Abstract
Intensification of animal production can be an important factor in the emergence of infectious diseases because changes in production structure influence disease transmission patterns. In 2004 and 2005, Thailand was subject to two highly pathogenic avian influenza epidemic waves and large surveys were conducted of the poultry sector, providing detailed spatial data on various poultry types. This study analysed these data with the aim of establishing the distributions of extensive and intensive poultry farms, based on the number of birds per holder. Once poultry data were disaggregated into these two production systems, they were analysed in relation to anthropogenic factors using simultaneous autoregressive models. Intensive chicken production was clustered around the capital city of Bangkok and close to the main consumption and export centres. Intensively-raised ducks, mainly free-grazing, showed a distinct pattern with the highest densities distributed in a large area located in the floodplain of the Chao Phraya River. Accessibility to Bangkok, the percentage of irrigated areas and human population density were the most important predictors explaining the geographical distribution of intensively-raised poultry. The distribution of extensive poultry showed a higher predictability. Extensive poultry farms were distributed more homogeneously across the country and their distribution was best predicted by human population density.
Keywords: Agricultural intensification, livestock mapping, disaggregation, livestock production systems, remote sensing, free-grazing ducks
Introduction
Intensive livestock production refers to elevated levels of inputs (for example concentrated feeds, elevated levels of health care and increased mechanization), resulting in increased outputs (meat, milk or eggs) per animal, and is often associated with large numbers of animals being concentrated on a small area of land. Intensification of livestock production can be achieved through one or a combination of: improved breeds, the use of feed, mechanization of labour, and investment in disease prevention and biosecurity (Otte et al., 2007). In turn, this allows a high turnover of animals and improved productivity. These innovations have enabled substantial increases in production to be realised since animal domestication (Mazoyer and Roudart, 2002). Intensification of livestock production has occurred particularly rapidly in recent decades, and some side-effects are of major concern at global level. Intensification affects nutrient cycles (Vitousek et al., 1997); is associated with deforestation, land degradation and water pollution (Berka et al., 2001); associated feed production competes with food crops for arable land (Steinfeld and Gerber, 2006); and it has been associated with emerging zoonoses such as Bovine Spongiform Encephalitis, Nipah and influenza viruses (Matson et al., 1997; Weiss and McMichael, 2004; Steinfeld, 2004). Characterizing and mapping the distribution of intensive livestock production is thus important at a range of spatial scales, in order better to assess its potential impact on agro-ecosystems (defined as units of agricultural activity in interactions with ecological, technological, and socio-economic factors (Francis et al., 2003)).
In veterinary epidemiology, previous work has suggested that regions in transition towards an intensified livestock sector may have greater susceptibility to disease emergence because changes in the production structure modifies patterns of disease transmission (Slingenbergh et al., 2004). Indeed, the evolutionary implications of intensive farming are receiving an increasing attention (Mennerat et al., 2010). Highly pathogenic avian influenza (HPAI) is an example of an important disease whose emergence can, in part, be attributed to rapid changes in poultry farming conditions. Laboratory studies have demonstrated that HPAI viruses can be produced from low pathogenic ones following consecutive passages through chickens of the same breed, under conditions that would be encountered in very large flocks of genetically homogeneous and susceptible birds raised in intensive production units (Ito et al., 2001). Rapid increases in the number of large-scale production units, not necessarily taking place with matching levels of increased bio-security, would hence favour the emergence of highly pathogenic strains from a pool of low pathogenic viruses maintained in wild or domestic birds. These conditions were encountered in the last few decades in several Asian countries, and it is in this context that HPAI of type H5N1 emerged in China in 1996 (Li et al., 2004) following several years of intensification of chicken and duck production. Since the HPAI H5N1 panzootic of 2004–2006, several studies have analysed the risk of HPAI H5N1 and found it to be correlated with indicators of intensive production such as the size of the flock (Otte et al., 2008) or the proximity between different livestock breeding facilities (Graham et al., 2008).
Previous studies on HPAI H5N1 epidemiology have also highlighted the key role played by domestic duck distributions (Anatidae can be healthy carriers of the H5N1 virus (Hulse-Post et al., 2005)), in the geographical distribution of HPAI H5N1 in Thailand and Vietnam (Gilbert et al., 2008), in India and Bangladesh (Gilbert et al. 2010) and in China (Martin et al., 2010). Because of the central role played by domestic ducks in the epidemiology of HPAI H5N1, efforts are been directed toward improving maps of duck distributions, particularly in regions with scarce census data such as Myanmar, India and several Chinese provinces (Van Boeckel et al., 2011). However despite these efforts to improve data, detailed investigations of the potential roles of intensive production in the emergence and spread of HPAI H5N1 have been prevented by a lack of detailed information on the spatial distribution of intensive production. Whilst several studies have focussed on mapping livestock distribution (Robinson et al., 2007; Wint and Robinson, 2007; Neumann et al., 2009; Prosser et al., 2011; Van Boeckel et al., 2011), only one published study distinguished extensive from Intensive production systems in the mapping procedure (Gerber et al., 2005). This first attempt was however limited by the spatial resolution and quality of the available data, and the authors were forced to make a number of simplifying assumptions; for example, any production falling between radii of 40 to 200 Kilometres from an urban area was classified as intensive, resulting in sharp transitions in predicted densities that did not reflect the more gradual nature of the actual distributions.
