Abstract
Background
Hepatitis B virus-related acute liver failure (HBV-ALF) may occur following acute HBV infection (AHBV-ALF) or during an exacerbation of chronic HBV infection (CHBV-ALF). Clinical differentiation of the two is often difficult if a prior history of hepatitis B is not available. Quantitative measurements of anti-hepatitis B core immunoglobulin M (IgM anti-HBc) titers and of HBV viral loads (VLs) might allow separation of acute from chronic HBV-ALF.
Methods
Of 1602 patients with ALF, 60 met clinical criteria for AHBV-ALF and 27 for CHBV-ALF. Sera were available on 47 and 23 patients, respectively. A quantitative immunoassay was used to determine IgM anti-HBc levels, and real-time polymerase chain reaction (rtPCR) to determine HBV VLs.
Results
AHBV-ALFs had much higher IgM anti-HBc titers than CHBV-ALFs, (signal to noise (S/N) ratio median 88.5, range 0–1,120, vs. 1.3, 0–750, p<0.001); a cut point for S/N ratio of 5.0 correctly identified 44/46 (96%) AHBV-ALFs and 16/23 (70%) CHBV-ALFs; the area under the receiver operator characteristic curve was 0.86, p<0.001. AHBV-ALF median admission VL was 3.9 (0–8.1) log10 IU/mL, vs. 5.2 (2.0–8.7) log10 IU/mL for CHBV-ALF, p<0.025. Twenty percent (12/60) of the AHBV-ALF group had no hepatitis B surface antigen (HBsAg) detectable on admission to study, while no CHBV-ALF patients experienced HBsAg clearance. Rates of transplant-free survival were 33% (20/60) for AHBV-ALF vs. 11% (3/27) for CHBV-ALF, p=0.030.
Conclusions
AHBV-ALF and CHBV-ALF differ markedly in IgM anti-HBc titers, in HBV VLs and in prognosis, suggesting that the two forms are indeed different entities that might each have a unique pathogenesis.
Keywords: antibody levels, adaptive immune response, polymerase chain reaction, acute hepatitis
Introduction
Hepatitis B virus-related acute liver failure constitutes 1% of those experiencing acute or chronic hepatitis B (1, 2). Patients who have acute HBV infection (AHBV-ALF) as well as those with an acute exacerbation (disease flare) of chronic HBV (CHBV-ALF) cannot be distinguished on clinical grounds without historical or histological evidence for chronicity which may be lacking in acutely ill patients. CHBV-ALF may occur spontaneously or due to the effect of immunosuppression on viral replication and immunity (1, 2). We postulated that serological or virological factors might better separate acute infections from acute exacerbations of chronic disease when they presents as acute liver failure, since the immune pathogenesis of each may be somewhat different.
During the natural history of hepatitis B infection, the immune response and the degree of liver injury as exemplified by aminotransferase levels are considered to be roughly inversely proportional to HBV viral loads (VLs) which vary widely from over a billion copies in immune tolerant patients, to barely detectable or negative in inactive carriers (3–5). A strong adaptive immune response results in rapid clearance of HBsAg and early detection of antibodies to HBsAg (anti-HBs) in some patients with HBV-ALF (6). In support of this, low or undetectable HBV VLs or HBsAg can be seen in about 20% of such patients (1,7). By contrast, in chronic hepatitis B infection, accompanied by immunosuppression, the virus may become directly cytopathic while liver injury in chronic infected patients who are not immunosuppressed is still presumed to be immune-mediated (8).
The detection of IgM anti-HBc measured by enzyme-linked immunosorbent assay (ELISA) is critical in differentiating acute from chronic HBV infection. However, patients with chronic hepatitis B sometimes demonstrate IgM anti-HBc positivity (9.10). Previous semi-quantitative assays described the longitudinal changes in IgM anti-HBc, but no studies have provided direct assessments of IgM anti-HBc quantitation in patients with ALF (11). Use of a semi-quantitative IgM anti-HBc ELISA, rather than a single cut-off value, might better distinguish AHBV-ALF from CHBV-ALF. In addition, measurement of viral loads across a wide dynamic range has not been studied extensively and might provide a second tool to separate AHBV-ALF from CHBV-ALF.
In the present study, we classified a large group of patients all of whom met criteria for HBV-related ALF, separating them on historical and clinical grounds into either AHBV-ALF or CHBV-ALF. We then determined whether quantitative measurement of IgM anti-HBc or HBV VLs (or a ratio combining the two) performed in blinded fashion could help to distinguish between AHBV-ALF and CHBV-ALF.
