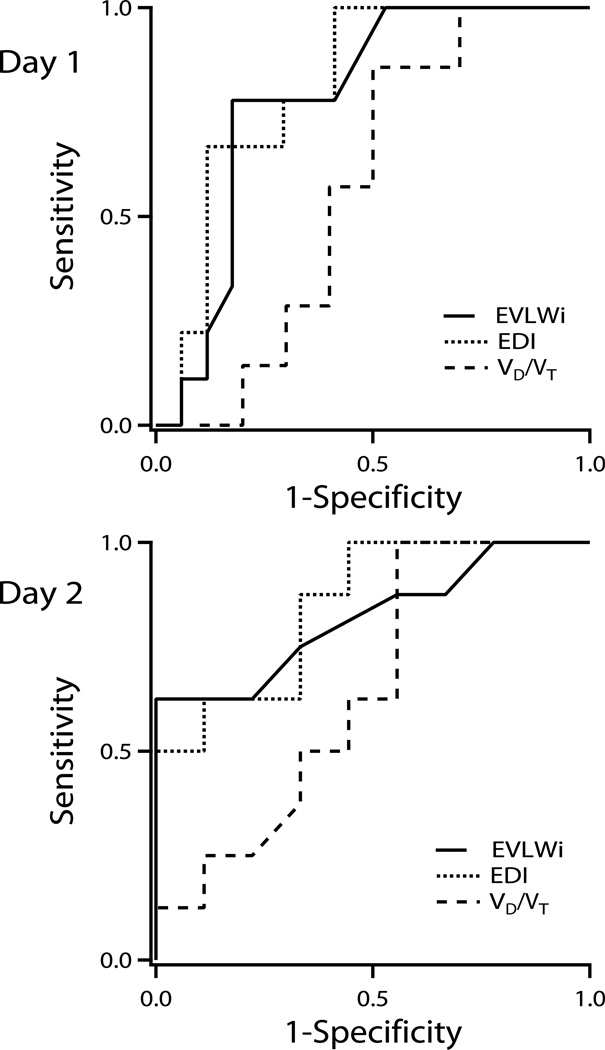
Receiver operator characteristic curves were created using the values on the first two days to determine the ability of EVLWi, VD/VT, PaO2/FiO2, and EDI to predict progression to ALI (Fig. 2 –3). The areas under the curves (± SEM) on day 1 were 0.75 ± 0.13, 0.57 ± 0.14, 0.71 ± 0.13, and 0.77 ± 0.12 for EVLWI, VD/VT, PaO2/FiO2, and EDI respectively. On the second day, the areas under the curves (± SEM) were 0.82 ± .11, 0.65 ± 0.14, 0.65 ± 0.14, and 0.85 ± 0.09 for EVLWi, VD/VT, PaO2/FiO2, and EDI, respectively. An EVLWi cutoff value of 11.5 ml/kg PBW on day one predicted progression to ALI on the first day with 57% sensitivity and 100% specificity. An EVLWi > 10 mL/kg PBW predicted progression to ALI with a sensitivity of 63%, and a specificity of 88%, positive predictive value 83%, negative predictive value 70%. The predictive value of EVLW improved on the second day with a value of 15.3 ml/kg PBW predicting progression to ALI with 63% sensitivity and 100% specificity.
Figure 2.
Receiver operator characteristic curves for extravascular lung water indexed for predicted body weight (EVLWi), PaO2/FiO2, and pulmonary dead space-tidal volume fraction (VD/VT) on days 1 and 2. For day 1, the areas under the curves were 0.75 ± 0.13, 0.71 ± 0.13, and 0.57 ± 0.14 for EVLWi, PaO2/FiO2, and VD/VT, respectively. For day 2, the areas under the curves were 0.82 ± 0.11, 0.65 ± 0.14, and 0.65 ± 0.14 for EVLWi, PaO2/FiO2, and VD/VT, respectively.
Figure 3.