During the HPAI H5N1 epidemic, Thailand implemented massive disease surveillance programmes which were referred to as “X-Ray surveys”, involving hundreds of thousands of inspectors searching door-to-door for evidence of disease presence and collecting detailed poultry population data. This has resulted in very detailed data on poultry in Thailand, opening up the possibility to analyse separately the distributions of extensive and intensive poultry farms and thus overcoming this limitation to previous studies.
The present analysis used the 2007 X-Ray survey data in order to disaggregate poultry data by extensive and intensive production systems, using the number of birds owned per holder as the discriminating variable. Subsequently, environmental and anthropogenic predictors of the spatial distributions of both extensive and intensive production systems were identified.
Material and Methods
Census Data
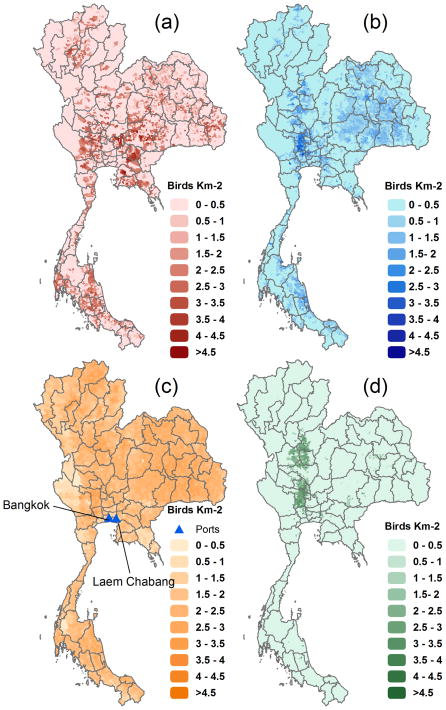
The 2007 X-Ray survey dataset contained the number of birds and number of holders in every sub-district (administrative level 3) of Thailand. More recent data were available, but 2007 was the last year when the distinctions were made among the 8 major classes of poultry. These included 3 types of chicken-broilers, layers, and Thai indigenous chickens (native); and 5 types of ducks- meat typed (MTD), egg typed (ETD), Muscovy (MD), meat typed free-grazing ducks (MTFGD), and egg typed free-grazing ducks (ETFGD). In subsequent years, some of these categories were pooled. Data were available for a total of 7,410 sub-districts with a median size of 16 km2. For each of these types, the respective number of holders was also known. Figure 1 of the supplementary information (SI) shows the relative composition of the chicken and duck sectors in 2007. Figure 1 shows the spatial distributions of four of these categories.
Figure 1.
Population density for: (a) broiler chickens, (b) egg type ducks, (c) native chickens, and (d) egg type free-grazing ducks per sub-district. Scale = Log10[(birds/km2) + 1])
Predictors
Predictor variables were chosen in order to include the most important anticipated predictors of poultry farming: i) availability of cheap feed available throughout the year (cropping intensity and irrigated areas), ii) access to markets (human population and travel time to main cities), and distribution of local producers/consumers (rural population). Furthermore, all variables were taken from global or regional datasets to ensure that the approach presented here could be replicated in other countries and the results compared. The variables investigated as predictors of intensification are listed in Table 1. The number of crops cultivated per year (Xiao et al., 2006; Biradar and Xiao, 2011) was derived from remote sensing measurements and predicts the number of cropping cycles. The percentage of irrigated areas (Siebert et al., 2007) is based both on remote sensing and sub-national water statistics. The human population densities were derived from the Global Rural Urban Mapping Project (GRUMP) database (CIESIN et al., 2005). Estimated travel times (accessibility) to Bangkok and to the closest provincial capital were derived from friction surface produced by Nelson (2008). Pre-processing of the predictors involved: i) re-sampling the irrigation database to 1 km resolution by nearest neighbour assignation, ii) averaging each predictors by sub-district.
Table 1.
Factors of livestock production intensification tested for correlation against disaggregated population density
| Variable Name | Reference | Resolution (Km) | Units |
|---|---|---|---|
| Urban Areas | Global Rural Urban Mapping Project (CIESIN, IPFRI, & CIAT 2005) | 1 | Binary |
| Accessibility to Province | Travel time to capital of province using cost surface from (Nelson, A. 2008) | 1 | Minute |
| Accessibility to Bangkok | Travel time to Bangkok using cost friction surface from (Nelson, A. 2008) | 1 | Minute |
| Irrigation | (Siebert et al. 2007) | 10 | % of Area |
| Average number of Crops/year | (Xiao et al. 2006) | 1 | crop cycles yr-1 |
| Population Density | Global Rural Urban Mapping Project (CIESIN et al. 2005) | 1 | Heads per Km-2 |
References
CIESIN, IPFRI & CIAT. (2005) Global Rural-Urban Mapping Project (GRUMP), Alpha Version. Center for International Earth Science Information Network (CIESIN), Columbia University; International Food Policy Research Institute (IPFRI); The World Bank; Centro Internacional de Agricultura Tropical (CIAT).