Materials and Methods
Patients
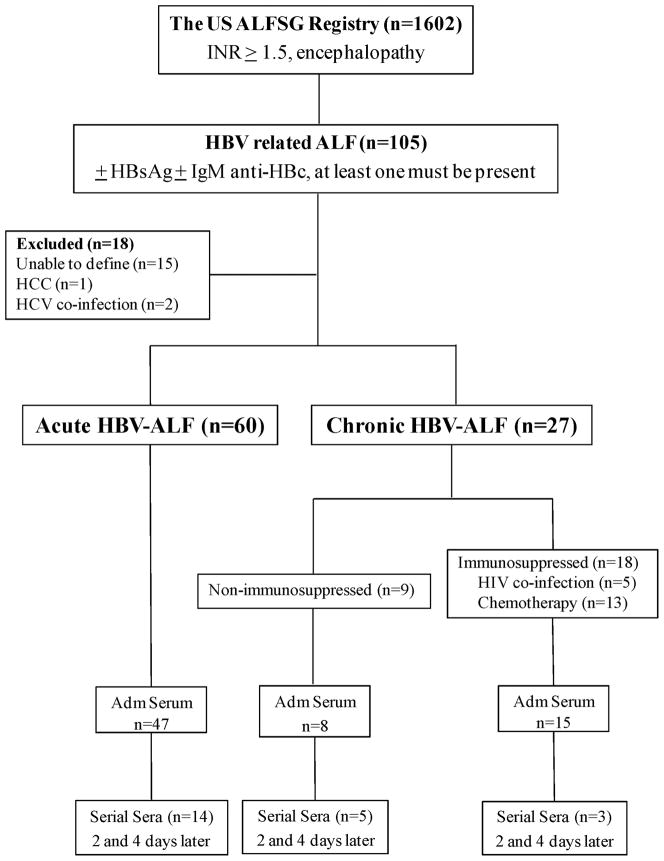
Between January 1998 and December 2009, 23 sites in the US ALF Study Group enrolled 1,602 patients with acute liver failure (ALF) comprising all etiologies, to study in a prospective fashion their clinical characteristics and outcomes. The definition of ALF included severe acute liver injury without known cirrhosis, with a duration of illness of <26 weeks accompanied by hepatic encephalopathy and coagulopathy (prothrombin time ≥15 seconds or international normalized ratio (INR) ≥1.5) (12); 105 patients were screened and 87 patients with HBV-ALF met criteria for enrollment as outlined in Figure 1. All patients were either IgM anti-HBc positive or HBsAg positive or both; 12 HBsAg negative/IgM anti-HBc positive patients were considered to represent early viral clearance (7). The clinical distinction between AHBV-ALF and CHBV-ALF was made after careful review of each case report form by one of us (WML) using specific criteria from the clinical history. Chronic patients either had a known history of having chronic disease (prior evidence of hepatitis B at least 6 months before admission to study) or, in the setting of immunosuppression or HIV infection acute liver failure was assumed to represent chronic infection. The AHBV-ALF group was characterized by age < 50 plus high risk behaviors: injection drug use, multiple sex partners or sex with a known hepatitis B carrier; in the absence of chronic disease or high risk behavior, older age (>50 years) and Asian ethnicity was deemed to indicate chronicity. Fifteen patients could not be characterized using these criteria. The reviewer was unaware of viral loads or quantitative IgM anti-HBc levels when the adjudication was made. Standard molecular analyses using polymerase chain reaction followed by standard consensus sequencing were used for viral genotyping (n=71) and determining the presence of HBeAg negative mutations (n=68).
Figure 1. Study schema.
Of the 1,602 acute liver failure (ALF) patients in the US ALF Study Group, there were 105 hepatitis B virus-related ALF (HBV-ALF) patients identified. Eighteen were excluded: 2 co-infected with hepatitis C virus, one determined to have hepatocellular carcinoma and 15 whom we were unable to define as either acute or chronic. Sixty were identified as AHBV-ALF of whom 47 had sera collected on admission and 14 had sera collected serially up to the 4th day. Twenty-seven patients were identified as CHBV-ALF; 9 appeared to show spontaneous exacerbation (non-immunosuppressed CHBV-ALF) and 18 were considered immunosuppressed; admission and serial sera over 4 days in these groups are also listed.
Since patients by definition were encephalopathic, informed consent was obtained from next of kin prior to the study enrollment. The study was approved by the local Institutional Review Board at each site. Detailed demographics, clinical and outcome data of all HBV-related ALF patients were available from the coordinating center, the University of Texas Southwestern Medical Center (UTSWMC) at Dallas. Serum samples were collected serially for up to 7 days following admission to study and were stored at –80°C prior to retrieval from the coordinating site for the study. Admission sera used for VL and IgM anti-HBc determinations were considered to be the first available serum samples after enrollment in the study (Figure 1). Spontaneous survival indicated survival without transplantation whereas overall survival includes all those surviving at 3 weeks after admission to study regardless of transplantation.
Laboratory Testing
Measurement of IgM anti-HBc titers
IgM anti-HBc titers were measured using the ADVIA®Centaur™ IgM Anti-HBc assay, Siemens Diagnostics, Tarrytown NY. Briefly, this assay is an indirect IgM capture immunoassay using a 2-step format including biotinylated anti-human IgM and a solid phase containing streptavidin-coated microparticles. An index value of ≥1 is considered to be reactive, 0.8–0.99 is a “gray zone” which requires re-testing, and <0.8 is non-reactive. Each serum sample of 200 μl was run according to assay instructions to determine the index value (signal to noise (S/N) ratio). Sample results that were beyond the dynamic range of 9.0 (index value range 0.05 – 9.0) were serially diluted with a 1:10 dilution using pooled serum that had been tested for HBc IgM antibody and found negative. The final index value was multiplied by the appropriate dilution factor to give a final “calculated” index result.
Quantification of HBV VL by real time polymerase chain reaction (rtPCR)
Sera collected in serial fashion on days 1 through 4 after admission to study were quantified using an established rtPCR protocol (13). Each serum sample was run in triplicate and the median value was selected. Viral DNA extracted from serum was amplified and quantified in a 7900HT Fast Real-Time PCR System. The dynamic range of the assay was 25 to 2×107 IU/mL.
Statistical analyses
Statistical analyses were performed using IBM® SPSS® Statistics 19.0 (SPSS Inc, Chicago, IL USA), SAS 9.2 (SAS Institute Inc., Cary, NC USA), and StatXact V8 (Cytel Inc., Cambridge, MA USA). The Mann-Whitney U Test was used to compare groups (AHBV-ALF versus all CHBV-ALF or only the non-immunosuppressed CHBV-ALF group) on the continuous measures including HBV viral load (VL) and IgM anti-HBc levels. Chi-square or Fisher’s Exact tests (when appropriate) were employed to compare groups on the categorical measures. The receiver operating characteristic curve and the test for the area under this curve (AUROC) was used to describe the relationship between the true-positive rate and the false positive rate in the prediction of group membership (AHBV-ALF versus overall CHBV-ALF) using the continuous measures IgM anti-HBc levels and the ratio of IgM anti-HBc to VL; standard errors for areas were estimated using a nonparametric method and testing the null hypothesis of the true area equal to 0.50. A mixed model analysis of covariance (ANCOVA) was used to determine the effects of time (baseline, day 2, and day 4) on log10 VLs for AHBV-ALF versus CHBV-ALF with subject at each time point treated as a random effect. Covariates considered in this model were patient’s age, admission IgM anti-HBc levels, and use of nucleoside (tide) analogue (NA); covariates remained in the model if p<0.15. Logistic regression analysis was employed to predict AHBV-ALF versus CHBV-ALF using admission log10 VLs, admission IgM anti-HBc titers, admission HBsAg status, patient age, and HBV genotypes (A, B, and C) as covariates; Hosmer-Lemeshow p values were provided to demonstrate the fit of the model to the data. AUROC analysis was used to examine the predictions made by the logistic regression analysis in distinguishing between the AHBV-ALF and CHBV-ALF groups. For all statistical tests, a p value < 0.05 was considered statistically significant unless otherwise stated. Viral load was expressed as median (range) (log10 IU/mL), unless otherwise stated.