Receiver operator characteristic curves for extravascular lung water indexed for predicted body weight (EVLWi), extravascular lung water – dead space tidal volume fraction (EDI), and dead space-tidal volume fraction (VD/VT) on days 1 and 2. For day 1, the areas under the curves were 0.75 ± 0.13, 0.77 ± 0.12, and 0.57 ± 0.14 for EVLWi, EDI, and VD/VT, respectively. For day 2, the areas under the curves were 0.82 ± 0.11, 0.85 ± 0.09, and 0.65 ± 0.14 for EVLWi, EDI, and VD/VT, respectively.
DISCUSSION
This study has shown that elevated EVLWi is a common feature of early ALI and that it predicts progression to ALI in patients at increased risk to develop it.
EVLWi predicts progression to ALI in patients at risk to develop it better than all markers of disease severity tested. Furthermore EVLWi predicted progression to ALI 2.6 days before these patients met AECC criteria for it. EVLWi has previously been shown to be a sensitive marker of disease severity in ALI, and to be associated with the degree of endothelial and epithelial injury and excess lung weight on post mortem examination (15, 16, 23, 35) in patients with pulmonary edema. Mean EVLWi was as elevated in those who progressed to ALI but did not yet meet AECC criteria as in those with ALI on the first day of the study. This supports the idea that EVLWi reflected injury that was present on the first day in those that went on to develop ALI and that EVLWi is more sensitive than the current AECC criteria to detect it. These 2.6 ± 0.3 days may then represent missed opportunity for therapeutic intervention such as initiation of lung protective ventilation and improvement of outcome.
Other markers of disease severity fail to predict progression to ALI
Physiologic dead space fraction has been found to predict mortality in ALI (13), but we have shown that it does not predict progression to ALI in those patients at risk.
A PVPI ≥ 3 has been has been found to discriminate between hydrostatic and non hydrostatic pulmonary edema with a 85% sensitivity and 100% specificity (38). But we have found that this value does not discriminate between those with ALI and those without ALI. Only 2 of our 19 patients with ALI had a PVPI ≥ 3. Therefore, a PVPI ≥ 3 does not appear to be useful in defining ALI. It is unclear if the PVPI should be used to predict progression to ALI, above and beyond simply measuring the EVLWi. The PVPI is not a measure of vascular permeability but rather an attempt to normalize lung water to differences in pulmonary blood volume (PVPI = EVLW/PBV). Since pulmonary blood volume was not significantly different among the various subgroups it is unclear if the PVPI would have better predicted progression to ALI had there been differences. As there was no difference in PBV we would expect the PVPI to have a similar predictive value for progression to ALI as EVLWi, as we found. Further studies are needed that include patients with varying PBV to more fully examine this.
The PaO2/FiO2 did not discriminate between patients that progressed to ALI and those that did. This is not surprising as the PaO2/FiO2 is confounded by variations in FiO2, ventilator mode, mean airway pressure, and PEEP and has not been found to be a sensitive indicator of lung injury or a reliable predicator of outcome in numerous studies (15, 39, 40). The Murray Lung Injury Score did discriminate between those that progressed to ALI and those that did not. However this did not reach statistical significance until day 2. Furthermore, treatment strategies utilizing EVLWi as a hemodynamic and preload metric have been previously shown to improve outcome in this disease (41, 42). It is unlikely the LIS could be similarly incorporated into such a treatment strategy.
The extravascular lung water physiologic dead space fraction product (EDI)
Increased EVLWi is believed to result from both changes in permeability and intravascular fluid extravasation, epithelial injury, and alveolar fluid clearance resulting in excess lung water. An increase in VD/VT in ARDS is believed to be due in part to endothelial injury, platelet activation and microthrombus formation. This injury tends to occur later in the disease process. We postulated that VD/VT and EVLW might then be combined to give a better indicator of overall lung injury – especially later in the disease – when EVLWi alone may lose sensitivity. We examined this by testing the ability of VD/VT to discriminate between those that progressed to ALI and those that did not on the first 5 days of the study. We combined EVLW and VD/VT as a simple product (EVLWi * VD/VT,) to give equal weight to both measures. We then compared VD/VT, PaO2/FiO2, EVLWi, and EDI in terms of their abilities to discriminate progression to ALI in those at risk in a receiver operator characteristic (ROC) curve. The area under the curve was essentially the same as EVLWi on day 1 but was greater by day 2 (Fig 3). In this preliminary study the small number of patients in each of our subgroups group impairs statistical comparison of these curves but examination of the same data over the course of the first three days (Fig. 1) illustrated excellent separation of those that progressed to ALI vs. those that did not using the EDI . Of note there were no significant differences in VD/VT, between those that progressed to ALI vs. those that did not in our study. Larger studies are needed to examine if differences in VD/VT occur among the various subgroups at risk for ALI and whether this would increase the trend for improved detection of injury, disease severity, and prediction to ALI using the EDI as compared to EVLWi alone.