Nelson, A. (2008) Travel time to major cities: A global map of Accessibility.
Siebert, S., Döll, P., Feick, S., Hoogeveen, J. & Frenken, K. (2007) Global map of irrigation areas version 4.0. 1. University of Frankfurt (Main), Germany, and FAO, Rome, Italy.
Xiao, X., Boles, S., Frolking, S., Li, C., Babu, J.Y., Salas, W. & Moore III, B. (2006) Mapping paddy rice agriculture in South and Southeast Asia using multi-temporal MODIS images. Remote Sensing of Environment, 100, 95–113.
Disaggregating survey data
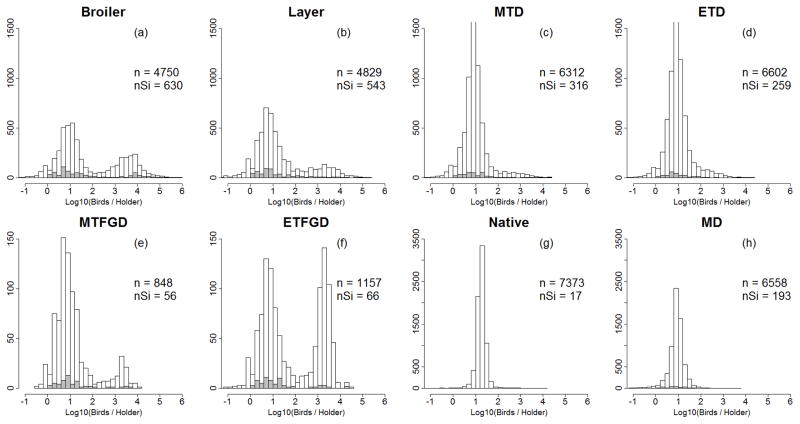
Exploratory analysis suggested that log10 transformed numbers of birds per holder was the best variable to discriminate intensive from extensive production systems for each poultry type, since a clear bimodal distribution of the number of birds per holder was observed for the poultry types for which intensive production is most important (Figure 2). Two distinct frequency distribution patterns were observed in the log10 transformed numbers of birds per holder.
Figure 2.
Histograms of the number of birds per holder for mixed poultry categories: (a) broiler chickens, (b) layer chickens, (c) meat type ducks,(d) egg type ducks, (e) meat type free grazing ducks, (f) egg type free grazing ducks, (g) native chickens, and (h) Muscovy ducks. Complete dataset (white) and isolated holders (grey).
First was a unimodal distribution with a peak of low numbers of birds per holder (e.g. MD and native). In this case, animals were categorized as being extensively produced without further analysis. This was supported by the observation that any increase in the number of birds per sub-district was in proportion to an increase in the number of holders (e.g. native chickens Figure 2g, see also the regression line in Figure 2 SI).
Second was a bimodal distribution, shown by the 6 poultry types: broiler, layer, MTD, ETD, MTFGD, ETFGD (e.g. Figure 2a & 2f). These poultry categories were disaggregated using four consecutive steps: i) sub-districts with mixed production were separated from those dominated by either extensive or intensive farms, ii) the groups dominated by one or other systems were used to estimate the average number of birds per holder in extensive (AE) and intensive (AI) systems, iii) the AE and AI parameters were fed into linear equations to predict the respective number of birds and owners in each (extensive and intensive) system in all sub-districts, and iv) numerical adjustments were applied for impossible values (e.g. negative numbers, non integer values). This procedure is detailed in protocol S1.
Finally, all chickens and ducks categorised as extensive or intensive were aggregated into four categories: intensive chickens, extensive chickens, intensive ducks and extensive ducks.
To assess the added value of using bimodal distributions for broilers, layers, MTD, ETD, MTFGD and ETFGD compared to a simple unimodal distribution, we used the difference in the Akaike Information Criterion (AIC):
where k is the number of parameters and LogLik the natural logarithm of the likelihood of the model used to fit the data. Both unimodal and bimodal models were fitted using maximum likelihood estimation. The difference in AIC (ΔAIC in Table 2) was defined as follows:
Table 2.
Disaggregation parameters for poultry categories from X-Ray Survey 2007
| Type | Heads | Holders | AE | Log10(sdE) | AI | Log10(sdI) | SingleHo | ΔAIC |
|---|---|---|---|---|---|---|---|---|
| Broiler Chicken | 114,235,177 | 56,233 | 9.227 | 0.5713 | 4829 | 0.5713 | 630 | +2791 |
| Layer Chicken | 40,265,293 | 38,336 | 6.125 | 0.6314 | 2526 | 0.6314 | 543 | +2231 |
| MTD (Meat Typed Duck) | 6,773,618 | 162,660 | 7.393 | 0.401 | 502.8 | 0.6072 | 316 | +2351 |
| ETD (Egg Typed Duck) | 6,633,887 | 165,827 | 7.659 | 0.392 | 220.7 | 0.5681 | 259 | +1584 |
| MTFGD (Meat Typed Free Grazing Duck) | 672,733 | 3,790 | 7.78 | 0.3197 | 2109 | 0.3197 | 56 | +560.5 |
| ETFGD (Egg typed Free Grazing Duck) | 8,825,219 | 6,382 | 9.87 | 0.2894 | 2157 | 0.2894 | 66 | +1006 |
| Native Chickena | 75,485,665 | 3,585,908 | 17.56 | 0.22 | - | - | 17 | - |
| MDb (Muscovy Duck) | 4,174846 | 443,019 | 8.406 | 0.33 | - | - | 193 | - |
a and b were classified in extensive systems without disaggregation
Positive values indicate that bimodal fitting performed better then unimodal fitting as regards the relative number of parameters
Statistical Analysis
Statistical models were built in order to analyse at the sub-district level the relationships between the densities of chickens and ducks by production system (birds km−2) and selected predictors.