Results
Analysis of the two groups: AHBV ALF and CHBV ALF
Initially, data on 105 patients were available: we excluded two HBV+HCV co-infected patients, one patient whose liver biopsy later revealed extensive hepatocellular carcinoma and 15 who could not be characterized as to their acute or chronic status (Figure 1). Comparing these 15 unclassified cases to the remaining 87, race (p=0.223) and gender (p=0.576) were not different, however the median age (range) for the 15 not included was significantly older than those that remained in the analysis (55.5 (40–69) versus 41 (17–71) respectively, p=0.001). Thus, 60 met criteria for the acute (AHBV-ALF) group and 27 for the CHBV-ALF group. No patient in either group was co-infected with hepatitis A, D or E viruses using standard tests. Liver histology, available for 31 of the overall group did not show evidence of cirrhosis in any patient.
The 27 patients within the overall CHBV-ALF group included 14 who were known to have chronic hepatitis B. Nine experienced spontaneous acute exacerbation (unexplained and without immunosuppression) while the remaining 18 experienced immunosuppression-related ALF: 13 had received either chemotherapy for leukemia or lymphoma (n=9), or corticosteroids for Crohn’s disease, asthma, Guillain-Barre syndrome and unknown (one each); 5 had concomitant HIV infection. Of interest, 6 of the 18 immunosupressed patients were unaware of their diagnosis of chronic hepatitis B at the time of presentation. The remaining 9 CHB-ALF patients with apparent acute on chronic disease included 5 known to have chronic hepatitis B. Among the 60 that were categorized as having true acute hepatitis B, 23 had a history of injection drug use (only), 19 had a history of high-risk sexual behavior or sex with a known hepatitis B individual; in 5 both risk factors were positive.
In general, clinical and laboratory features such as length of illness and International Normalized Ratio (INR) levels, aspartate aminotransferase (AST) levels, bilirubin, and creatinine did not differ between the 60 patients considered to have AHBV ALF and the 27 in the overall CHBV ALF group (Table 1). However, alanine aminotransferase (ALT) levels and albumin levels were higher in the AHBV-ALF patients than the CHBV-ALF patients. There were few apparent differences in virologic or host features found between the two chronic subgroups (9 non-immunosuppressed vs. 18 patients with immunosuppression), although viral loads at admission were lower in the non-immunosuppressed group. For the remaining statistical analyses, we combined the CHBV ALF subgroups except where noted.
Table 1.
Demographic and Baseline Characteristics of the Study Patients*
| Characteristics | AHBV-ALF (n=60) | CHBV-ALF (n=27) | P value# | CHBV-ALF-non-immunosuppressed (n=9) | P value¥ |
|---|---|---|---|---|---|
| Age (yr) | 36 (17–64) | 53 (33–71) | <0.001 | 60 (46–69) | <0.001 |
| Race | |||||
| African American | 37 (22/60) | 22 (6/27) | <0.001 | 0 | <0.001 |
| Asian | 3 (2/60) | 52 (14/27) | 67 (6/9) | ||
| Caucasian | 52 (31/60) | 22 (6/27) | 22 (2/9) | ||
| Other | 8 (5/60) | 4 (1/27) | 11 (1/9) | ||
| Female Sex | 47 (28/60) | 37 (10/27) | 0.402 | 22 (2/9) | 0.281 |
| ALT (IU/L) | 1,954 (87–11,100) | 1,008 (94–7,856) | 0.018 | 826 (117–7,774) | 0.069 |
| AST (IU/L) | 1,024 (66–9,901) | 719 (78–8,615) | 0.248 | 530 (98–7,420) | 0.250 |
| Bilirubin (mg/dL) | 18.4 (3.6–62.4) | 20.2 (5.7–36.0) | 0.319 | 21.4 (7.5–30.4) | 0.392 |
| Albumin (g/dL) | 2.8 (1.7–4.0) | 2.5 (1.7–3.3) | 0.006 | 2.4 (2.0–3.3) | 0.056 |
| INR | 2.9 (1.2–20.1) | 3.2 (0.9–10.3) | 0.456 | 2.7 (0.9–9.4) | 0.679 |
| Hepatic Coma Grade | |||||
| I & II | 48 (29/60) | 41 (11/27) | 0.511 | 33 (3/9) | 0.489 |
| III & IV | 52 (31/60) | 59 (16/27) | 67 (6/9) | ||
| Creatinine (mg/dL) | 1.1 (0.4–8.6) | 1.1 (0.6–10.5) | 0.508 | 1.8 (0.6–6.6) | 0.269 |
| Days from Jaundice to Onset of Hepatic Coma | 5 (0–46) | 9.5 (0–28) | 0.277 | 13 (1–28) | 0.202 |
| HBsAg Negativity | 20 (12/60) | 0 (0/27) | 0.015 | 0 (0/9) | 0.342 |
| HBeAg Positivity | 26 (15/57) | 27 (7/26) | 0.954 | 11 (1/9) | 0.436 |
| Anti-HBs Positivity | 28 (17/60) | 15 (4/27) | 0.173 | 11 (1/9) | 0.428 |
| HBV Genotype | |||||
| A | 51 (24/47) | 29 (7/24) | 0.003 | 13 (1/8) | <0.001 |
| B | 9 (4/47) | 46 (11/24) | 88 (7/8) | ||
| C | 4 (2/47) | 8 (2/24) | 0 | ||
| D | 30 (14/47) | 17 (4/24) | 0 | ||
| Other | 6 (3/47) | 0 | 0 | ||
| HBV Basal Core Promoter Mutations (A1762T/G1764A) | 31 (15/48) | 25 (5/20) | 0.606 | 29 (2/7) | >0.999 |
| HBV Pre-Core Mutation (G1896A) | 21 (10/47) | 50 (10/20) | 0.019 | 86 (6/7) | 0.002 |
| HBV Pre-Core ± Basal Core | 40 (19/47) | 60 (12/20) | 0.141 | 86 (6/7) | 0.041 |
| Promoter Mutations | |||||
| HBV Viral Load - log10 (IU/mL) | 3.9 (0.0–8.1) | 5.2 (2.0–8.7) | 0.025 | 3.8 (2.5–8.7) | 0.982 |
| IgM Anti-HBc (Index value) | 88.5 (0–1,120) | 1.3 (0–750) | <0.001 | 1.9 (0–28.9) | <0.001 |
| N-acetylcysteine Treatment | 51 (30/59) | 15 (4/27) | 0.002 | 44 (4/9) | >0.999 |
| Acetaminophen Use Mentioned | 80 (28/35) | 60 (6/10) | 0.228 | 67 (2/3) | 0.519 |
| Alcohol Use | 43 (25/58) | 31 (8/26) | 0.285 | 33 (3/9) | 0.724 |
| Nucleoside (tide) Analogues Use | 53 (31/59) | 78 (21/27) | 0.026 | 89 (8/9) | 0.068 |
| Spontaneous Survival | 33 (20/60) | 11 (3/27) | 0.030 | 11 (1/9) | 0.258 |
| Overall 3-week Survival | 72 (43/60) | 44 (12/27) | 0.015 | 44 (4/9) | 0.132 |
Continuous data are shown as median (range) and categorical data are % (numerator/denominator).