The American-European definition of ALI is insensitive for detecting lung injury
Among the patients who did not meet clinical criteria for ALI at the time of enrollment who then progressed to ALI, 2 of the 8 had chest radiographs that were consistent with ALI on day 1, but the PaO2/FiO2 were greater than 300 at that time (Table 2). The remaining 6 patients developed pulmonary edema on chest radiograph on day 2. Three of these patients did not meet PaO2/FiO2 criteria until day 3, one until day 4. In patients who went on to develop ALI, EVLWi was elevated on day 1 and remained elevated an average of 2.6 days before the patients met consensus criteria for ALI. This would seem to indicate that lung injury was present at presentation in those that progressed to ALI and that EVLWi is a sensitive indicator of that injury. Whether this represents a therapeutic window in which to improve outcome will require a large interventional trial to discern. But it is clear from this and preceding studies that EVLWi is a sensitive marker of lung injury, better predicts progression to ALI, and thus may be a more sensitive marker of lung injury than criteria presently used in our definition of ALI. Therefore, perhaps we should ask: do we need to change the definition of ALI and should we consider including measured EVLWi?
Table 2.
Patients who progressed to ALI
| Age (years), Gender | Day of qualifying radiograph |
Day of qualifying PaO2/FiO2 |
Day all criteria for ALI met |
|---|---|---|---|
| 55, M | Day 2 | Day 3 | Day 3 |
| 65, M | Day 1 | Day 2 | Day 2 |
| 86, M | Day 1 | Day 2 | Day 2 |
| 59, F | Day 2 | Day 3 | Day 3 |
| 37, F | Day 2 | Day 1 | Day 2 |
| 72, F | Day 2 | Day 2 | Day 2 |
| 64, F | Day 2 | Day 3 | Day 3 |
| 50, F | Day 2 | Day 4 | Day 4 |
The amount of EVLWi results from a dynamic balance between factors causing fluid to enter the lung vs. factors that carry the fluid away. Changes in vascular permeability, hydrostatic pressure, oncotic pressure, white blood cell emigration and inflammation, alveolar epithelial injury and alveolar fluid clearance, disruption in the architecture of the interstitial matrix, and lymph clearance all contribute to changes in EVLWi. Changes in all of these can and do occur in ALI; it is not surprising then that in the appropriate clinical setting elevated EVLWi has been found to be a highly sensitive marker of lung injury, and predicts progression to and outcome in, ALI.
It is our opinion that the AECC criteria should be revisited, that more mechanistic criteria should be incorporated in the definition, and that EVLW may have a role to play. People with heart failure can develop ALI. Sepsis related ALI can result in cardiomyopathy and acute heart failure. The two conditions are not mutually exclusive. Furthermore, the PaO2/FiO2 ratio is non-linear at a FiO2 >0.6, is confounded by changes in PEEP and mean airway pressure.
This study has shown that elevated EVLWi is a common feature of ALI, that it detects the presence of lung injury early and predicts progression to ALI some 2.6 days before current criteria are met. Our study suggests that this is because EVLWi is a more sensitive indicator of the presence of lung injury. Given these findings, bedside determination of EVLWi may have a role in helping to better define ALI. EVLWi can be elevated (EVLWi > 7ml/kg) in patients who may not have severe enough lung injury to warrant lung protective ventilation and other therapeutic interventions for ALI. We propose using that value that we have found best predicts progression to ALI, an EVLW > 10ml/kg, in a definition that also includes consideration of the clinical setting.