Poultry classified as intensive showed a zero inflated distribution (ZID). Therefore two separate sets of analysis were carried out. Firstly, a logistic regression was used to identify predictors associated with the presence (> 0) of animals raised in intensive systems. Secondly, in sub-districts where intensive production was present, the log10 transformed number of birds was analysed using a regression model assuming a normal distribution of errors.
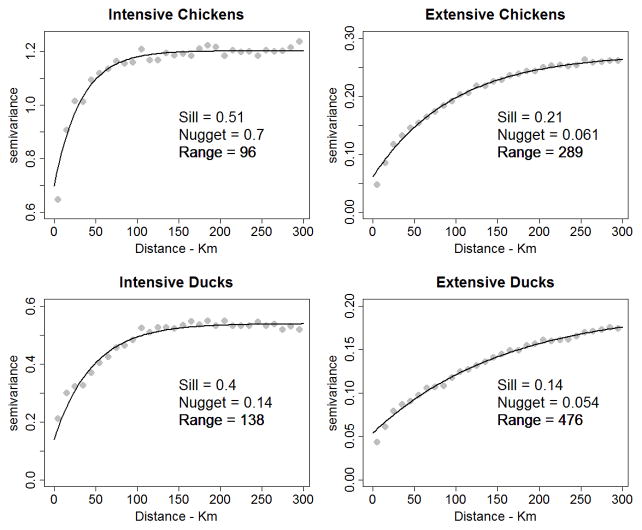
Preliminary analyses indicated that the four categories (intensive chickens, extensive chickens, intensive ducks and extensive ducks) showed distinct levels of spatial autocorrelation (SA) (Figure 3). Exponential variograms, including a nugget component, were used to quantify the level of SA in the model residuals. No significant SA was found in the residuals of the logistic regression models used to analyse the presence or absence of intensively-raised birds, whereas high levels of SA were observed in the residuals of the bird density regression models. Accounting for SA was achieved through a mixed simultaneous autoregressive model (SARM) (Bivand et al., 2008) for the following reasons: first, SARM was recently shown to provide better estimates of coefficients than the classic autocovariate method (Dormann, 2007); second, SARM would converge in a reasonable time, despite having a relatively large dataset; third, SARM accounts for SA both within the response variable and within the predictor variables, within most of which there was assumed to be considerable SA. For computational reasons, SARM was applied to a sub-sample of each category. Two thousand points were randomly distributed over the entire territory and, at each location, the bird density from the corresponding sub-district was extracted.
Figure 3.
Semi-variogram of for: (a) intensive chickens, (b) intensive ducks, (c) extensive chickens, and (d) extensive ducks. Extensive systems show a higher degree of spatial autocorrelation.
Since the average radius of a sub-district was 3.82 km, the neighbourhood radius value used in the SARM was 20 km around each sub-district’s centroid to ensure that the closest neighbours of a sub-district would contribute to the autoregressive term. Finally, the stability of the values of the estimated coefficients was assessed by repeating the analysis for five sub-samples of 2,000 randomly distributed observations.
A selection of goodness of fit (GOF) indices was derived from fitted values of the models to measure the overall degree of agreement of each model. More specifically, a correlation coefficient (COR), and root mean square error (RMSE) were estimated for the SARM models. Two GOF indices were calculated to measure the accuracy of the multiple logistic regressions: Cohen’s Kappa and the area under the curve (AUC) of the receiver operator characteristic (ROC) curve. The AUC is a measure of the capacity of the model to predict correctly the absence or presence of poultry, its value can range from 0.5 (random prediction) to 1 (perfect prediction). To calculate Cohen’s Kappa a cut-off value was first applied to the predicted probabilities of presence of poultry to classify these into two classes (present or absent). The threshold value chosen was the optimal cut-point probability obtained from the ROC curve, since this value represents the best possible cut-point (where the rate of true positives is optimized, compared to that of false positives). Finally, the relative contribution of each predictor to the model was quantified by the difference in deviance (DD) after sequential exclusion of each predictor from the model including all predictor variables.