Comparison between AHBV-ALF vs. Overall CHBV-ALF
Comparison between AHBV-ALF vs. CHBV-ALF without immunosuppression (CHBV-ALF)
Demographics and HBV genotypes
Certain important demographic and virologic characteristics differed between the two main groups: AHBV-ALF patients were younger (median, 36; range, 17–64 years old) than patients with CHBV-ALF (median, 53; range, 33–71 yrs, p<0.001, Table 1). The AHBV-ALF group was comprised mostly of Caucasians (31/60, 52%) and African Americans (22/60, 37%) whereas Asians accounted for only 2/60 (3%) of AHBV-ALFs. The AHBV-ALF group included primarily genotypes A, 24/47 (51%) and D, 14/47 (30%) with only 4 and 2 patients, respectively, classified as genotype B or C.
By contrast, the CHBV-ALF group included a much larger number of Asians (14/27, 52%) and, as expected, mainly genotypes B, 11/24 (46%) and A, 7/24 (29%). Mutations in the core promoter region alone did not differ between the two groups: AHBV-ALFs, 15/48 (31%) versus CHBV-ALFs, 5/20, (25%) p=0.606, while pre-core mutations were significantly more common in CHBV-ALF, 10/20 (50%) versus 10/47 (21%) of AHBV-ALFs, (p=0.019).
Admission titers and cut off value of IgM anti-HBc in AHBV-ALFs versus CHBV-ALFs
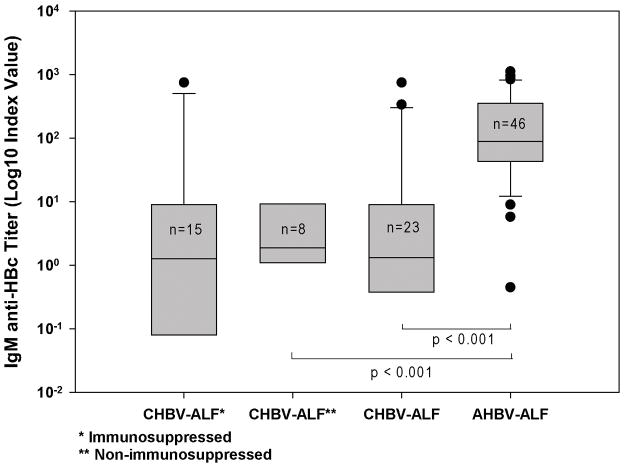
Ninety six percent (44/46) of the AHBV-ALF patients had positive IgM anti-HBc tests (index value ≥ 1.0), while 15/23 (65%) of those deemed to have CHBV-ALF tested positive for IgM anti-HBc. The admission IgM anti-HBc index values in AHBV-ALFs (n = 46; median, 88.5; range, 0–1,120) were significantly higher than those of the CHBV-ALF group (n=23; median, 1.3; range 0–750), p<0.001 (Figure 2). Among patients with CHBV-ALF, 70% (16/23) had index values <5. By contrast, 44/46 (96%) of AHBV-ALFs demonstrated IgM anti-HBc index values ≥5. Based on these data, the proposed cut off value of IgM anti-HBc to best differentiate AHBV-ALF from CHBV-ALF was 5.0. Using this cut off, the percent correct for the overall group was 87 with the area under the receiver operator characteristic curve (AUROC) of 0.86 (p < 0.001), curve not shown.
Figure 2. IgM anti-HBc levels for the various groups.
Admission IgM anti-HBc levels were much higher in AHBV-ALFs than in the overall CHBV-ALF group. Median IgM anti-HBc index value (signal/noise), on admission to hospital in the 46 AHBV-ALFs was 88.5 (0–1,120), significantly higher than that of the 23 overall CHBV-ALFs [1.30 (0–750), p<0.001], or the 8 non-immunosuppressed CHBV-ALFs [**], 1.9 (0–28.9), p<0.001]. The median (range) value for the 15 immunosuppressed CHBV-ALFs [*] was 1.27 (0–750). There was no difference observed between the two CHBV-ALF sub-groups.