The late Dr. Daniel Schuster has said, "The diagnosis of ARDS (ideally) should not be a diagnosis of exclusion but instead depend on some direct measure of lung injury." He thought measured vascular permeability might be the desired direct measure of injury. Since that time we and others have found that bedside determination EVLWi is a much simpler measurement to perform as well as being a highly sensitive marker of injury, disease severity, and predictor of outcome. The findings of our current study need to be confirmed in larger studies before we may consider using EVLWi in a new definition for ALI. But if confirmed, what would such a definition of ALI that incorporates EVLWi look like? Here we present one idea that borrows heavily from Dr Shuster's approach while using EVLWi as the direct measure of lung injury.
An appropriate clinical setting - sepsis, severe trauma, pneumonia, massive transfusion, etc.
Bilateral chest radiograph opacities consistent with pulmonary edema.
EVLWi > 10ml/kg
We include the chest radiograph because our data show that all patients who progressed to ALI had chest radiograph findings consistent with ALI no later than day two. It was the PaO2/FiO2 that most delayed meeting criteria for ALI.
Aggressive fluid resuscitation may be detrimental
While there was no statistically significant difference in fluid balance between individuals who developed ALI and those who did not, there was a trend toward higher fluid balance in the group that developed ALI. In fact, the daily fluid balance was 2–4 liters greater in the group that developed ALI versus those that did not. It is unlikely that the individuals who developed ALI received more fluid because they were "sicker" as the APACHE II and SAPS scores were not different between the two groups. This perhaps suggests that employing a fluid restrictive strategy earlier may have been beneficial.
Limitations
This preliminary study was limited by small numbers of patients in the subgroups. Thus we could not determine if elevated EVLWi is an independent risk factor for progression to ALI in all patients at risk to develop it and larger studies are needed to discern this. Despite being blinded to the study results there was large interobserver variability in chest radiograph determinations, and this may have influenced the study results. However the large inter-observer variability is in line with those previously reported and with clinical practice.
CONCLUSIONS
Elevated EVLWi is a common feature of early ALI and discriminates between those with ALI and those without. EVLWi predicts progression to ALI in patients at risk to develop it 2.6 ± 0.3 days before the patients met AECC criteria for it. EVLWi was similarly elevated in those with ALI as in those that progressed to it, but significantly less in those that never developed it, supporting the idea that significant lung injury was present in those that went on to develop ALI and that EVLWi is more sensitive than the current AECC criteria in detecting it. These 2.6 ± 0.3 days may then represent missed opportunity for therapeutic intervention to limit ventilator associated lung injury and improve outcome.
There is a trend toward greater predictive value in identifying patients at risk of developing ALI using a new index the EDI (VD/VT * EVLW). Larger studies are needed to determine if elevated EVLWi and EDI are independent risk factors for progression to ALI in all patients at risk to develop it and whether initiation of lung protective ventilator strategies and/or goal-directed fluid management based on early changes in EVLWi or EDI improves outcome.
ACKNOWLEDGEMENTS
We acknowledge the contribution of the patients and their families in allowing us to conduct this research, as well as the physicians, nurses, and support staff in our intensive care units, who made this project possible.
This research was supported by the Medical Research Foundation of Oregon and, in part, by a grant from the Center of Excellence in Human Research, Oregon Opportunity Funds, Oregon Health & Science University, Portland Oregon and support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Grant support: Medical Research Foundation, Oregon Health &Science University, Portland OR; Center of Excellence in Human Research, Oregon Opportunity Funds, Oregon Health & Science University, Portland Oregon and support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
This study was funded, in part, by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Phillips consulted for and received honoraria/speaking fees from MAB. The remaining authors have not disclosed any potential conflicts of interest.