All analyses were implemented in the open source software R × 64 2.12 (R Development Core Team, 2010)
Results
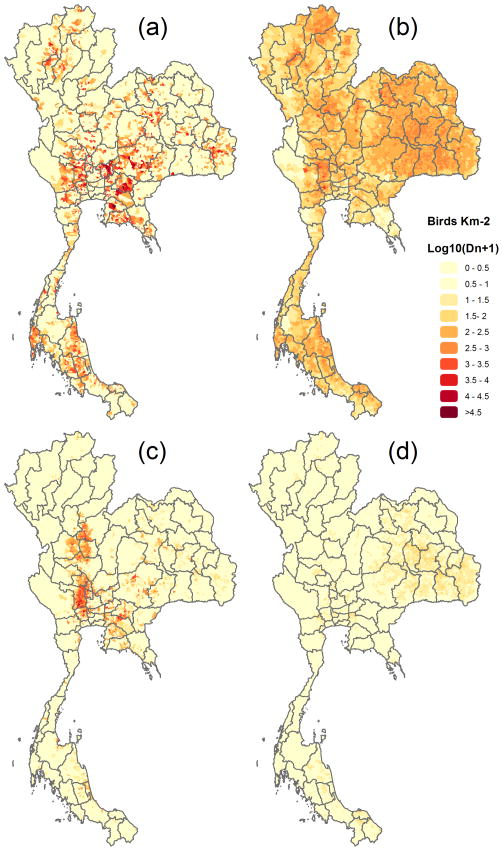
The delta AIC of the unimodal vs. the bimodal test of poultry categories presented in Table 2 (except native and MD) was always positive and the likelihood ratio test was highly significant in all cases, indicating a bimodal pattern and justifying their disaggregation into extensive and intensive production. The results of these disaggregations and the ensuing spatial distribution of ducks and chickens in intensive and extensive systems are presented in Table 2 and Figure 4. Figure 3 presents the semi-variograms of ducks and chickens in intensive and extensive production systems, and Tables 3a and 3b present the result of the regression models.
Figure 4.
Population densities of poultry disaggregated by production systems: (a) intensive chickens, (b) extensive chickens, (c) intensive ducks and (d) extensive ducks.
Table 3a.
Logistic Regressions G.O.F.
| Chicken Intensive: | |||||
|---|---|---|---|---|---|
| Predictor | Coef | p-value | DD | Kappa | AUC |
| Urban Areas | −0.5754 | 7.1e-02 | 3.34 | 0.156 | 0.644 |
| Province accessibility | −0.0036 | 3.3e-01 | 0.94 | ||
| Bangkok accessibility | −0.0072 | <0.001*** | 12.98 | ||
| Irrigation | 0.7405 | 9.8e-03** | 6.61 | ||
| Number of crops | −0.6905 | <0.001*** | 24.34 | ||
| Population Density | 0.6252 | <0.001*** | 13.55 | ||
|
| |||||
| Duck Intensive | |||||
| Predictor | Coef | p-value | DD | Kappa | AUC |
|
| |||||
| Urban Areas | −0.7695 | 1.8e-02* | 5.6 | 0.274 | 0.725 |
| Province accessibility | 0.0068 | 7.0e-02 | 3.2 | ||
| Bangkok accessibility | −0.0091 | <0.001*** | 18.2 | ||
| Irrigation | 1.6537 | <0.001*** | 30.3 | ||
| Number of crops | 0.5578 | <0.001*** | 14.5 | ||
| Population Density | 1.0961 | <0.001*** | 37.5 | ||
Table 3b.
Multivariate Simultaneous Autoregressive Regressions G.O.F.
| Chicken Intensive: | ||||||
|---|---|---|---|---|---|---|
| Predictor | Coef | p-value | DD | CORL | RMSE | rho |
| Urban Areas | −0.43369 | 0.0086*** | 3.78 | 0.568 | 0.574 | 0.286 |
| Province accessibility | 0.00026 | 9.4e-01 | 1.06 | |||
| Bangkok accessibility | −0.00543 | 0.01* | 4.17 | |||
| Irrigation | 0.38946 | 7.5e-02 | 1.15 | |||
| Number of crops | 0.07339 | 5.0e-01 | 0.43 | |||
| Population Density | 0.51642 | <0.001*** | 9.98 | |||
|
| ||||||
| Chicken Extensive | ||||||
| Predictor | Coef | p-value | DD | CORL | RMSE | rho |
|
| ||||||
| Urban Areas | −0.2929 | 1.4e-11*** | 3.2 | 0.876 | 0.255 | 0.617 |
| Province accessibility | −0.0055 | <0.001*** | 3.3 | |||
| Bangkok accessibility | 0.0043 | <0.001*** | 1.4 | |||
| Irrigation | 0.2609 | <0.001*** | 1.4 | |||
| Number of crops | 0.1246 | <0.001*** | 1.9 | |||
| Population Density | 0.6715 | <0.001*** | 57.8 | |||
|
| ||||||
| Duck Intensive | ||||||
| Predictor | Coef | p-value | DD | CORL | RMSE | rho |
|
| ||||||
| Urban Areas | −0.27134 | 0.05 | 1.163 | 0.761 | 0.549 | 0.438 |
| Province accessibility | −0.00099 | 7.6e-01 | 0.017 | |||
| Bangkok accessibility | −0.00348 | 7.8e-02 | 1.678 | |||
| Irrigation | 0.60887 | 2.5e-03 ** | 7.176 | |||
| Number of crops | 0.21223 | 1.1e-02 | 2.434 | |||
| Population Density | 0.43802 | <0.001*** | 8.102 | |||
|
| ||||||
| Duck Extensive | ||||||
| Predictor | Coef | p-value | DD | CORL | RMSE | rho |
|
| ||||||
| Urban Areas | −0.0489 | 0.26232 | 0.45 | 0.825 | 0.247 | 0.625 |
| Province accessibility | 0.0013 | 0.31080 | 0.12 | |||
| Bangkok accessibility | −0.0016 | 0.15269 | 0.28 | |||
| Irrigation | 0.2272 | <0.001*** | 0.88 | |||
| Number of crops | 0.0492 | 0.07279 | 0.39 | |||
| Population Density | 0.3481 | <0.001*** | 11.51 | |||
Intensive chicken production showed a highly heterogeneous distribution, with production concentrated in a limited number of sub-districts (Figure 4a). Sub-districts with high levels of intensive chicken production were located around Bangkok and close to the main consumption and exportation centres (Port of Bangkok and Port Laem Chabang, in particular). Intensive chicken production included several categories presenting very high numbers of birds per holder (Table 2), such as broiler chickens (AI = 4,829 birds). The semi-variogram model of intensively-raised chickens shows a relatively short range of spatial dependence compared to other poultry (Figure 3), which quantitatively confirms the short-range patchiness that can be observed in the distribution map. The main predictor associated with the presence of intensively-produced birds was the number of crop cycles, followed by the human population density and accessibility to Bangkok (Table 3a); whilst the main predictors associated with the abundance of raised birds were the human population density, the accessibility to Bangkok and percentage of urban area (Table 3b). Predictability of both the presence and abundance was low to moderate, with an AUC of 0.644, and a correlation coefficient of predicted vs. observed numbers of 0.568.