Admission and serial HBV VLs in AHBV-ALF vs. CHBV-ALF
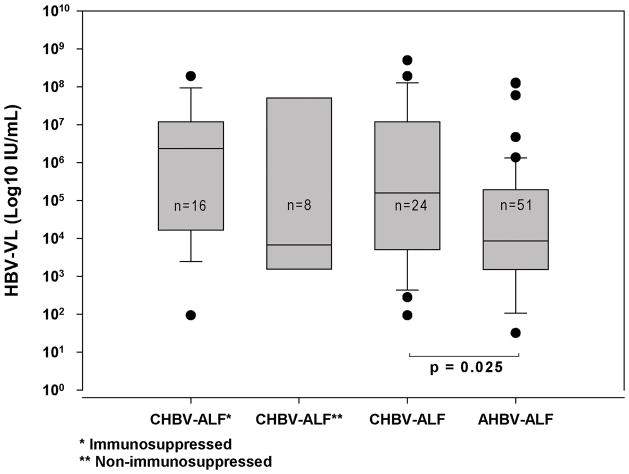
Patients with AHBV-ALF demonstrated lower admission log10 VLs (n=51; median, 3.9; range, 0–8.1 log10 IU/mL) than those in the overall CHBV-ALF group (n=24; median, 5.2; range, 2.0–8.7 log10 IU/mL, Figure 3); the difference in the median levels between the groups was between 1 and 2 logs. Of note, there was considerable overlap of admission VLs between AHBV-ALFs and CHBV-ALFs. There were 4 patients in the AHBV-ALF category who had undetectable admission VLs by our assay (LLD 25 IU/mL), compared to none with CHBV-ALF. A difference was observed in the viral loads for the two CHBV-ALF sub-groups in that the non-immunosuppressed group median viral load was similar to that of the acute group and less than that of the immunosuppressed group, as might be expected.
Figure 3. Hepatitis B viral loads for the various groups.
Median admission viral load (VL) in 51 patients with AHBV-ALF was 3.9 (0–8.1) log10 IU/mL, significantly lower than observed for the 24 patients in the overall CHBV-ALF group [5.2 (2.0–8.7) log10 IU/mL, p=0.025], but not for the 8 non-immunosuppressed CHBV-ALF patients [3.8 (2.5–8.7) log10 IU/mL, p=0.982]. The median (range) for the 16 in the immunosuppressed subgroup was 6.28 (1.97–8.28) log10 IU/mL. There were no significant differences in admission VLs between the two CHBV-ALF subgroups; horizontal line in each bar graph represents median VL.
Overall, high IgM anti-HBc and low VLs characterized the AHBV-ALF group, while the opposite was true of the CHBV-ALF subjects (Figures 2 and 3).
Changes in viral loads over time
The mean log10 VLs declined significantly for both groups at 3 time points over 4 days (p<0.001, Figure 4). With relatively low initial levels, the VLs in AHBV-ALF continued to decline and were consistently lower at all time points than the mean CHBV-ALF log values (p<0.001). The interaction between the two groups over time was non-significant (p=0.360) and the only covariate remaining in the ANCOVA model (p < 0.150) was admission IgM anti-HBc levels (p=0.137).
Figure 4. ANCOVA for VLs measured over time for AHBV-ALF and the overall CHBV-ALF groups adjusting for baseline IgM anti-HBc levels.
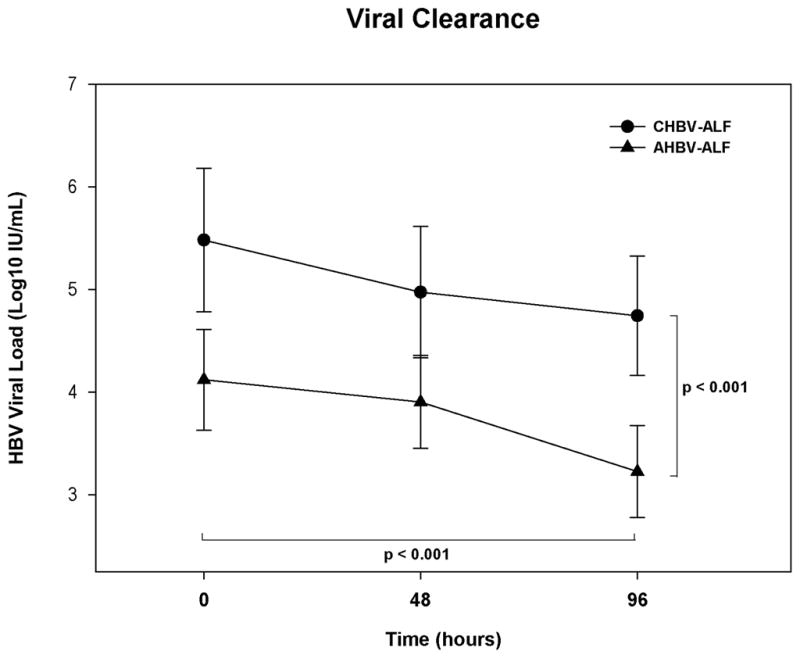
The decrease in VLs was significant for each of the two groups (p<0.001). VLs in AHBV-ALFs were consistently lower than in CHBV-ALFs at all time points (p<0.001). Admission IgM anti-HBc level was the only covariate that remained significant in the ANCOVA model (p=0.137). Error bars are 95% confidence intervals.
Ratio of IgM anti-HBc to HBV VL
The ratio of IgM anti-HBc to HBV VL calculated using admission values for each patient was significantly higher in AHBV-ALF (median 9.2 × 10−3, range 0–1.1), than in CHBV-ALF (median 1.0 × 10−5, range 0–2.0 × 10−2, p<0.001; AUROC 0.86, p > 0.001). Logistic regression analysis considering VLs, IgM anti-HBc titers, age, HBV genotypes (A, B, and D), and HBsAg status upon admission was employed to determine independent predictors of AHBV-ALF versus CHBV-ALF. Admission log10 VLs [p=0.022, OR (0.569), 95% CI (.352–.921)], IgM anti-HBc [p=0.005, OR (1.006), 95% CI (1.002–1.010)], and age [p<0.001, OR (0.855), 95% CI (0.788–0.929)] were independent determinants that distinguished the groups (Hosmer-Lemeshow p = 0.812; AUROC = 93%, p < 0.001). There were 3 ‘outlier’ patients in the CHBV-ALF group who exhibited high IgM anti-HBc titers of 248, 337, or 750 index values with correspondingly high HBV VLs on admission (1.21 × 103, 20.05 × 106, and 9.70 × 106). However, the ratios of IgM anti-HBc to HBV VLs were 2.04 × 10−2, 1.68 × 10−5, and 7.73 × 10−5 respectively, suggesting that they may indeed belong to the CHBV-ALF group. Two were receiving an immunosuppressive agent and the third had HIV co-infection.