REFERENCES
- 1.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Eaton S, Mealer M, et al. Extravascular lung water in patients with severe sepsis: A prospective cohort study. Crit Care. 2005;9:R74–R82. doi: 10.1186/cc3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips CR, Smith SM. Predicted body weight-indexed extravascular lung water is elevated in acute respiratory distress syndrome. Crit Care Med. 2009;37:377–378. doi: 10.1097/CCM.0b013e31818f29d5. [DOI] [PubMed] [Google Scholar]
- 4.Davey-Quinn A, Gedney JA, Whiteley SM, et al. Extravascular lung water and acute respiratory distress syndrome--oxygenation and outcome. Anaesth Intensive Care. 1999;27:357–362. doi: 10.1177/0310057X9902700404. [DOI] [PubMed] [Google Scholar]
- 5.Rubenfeld GD, Caldwell E, Granton J, et al. Interobserver variability in applying a radiographic definition for ards*. Chest. 1999;116:1347–1353. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 6.Meade MO, Cook RJ, Guyatt GH, et al. Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:85–90. doi: 10.1164/ajrccm.161.1.9809003. [DOI] [PubMed] [Google Scholar]
- 7.Gowda MS, Klocke RA. Variability of indices of hypoxemia in adult respiratory distress syndrome. Crit Care Med. 1997;25:41–45. doi: 10.1097/00003246-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Whiteley JP, Gavaghan DJ, Hahn CE. Variation of venous admixture, sf6 shunt, pao2, and the pao2/fio2 ratio with fio2. Br J Anaesth. 2002;88:771–778. doi: 10.1093/bja/88.6.771. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson ND, Kacmarek RM, Chiche JD, et al. Screening of ards patients using standardized ventilator settings: Influence on enrollment in a clinical trial. Intensive Care Med. 2004;30:1111–1116. doi: 10.1007/s00134-004-2163-2. [DOI] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, et al. Apache ii: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 11.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (saps ii) based on a european/north american multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 12.Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 13.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 14.Seeley E, McAuley DF, Eisner M, et al. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63:994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips CR, Chesnutt MS, Smith SM. Extravascular lung water in sepsis-associated acute respiratory distress syndrome: Indexing with predicted body weight improves correlation with severity of illness and survival. Crit Care Med. 2008;36:69–73. doi: 10.1097/01.CCM.0000295314.01232.BE. [DOI] [PubMed] [Google Scholar]
- 16.Sakka SG, Klein M, Reinhart K, et al. Prognostic value of extravascular lung water in critically ill patients. Chest. 2002;122:2080–2086. doi: 10.1378/chest.122.6.2080. [DOI] [PubMed] [Google Scholar]
- 17.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 18.Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz M, Keegan MT, Iscimen R, et al. Toward the prevention of acute lung injury: Protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med. 2007;35:1660–1666. doi: 10.1097/01.CCM.0000269037.66955.F0. quiz 1667. [DOI] [PubMed] [Google Scholar]
- 20.Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: A randomized controlled trial. JAMA. 2010;304:2620–2627. doi: 10.1001/jama.2010.1796. [DOI] [PubMed] [Google Scholar]
- 21.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: A preventive randomized controlled trial. Crit Care. 2010;14:R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirov MY, Kuzkov VV, Kuklin VN, et al. Extravascular lung water assessed by transpulmonary single thermodilution and postmortem gravimetry in sheep. Crit Care. 2004;8:R451–R458. doi: 10.1186/cc2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig TR, Duffy MJ, Shyamsundar M, et al. Extravascular lung water indexed to predicted body weight is a novel predictor of intensive care unit mortality in patients with acute lung injury. Crit Care Med. 2010;38:114–120. doi: 10.1097/CCM.0b013e3181b43050. [DOI] [PubMed] [Google Scholar]