In contrast, extensive chicken production was found to be more homogeneously distributed throughout the country with a few notable exceptions such as the Khorat Plateau in the NorthEastern Region and a few high density spots around the provincial capitals of Chiang Rai, Nan and Kanchanaburi (Figure 4b). The number of birds per holder was relatively constant across the poultry types with values close to 10 birds per holder with the exception of 17 birds per person for the native chickens (Table 2). The semi-variogram model of extensive poultry numbers had a much wider range, confirming the more homogeneous pattern of distribution. The main predictor associated with the abundance of extensively-raised chickens was human population density (Table 3b). One can note that the difference of deviance (DD) associated with the removal of the human population density in the model ranged from 17 to 18 times the values associated with removing the next most important predictor variables: the accessibility to Bangkok and the urban areas. The predictability of the number of extensively-raised chickens was considerably greater than that of intensively-raised chickens, with a predicted vs. observed correlation coefficient of 0.879.
An unexpected result was the intensive nature of free-grazing duck farming; with mean numbers of Ai = 2,109 and Ai = 2,157 for meat type and egg type free-grazing ducks respectively, and with spatial distributions differing markedly from those of chickens.
Intensively-raised ducks were found to be mainly distributed in a large area located in the central floodplain of the Chao Phraya river (Figure 4c). There was considerable spatial clustering over a relatively short range, as quantified by the range of the semi-variogram model (Figure 3). The most important predictor of the presence of intensive duck production was the population density, followed by the percentage of irrigated areas and accessibility to Bangkok. The main predictors associated with the abundance of intensively-raised ducks were the human population density, percentage of irrigated areas and cropping intensity (Table 3b). Predictability intensively-raised ducks was moderate, and higher than that of intensively-raised chickens but lower than for extensively-raised chickens.
Finally, extensively-raised ducks were distributed more homogeneously than intensively-raised ducks, occurred at lower densities in general, with a relative abundance in the north-eastern part of Thailand (Figure 4d). The semi-variogram model had a very long range, again confirming the spatial homogeneity apparent in the distribution map. The main predictors of the abundance of extensively-raised ducks were human population density, the percentage of irrigated areas and urban areas. Predictability was good; slightly lower than for extensive chickens, despite the relatively low number of significant predictor variables.
Discussion
This study aimed (i) to produce maps of chicken and duck population densities, distinguishing intensive from extensive production based on the number of birds per holder, and (ii) to explore the associations between the densities of birds in each category and some anthropogenic predictor variables.
The vast majority (>99%) of ducks in Thailand are raised in the free-grazing production systems and were categorized as intensive using this approach. This may seem surprising since the practice of free-grazing is usually thought to represent a low-input, low-output system. However, beyond the number of birds per holder, used here as index to allocate birds to the intensive production category, other arguments support the results that free-grazing duck production in Thailand is more intensive than extensive: i) free-grazing production systems involve flocks of several thousands of individuals and tens of duck herders clustered on small areas of land; ii) the movement of the ducks from one field to another involves motorised transportation (Songserm et al., 2006; Henning et al., 2009); iii) ducklings are purchased in high numbers from dedicated intensive producers (Minh et al., 2010); and iv) the management of eggs produced by millions of free-grazing ducks, laying daily, requires intensive logistics; as does the transportation, processing and storage of duck meat products (Heft-Neal et al., 2008). The main factors in free-grazing duck farming that fall outside the definition of intensive production presented in the introduction are the absence of high investments in bio-security, and that bought feed is not provided. The absence of bio-security measures associated with high production levels of free-grazing duck products had important epidemiological consequences in term of risk of HPAI H5N1 transmission (Otte et al., 2006; Paul et al., 2011).