HBsAg status and outcomes
All CHBV-ALF patients were HBsAg positive, whereas 20% (12/60) of AHBV-ALF had undetectable HBsAg on admission (p=0.015). Median IgM anti-HBc index values and log10 VL for the 12 HBsAg negative patients were 125 (17.2–840) and 3.55 (range 0–8.09) log10 IU/mL respectively. Spontaneous survival was significantly higher for AHBV-ALF patients (33%, 20/60) than for those with CHBV-ALF (11%, 3/27), p=0.030. Overall survival was higher for AHBV-ALF patients (72%, 43/60) than for those with CHBV-ALF (44%, 12/27), p=0.015. Admission HBeAg, Anti-HBs positivity, and coma grade were comparable between the two groups (Table 1). Follow-up (beyond 3 weeks) was available on 15/22 with AHBV-ALF who had survived without grafting. This group would be expected to clear HBsAg if they represented ‘true acute’ infections: 4 had died after 3 weeks, including 2 who had undergone liver grafting, 4 had cleared their infection as indicated by negative HBsAg at 3 weeks to 18 months following infection (no earlier visits for this patient). In 7, further follow up failed to include information on HBsAg clearance and 7 others were lost to follow up.
Discussion
This study sought to describe in more detail the clinical and immunological features of patients with acute or chronic hepatitis B-related ALF in relation to their serological features and molecular biology, since distinguishing between the two forms might well have clinical and pathogenetic significance. There were at least two distinct groups within the overall HBV-ALF cohort, based on history obtained and certain key clinical and histological features: one with newly acquired acute HBV infection leading to ALF (illness <6 months, usually <2–4 weeks) and one in which ALF had occurred in the setting of definite or presumed chronic disease. The source of confusion has been that the two forms resemble each other remarkably in clinical and biochemical features: apparent rapid onset of severe disease, advanced grades of encephalopathy, high aminotransferases and prolonged INRs, and thus cannot be distinguished readily without historical information of chronicity or, by contrast, of recent HBV exposure, both of which are often lacking. Although we had made initial assessments on overall gestalt, we revisited the data on each CRF using the algorithm described under Methods, including as primary data confirming CHBV-ALF with either prior knowledge of chronic hepatitis B infection, whether the patient was receiving chemotherapy or had HIV co-infection. Next we considered evidence of high risk behavior, age and ethnicity to complete the picture. This was not a 100% accurate profiling procedure since it was performed blindly with only clinical historical data, possible proof being that by this technique two with negative IgM anti-HBc were classified as being acute. However, at least 85% of our assessments are likely to be accurate based on these criteria. Among those misclassified might be, for example, a young patients with high risk behavior who might have chronic hepatitis B. We were forced to exclude 15 patients from the original cohort because features delineating acute from chronic could not be found. It is well-known however, that ALF patient histories are often limited by the presence of encephalopathy on arrival at the referring hospital.
The acute form of HBV-ALF accounted for two thirds of the overall group; CHBV-ALF comprised the rest, 2/3 of whom were considered immunosuppressed. Other acute-on-chronic patients in the cohort might have been excluded because they lacked adequate history of chronicity. CHBV-ALF subjects receiving immunosuppressive agents or co-infected with HIV were considered together in this analysis. The immunosuppressed group differed in only minor respects from the remainder of the CHBV-ALF group. However, one differentiating feature was viral load, which was lower in the non-immunosuppressed group.
HBV viral load, IgM anti-HBc titer or the ratio of the two (if both values are available) effectively distinguished the two forms of ALF resulting from hepatitis B infection, particularly the quantitation of IgM anti-HBc levels with an AUROC of 86%. In practice, the presence of IgM anti-HBc positivity is associated with acute infection and is necessary but not sufficient to diagnose acute hepatitis B, since IgM anti-HBc is also observed in some patients with exacerbation of chronic infection (10,14). Higher IgM anti-HBc titers have been suggested to be associated with a highly active host immune response. Quantitative IgM anti-HBc testing more accurately distinguishes between acute and non-acute cases. Fink et al showed that a strong immunologic response promotes B cell differentiation into IgM-producing plasmablasts and high titers of IgM antibody, whereas moderate or weak stimulation drives differentiation into memory or plasma cells that mostly produce the IgG isotype (15). Moreover, clinical studies have consistently suggested that higher titers of IgM anti-HBc are seen in new acute HBV infection than in chronic HBV (16). The cutoff index value for IgM anti-HBc of 5.0 in our study effectively differentiated AHBV-ALF from CHBV-ALF with a PPV of 86% and a NPV of 89%. While 35% of CHBV-ALF patients demonstrated levels of IgM anti-HBc between 1.0 and 5.0 using the Advia® assay, 70% were below the index value of 5.0. Given our current knowledge, we suggest that determining the IgM anti-HBc level with a quantitative assay or at least a higher cut-off value than 1.0, is a readily available single test with excellent predictive capacity.
Earlier studies describing rapid viral clearance with undetectable HBsAg and VLs in hepatitis B-related ALF were based on insensitive tests, such as immunoelectrophoresis, radioimmunoassay and, for VLs, dot blot hybridization with a lower limit of detection (LLD) of approximately 100,000 IU/mL (5,7). We could not find recent studies that compared viral loads in either acute or fulminant hepatitis B using current highly sensitive assays. Thus, we did not have available known ranges for VL during acute or chronic HBV infection leading to liver failure. Using a real time-PCR assay with LLD of 25 IU/mL nearly all (92%, 47/51) of our AHBV-ALF patients had a detectable HBV viral load on admission although many had remarkably low values. AHBV-ALF subjects had significantly lower VLs than CHBV-ALFs subjects although there was some overlap between groups. The reasons for this overlap included possible inaccuracy of the initial clinical assessment that categorized the patients and the overall heterogeneity in timing of admission to study.