- 24.Berkowitz DM, Danai PA, Eaton S, et al. Accurate characterization of extravascular lung water in acute respiratory distress syndrome. Crit Care Med. 2008;36:1803–1809. doi: 10.1097/CCM.0b013e3181743eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard GR, Artigas A, Brigham KL, et al. The american-european consensus conference on ards. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 26.Rossi P, Wanecek M, Rudehill A, et al. Comparison of a single indicator and gravimetric technique for estimation of extravascular lung water in endotoxemic pigs. Crit Care Med. 2006;34:1437–1443. doi: 10.1097/01.CCM.0000215830.48977.29. [DOI] [PubMed] [Google Scholar]
- 27.Katzenelson R, Perel A, Berkenstadt H, et al. Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit Care Med. 2004;32:1550–1554. doi: 10.1097/01.ccm.0000130995.18334.8b. [DOI] [PubMed] [Google Scholar]
- 28.Tagami T, Kushimoto S, Yamamoto Y, et al. Validation of extravascular lung water measurement by single transpulmonary thermodilution: Human autopsy study. Crit Care. 2010;14:R162. doi: 10.1186/cc9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuzkov VV, Kirov MY, Sovershaev MA, et al. Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury. Crit Care Med. 2006;34:1647–1653. doi: 10.1097/01.CCM.0000218817.24208.2E. [DOI] [PubMed] [Google Scholar]
- 30.Bognar Z, Foldi V, Rezman B, et al. Extravascular lung water index as a sign of developing sepsis in burns. Burns. 2010;36:1263–1270. doi: 10.1016/j.burns.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Giraud R, Siegenthaler N, Park C, et al. Transpulmonary thermodilution curves for detection of shunt. Intensive Care Med. 2010;36:1083–1086. doi: 10.1007/s00134-010-1876-7. [DOI] [PubMed] [Google Scholar]
- 32.Benington S, Ferris P, Nirmalan M. Emerging trends in minimally invasive haemodynamic monitoring and optimization of fluid therapy. Eur J Anaesthesiol. 2009;26:893–905. doi: 10.1097/EJA.0b013e3283308e50. [DOI] [PubMed] [Google Scholar]
- 33.Joshi D, Sizer E, Bernal W, et al. High values of intrathoracic blood volume in hepatopulmonary syndrome. Crit Care Resusc. 2009;11:129–131. [PubMed] [Google Scholar]
- 34.Lubrano R, Cecchetti C, Elli M, et al. Prognostic value of extravascular lung water index in critically ill children with acute respiratory failure. Intensive Care Med. 2010 doi: 10.1007/s00134-010-2047-6. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Mondejar E, Rivera-Fernandez R, Garcia-Delgado M, et al. Small increases in extravascular lung water are accurately detected by transpulmonary thermodilution. J Trauma. 2005;59:1420–1423. doi: 10.1097/01.ta.0000198360.01080.42. discussion 1424. [DOI] [PubMed] [Google Scholar]
- 36.Fagon JY, Chastre J, Wolff M, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132:621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- 37.Kallet RH, Daniel BM, Garcia O, et al. Accuracy of physiologic dead space measurements in patients with acute respiratory distress syndrome using volumetric capnography: Comparison with the metabolic monitor method. Respir Care. 2005;2005(50):462–467. [PubMed] [Google Scholar]
- 38.Monnet X, Anguel N, Osman D, et al. Assessing pulmonary permeability by transpulmonary thermodilution allows differentiation of hydrostatic pulmonary edema from ali/ards. Intensive Care Med. 2007;33:448–453. doi: 10.1007/s00134-006-0498-6. [DOI] [PubMed] [Google Scholar]
- 39.Krafft P, Fridrich P, Pernerstorfer T, et al. The acute respiratory distress syndrome: Definitions, severity and clinical outcome. An analysis of 101 clinical investigations. Intensive Care Med. 1996;22:519–529. doi: 10.1007/BF01708091. [DOI] [PubMed] [Google Scholar]
- 40.Doyle RL, Szaflarski N, Modin GW, et al. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med. 1995;152:1818–1824. doi: 10.1164/ajrccm.152.6.8520742. [DOI] [PubMed] [Google Scholar]
- 41.Eisenberg PR, Hansbrough JR, Anderson D, et al. A prospective study of lung water measurements during patient management in an intensive care unit. Am Rev Respir Dis. 1987;136:662–668. doi: 10.1164/ajrccm/136.3.662. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell JP, Schuller D, Calandrino FS, et al. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis. 1992;145:990–998. doi: 10.1164/ajrccm/145.5.990. [DOI] [PubMed] [Google Scholar]