Most of Thailand’s intensive poultry farming was seen to be clustered in the central region of the Chao Phraya, corroborating conclusions drawn from previous works on Thailand’s poultry sector (Costales, 2004). However different distribution patterns were observed for chickens and ducks. Intensive duck production was concentrated in the Chao Phraya river central flood plain, mainly because this supports multiple rice production cycles per year (a condition necessary to sustain free-grazing duck production (Gilbert et al., 2007)). Intensive chicken production was a lot more scattered throughout the country (Figure 4), but with highest densities in provinces surrounding Bangkok: the largest market for national consumption, but also an important commercial hub for exports. Broiler production is primarily located close to hatcheries, feed mills and processing plants while large integrated layer farms are distributed more evenly across regions (Costales, 2004; NaRanong, 2007).
These observations were further supported by the results of the regression analyses. The strong relationship between intensively-farmed chickens and human population density and proximity to Bangkok reflects the huge demand for chicken meat in the Thai capital and the importance of Bangkok as an exportation hub. For intensively-raised ducks, human population density was also found to be an important predictor; but so was the number of crop cycle per year, reflecting the importance of available feeding resources, provided by proximity to paddy fields with multiple cropping as reported in previous studies (Gilbert et al., 2007). Unexpectedly the number of crop cycles showed a negative correlation with the presence of intensively-raised chickens, whereas a positive contribution from the percentage of irrigated areas was observed (Table 3b). Intensive chicken farming is less closely associated with the land than is intensive duck farming. The counterintuitive sign of both coefficients may be explained by the fact that intensive chicken farming occurs within the broad area of the central floodplain, i.e. an area with a high proportion of land under irrigation, but not right next to the paddy fields themselves. This observation could also have arisen due to scale: the percentage of irrigated area is derived from a 10 km resolution image, whilst the number of crop cycles was derived from a 500 m resolution image (Xiao et al., 2006). Both were subsequently aggregated to 1 km pixels. More surprising was the fact that sub-districts dominated by urban areas supported large numbers of intensively-raised poultry, possibly corroborating the idea that intensive poultry farming is increasingly urban in nature (Rushton et al., 2005).
Intensive production was found to be strongly clustered (as indicated by the short range of semi-variogram models), and difficult to predict through regression analysis. Both results can be interpreted relatively easily. Firstly, a wide range of parameters influence decisions on where to set up intensive or industrial farms (Neumann et al., 2009). This includes variables such as the cost of the land, local government incentives or tax regimes, which are difficult to integrate into this type of modelling because of a lack of detailed data. Secondly, from a producer perspective, the geographical concentration of production units results in economies of scales for the provision of inputs (low-cost feed and day-old chicks or ducklings) and the collection and transportation of outputs. For two sub-districts which are equally suitable for the establishment of a chicken farm, the decision to set up a farm may have depended initially on some unknown factor such as the availability of a parcel of land to buy. Then subsequent farm establishment may occur in the vicinity of that original farm in order to benefit from economies of scale. Over time, this would result in two sub-districts with apparently identical conditions (in terms of accessibility to Bangkok, etc.) but one having a cluster of intensive production units whist the other may have none. This situation would typically result in a clustered distribution of production, whose distribution may be difficult to predict using spatial, environmental variables. This is further exacerbated by the fact that chicken production can be fully detached from the land resource, which restricts the extent to which its distribution can be explained by environmental variables (Naylor et al., 2005).
Extensive production showed a much more homogeneous and predictable distribution for both chickens and ducks, and was found to be strongly correlated with human population density and much less influenced by the accessibility to Bangkok and other urban areas. This supports the idea that extensive production mostly comprises subsistence farming in rural areas. Extensive production still dominates Thailand’s poultry sector in terms of number of holders (Poapongsakorn et al., 2003) and their competitiveness with respect to larger-scale producers may be explained by their ability to maintain low production costs, often using family labour and with reduced overhead costs. Among the rural population, which represent about 66% of the total, 73% of poultry farms (UN, 2010) are traditional, low-input, backyard farms, rearing native chickens and fighting cocks in free-range systems (Otte et al., 2006). Only recently have native chickens started to be produced by larger commercial producers (Chang et al., 2004).
The relatively good predictability of extensive production, with few spatial predictor variables, opens up the possibility for separating extensive from intensive production in other countries that do not have detailed census data such as is available for Thailand. Indeed, given the weight of human population in the prediction of extensively-raised poultry, it could be realistically assumed that extensive poultry production could be predicted based solely on rural population maps, and that the amount of intensively-raised birds could then be deduced from the difference with census totals. Approaches based on these principles are reviewed in Robinson et al. (2011) and call for detailed follow-up studies to test and validate them.
The approach developed in this paper could be improved in several ways. First, the methodology would benefit from the addition of information derived from expert opinion and, if available, from a selected sets of livestock-oriented, socio-economic predictors. One example is the value of the land, which could be an important factor in deciding where farms should be established. Second, the SARM method used to investigate links between the densities of the different poultry categories and a suite of predictor variables raised some issues. First, models were based only on a sub-sample of 2,000 locations randomly distributed across the country, possibly missing some important relationships. Second, the SARM does not explicitly account for possible interaction among predictor variables. There remains considerable scope to evaluate other modelling approaches such as, for example, Boosted Regression Trees (Elith et al., 2008), which may better be able to describe nonlinear relationships between poultry densities and covariates.