Lower VLs in AHBV-ALF, like higher IgM anti-HBc levels, suggest a more robust immune response. While the CHBV-ALF group demonstrated higher admission VLs (around 6 log10 IU/mL, compared to 3–4 log10 IU/mL), nearly all values declined during the study (Figure 4). The ratio of IgM anti-HBc to viral load, had a remarkably high AUROC of 0.86 (p<0.001), but was no better than IgM anti-HBc alone (AUROC = 86%, p<0.001).
Spontaneous survival differed between the two groups: 11% in the CHBV-ALF group vs. 33% in the AHBV-ALF patients, which might be due to differences in survival with older age. Overall survival was similar between the two groups, largely due to the increased numbers of CHBV-ALF patients receiving a liver allograft.
A third feature of AHBV-ALF is early HBsAg clearance observed in 20% (12/60) of patients with AHBV-ALF and in none with CHBV-ALF (7). Those who cleared HBsAg demonstrated higher median IgM anti-HBc titers and lower HBV VLs than their peers. The combination of lower VL, high IgM anti-HBc titers, and low or undetectable HBsAg on admission in AHBV-ALF suggests that a more robust host immune response occurs in true acute patients than is seen in chronic HBV presenting as ALF. Based on these data, it may be useful for clinicians to differentiate AHBV-ALF and CHBV-ALF on clinical and serological grounds, either by use of the IgM anti-HBc titers, viral loads or, if available, the ratio of the two. As a single value, IgM anti-HBc appears to be robust using the 5.0 cut-off value. Of interest, the immunosuppressed chronic group were mixed in ethnicity while virtually all remaining acute-on-chronic non-immunosuppressed patients were of Asian heritage, B genotype (7/8) and demonstrated pre-core (6/7) and/or basal core promoter mutations.
As expected, the algorithm we used resulted in an increased number of Asian patients in the CHBV-ALF group and nearly 50% of CHBV-ALF patients had genotype B. Studies from Asia suggest that genotype B is associated with more frequent acute exacerbations and a higher risk of hepatic decompensation and mortality compared to genotype C, while genotype C is associated with more liver cirrhosis and hepatocellular carcinoma (17,18). Among Asian patients with presumed acute HBV infection, more genotype B patients developed a fulminant course; this was confirmed in our study with 7 of 8 being genotype B (19).
Pre-core (G1896A) and core promoter (A1762T/G1764A) mutations have been considered drivers of severe disease, although these mutations are also found in chronic or asymptomatic HBV infection (20). A low prevalence of these mutations was detected in two prior series of HBV-related ALF (21). However, once the groups were separated in our study, it appeared that 60% of CHBV-ALFs (and all but one of the non-immunosuppressed CHBV-ALFs) presented with either pre-core, core promoter mutations or both, as compared to 40% of AHBV-ALFs—this was not statistically significant. These findings may point to the different immunopathogenesis in CHBV-ALF compared to the truly acute cases. While these data were obtained on consecutive United States patients, the patient characteristics seen here are likely unique to the United States and not readily extrapolated to other populations.
Our study has several limitations. Histories are often limited in rapidly evolving severe illnesses such as ALF where altered mentation is a criterion for study entry. The case selection process likely had inaccuracies as noted since the distinction between AHBV-ALF and CHBV-ALF was based solely on patient history and demographics. Finally, the ADVIAR Centaur IgM Anti-HBc assay used is considered to be semi-quantitative and was adapted for the quantitation performed here. Nevertheless, the wide differences observed in IgM anti-HBc between the two groups are clear, since the majority of CHBV-ALF patients demonstrated low IgM anti-HBc titers that were within the 1st dynamic range (no dilution needed).
In conclusion, AHBV-ALF can be separated from CHBV-ALF on clinical grounds when valid historical data are available, but the two may be more readily distinguished by quantitative IgM anti-HBc, viral loads and/or IgM anti-HBc/VL ratios. Additional indicators such as HBsAg negativity, younger age, non-Asian, and genotype non-B provide indirect evidence of acute hepatitis B-related ALF. By contrast, a low or undetectable IgM anti-HBc level, elevated HBV-DNA VL to >5 log10 IU/mL, in a patient with what appears to be an acute illness, suggest that this patient probably actually suffers from chronic HBV-related ALF. Overall, HBV-related ALF patients carry a poor prognosis, although those with new acute infection appear to fare somewhat better than those with chronic disease. Differentiation between the two subtypes within HBV-ALF may be helpful in determining more appropriate therapeutic strategies.
Acknowledgments
We gratefully acknowledge the support provided by the members of The Acute Liver Failure Study Group 1998–2008. This study was funded by NIH grant DK U-01 58369 for the Acute Liver Failure Study Group provided by the National Institute of Diabetes, Digestive and Kidney Disease. Additional funding provided by the Tips Fund of Northwestern Medical Foundation and the Jeanne Roberts and Rollin and Mary Ella King Funds of the Southwestern Medical Foundation, and T-32 DK007745-12 to DD. *Members and institutions participating in the Acute Liver Failure Study Group 1998–2009: W.M. Lee, MD (Principal Investigator), George A. Ostapowicz, MD, Frank V. Schiødt, MD, Julie Polson, MD, University of Texas Southwestern, Dallas, TX; Anne M. Larson, MD, University of Washington, Seattle, WA; Timothy Davern, MD, University of California, San Francisco, CA (current address: California Pacific Medical Center, San Francisco); Michael Schilsky, MD, Mount Sinai School of Medicine, NY, NY (current address: Yale University, New Haven, CT; Timothy McCashland, MD, University of Nebraska, Omaha, NE; J. Eileen Hay, MBBS, Mayo Clinic, Rochester, MN; Natalie Murray, MD, Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, MD, University of Pittsburgh, Pittsburgh, PA; Andres Blei, MD, Northwestern University, Chicago, IL (deceased); Atif Zaman, MD, University of Oregon, Portland, OR; Steven H.B. Han, MD, University of California, Los Angeles, CA; Robert Fontana, MD, University of Michigan, Ann Arbor, MI; Brendan McGuire, MD, University of Alabama, Birmingham, AL; Raymond T. Chung, MD, Massachusetts General Hospital, Boston, MA; Alastair Smith, MB, ChB, Duke University Medical Center, Durham, NC; Robert Brown, MD, Cornell/Columbia University, NY, NY; Jeffrey Crippin, MD, Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, MBBS, Medical University of South Carolina, Charleston, SC; Santiago Munoz, MD, Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, MD, University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, MD, Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, MD, University of California Davis, Sacramento, CA; Raj Satyanarayana, MD, Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, MD, University of California, San Diego, CA. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia, and Corron Sanders, PhD and the Statistics and Data Management Group included, Joan S. Reisch, PhD, Linda S. Hynan, PhD, Janet P. Smith, Joe W. Webster, and Mechelle Murray. We further acknowledge all the coordinators from the study sites as well as the patients and their families who participated in this study.
References
- 1.Inoue K, Yoshiba M, Sekiyama K, Okamoto H, Mayumi M. Clinical and molecular virological differences between fulminant hepatic failures following acute and chronic infection with hepatitis B virus. J Med Virol. 1998;55:35–41. doi: 10.1002/(sici)1096-9071(199805)55:1<35::aid-jmv7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Umemura T, Tanaka E, Kiyosawa K, Kumada H. Mortality secondary to fulminant hepatic failure in patients with prior resolution of hepatitis B virus infection in Japan. Clin Infect Dis. 2008;47:e52–56. doi: 10.1086/590968. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 4.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 5.Krogsgaard K, Kryger P, Aldershvile J, Andersson P, Brechot C. Hepatitis B virus DNA in serum from patients with acute hepatitis B. Hepatology. 1985;5:10–13. doi: 10.1002/hep.1840050104. [DOI] [PubMed] [Google Scholar]
- 6.Trepo CG, Robert D, Motin J, Trepo D, Sepetjian M, Prince AM. Hepatitis B antigen (HBsAg) and/or antibodies (anti-HBs and anti-HBc) in fulminant hepatitis: pathogenic and prognostic significance. Gut. 1976;17:10–13. doi: 10.1136/gut.17.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolf IL, El Sheikh N, Cullens H, Lee WM, Eddleston AL, Williams R, Zuckerman AJ. Enhanced HBsAb production in pathogenesis of fulminant viral hepatitis type B. Br Med J. 1976;2:669–671. doi: 10.1136/bmj.2.6037.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156–165. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M, Ohyama M, Takahashi Y, Udo K, Kojima M, Kametani M, Tsuda F, et al. Immunoglobulin M antibody against hepatitis B core antigen for the diagnosis of fulminant type B hepatitis. Gastroenterology. 1983;84:604–610. [PubMed] [Google Scholar]
- 10.Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001;120:1009–1022. doi: 10.1053/gast.2001.22461. [DOI] [PubMed] [Google Scholar]
- 11.Colloredo G, Bellati G, Leandro G, Colombatto P, Rho A, Bissoli F, et al. Quantitative analysis of IgM anti-HBc in chronic hepatitis B patients using a new “gray-zone” for the evaluation of borderline” values. J Hepatol. 1996;25:644–48. doi: 10.1016/s0168-8278(96)80233-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 13.Garson JA, Grant PR, Ayliffe U, Ferns RB, Tedder RS. Real-time PCR quantitation of hepatitis B virus DNA using automated sample preparation and murine cytomegalovirus internal control. J Virol Methods. 2005;126:207–213. doi: 10.1016/j.jviromet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Sjogren M, Hoofnagle JH. Immunoglobulin M antibody to hepatitis B core antigen in patients with chronic type B hepatitis. Gastroenterology. 1985;89:252–258. doi: 10.1016/0016-5085(85)90323-3. [DOI] [PubMed] [Google Scholar]
- 15.Fink K, Manjarrez-Orduno N, Schildknecht A, Weber J, Senn BM, Zinkernagel RM, Hengartner H. B cell activation state-governed formation of germinal centers following viral infection. J Immunol. 2007;179:5877–5885. doi: 10.4049/jimmunol.179.9.5877. [DOI] [PubMed] [Google Scholar]
- 16.Huang YW, Lin CL, Chen PJ, Lai MY, Kao JH, Chen DS. Higher cut-off index value of immunoglobulin M antibody to hepatitis B core antigen in Taiwanese patients with hepatitis B. J Gastroenterol Hepatol. 2006;21:859–862. doi: 10.1111/j.1440-1746.2006.04280.x. [DOI] [PubMed] [Google Scholar]
- 17.Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554–559. doi: 10.1016/s0016-5085(00)70261-7. [DOI] [PubMed] [Google Scholar]
- 18.Yuen MF, Sablon E, Wong DK, Yuan HJ, Wong BC, Chan AO, Lai CL. Role of hepatitis B virus genotypes in chronic hepatitis B exacerbation. Clin Infect Dis. 2003;37:593–597. doi: 10.1086/376988. [DOI] [PubMed] [Google Scholar]
- 19.Ozasa A, Tanaka Y, Orito E, Sugiyama M, Kang JH, Hige S, Kuramitsu T, et al. Influence of genotypes and precore mutations on fulminant or chronic outcome of acute hepatitis B virus infection. Hepatology. 2006;44:326–334. doi: 10.1002/hep.21249. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto H, Yotsumoto S, Akahane Y, Yamanaka T, Miyazaki Y, Sugai Y, Tsuda F, et al. Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J Virol. 1990;64:1298–1303. doi: 10.1128/jvi.64.3.1298-1303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang TJ, Hasegawa K, Munoz SJ, Shapiro CN, Yoffe B, McMahon BJ, Feng C, et al. Hepatitis B virus precore mutation and fulminant hepatitis in the United States. A polymerase chain reaction-based assay for the detection of specific mutation. J Clin Invest. 1994;93:550–555. doi: 10.1172/JCI117006. [DOI] [PMC free article] [PubMed] [Google Scholar]