Poultry data broken down by production system have rarely been used in epidemiological risk factor analyses. An important follow-up to this work will be to look at data on historical HPAI H5N1 epidemics in Thailand in relation to detailed poultry systems information. Poultry data, broken down by production systems, could also be used in knowledge-based approaches such as multi-criteria decision analysis (MCDA), in order to characterize better the regions where the interactions between people and poultry are highest.
Beyond these epidemiological applications, such poultry maps disaggregated by production system have a wide range of potential uses, in fields such as: soil and water pollution (Long et al., 2004), modelling nutrient transport (Karr et al., 2001), environmental planning (Haas et al., 2001), conservation policy, and economic geography (Pritchard, 2000). This calls for complementary studies extending the work reported here to other countries and regions, and adapting the methodology to other species.
Supplementary Material
Highlights.
This study analysed the geographical distribution of poultry farming in Thailand in 2007
Poultry data were disaggregated into two systems, extensive and intensive, based on the number of birds per owner
The spatial distributions of extensive and intensive production were analysed in relation to anthropogenic factors
Human population was the main determinant of extensive poultry distribution
Human population and accessibility best explained intensive poultry distribution.
Acknowledgments
This work was partly supported by the National Institutes of Health Fogarty International Centre through the NSF/NIH Ecology of Infectious Diseases program (7R01TW007869-04).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berka C, Schreier H, Hall K. Linking water quality with agricultural intensification in a rural watershed. Water, Air, & Soil Pollution. 2001;127:389–401. [Google Scholar]
- Biradar CM, Xiao X. Quantifying the area and spatial distribution of double-and triple-cropping croplands in India with multi-temporal MODIS imagery in 2005. International Journal of Remote Sensing. 2011;32:367–386. [Google Scholar]
- Bivand RS, Pebesma EJ, Gómez-Rubio V, Corporation E. Applied spatial data analysis with R. Springer; New York: 2008. [Google Scholar]
- Chang HS Agricultural, U. of N.E.G.S. of, Economics, R. Cross-sector comparisons of poultry production in the Philippines. University of New England, Graduate School of Agricultural and Resource Economics; 2004. [Google Scholar]
- CIESIN, IPFRI, CIAT. Global Rural-Urban Mapping Project (GRUMP), Alpha Version. Center for International Earth Science Information Network (CIESIN) Columbia University; International Food Policy Research Institute (IPFRI); The World Bank; Centro Internacional de Agricultura Tropical (CIAT); 2005. [Google Scholar]
- Costales A. Livestock Sector Report–Thailand elaborated for the FAO–AGAL. 2004. A review of the Thailand poultry sector. [Google Scholar]
- Dormann CF. Assessing the validity of autologistic regression. Ecological Modelling. 2007;207:234–242. [Google Scholar]
- Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- Francis C, Lieblein G, Gliessman S, Breland TA, Creamer N, Harwood R, Salomonsson L, Helenius J, Rickerl D, Salvador R, et al. Agroecology: the ecology of food systems. Journal of sustainable agriculture. 2003;22:99–118. [Google Scholar]
- Gerber P, Chilonda P, Franceschini G, Menzi H. Geographical determinants and environmental implications of livestock production intensification in Asia. Bioresource Technology. 2005;96:263–276. doi: 10.1016/j.biortech.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Xiao X, Chaitaweesub P, Kalpravidh W, Premashthira S, Boles S, Slingenbergh J. Avian influenza, domestic ducks and rice agriculture in Thailand. Agriculture, Ecosystems and Environment. 2007;119:409–415. doi: 10.1016/j.agee.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JP, Leibler JH, Price LB, Otte JM, Pfeiffer DU, Tiensin T, Silbergeld EK. The Animal-Human Interface and Infectious Disease in Industrial Food Animal Production: Rethinking Biosecurity and Biocontainment. Public Health Rep. 2008;123:282–299. doi: 10.1177/003335490812300309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas G, Wetterich F, Köpke U. Comparing intensive, extensified and organic grassland farming in southern Germany by process life cycle assessment. Agriculture, Ecosystems & Environment. 2001;83:43–53. [Google Scholar]
- Heft-Neal S, Otte J, Pupphavessa W, Roland-Holst D, Sudsawasd S, Zilberman D. Supply Chain Auditing for Poultry Production in Thailand RR. 2008. [Google Scholar]
- Henning J, Henning K, Vu LT, Yulianto D, Meers J. The Role Of Moving Duck Flocks In The Spread Of Highly Pathogenic Avian Influenza (HPAI) Virus In Viet Nam And Indonesia. Proceedings of the 12th Symposium of the International Society for Veterinary Epidemiology and Economics; Durban, South Africa. 2009. [Google Scholar]
- Hulse-Post DJ, Sturm-Ramirez KM, Humberd J, Seiler P, Govorkova EA, Krauss S, Scholtissek C, Puthavathana P, Buranathai C, Nguyen TD, Long HT, Naipospos TSP, Chen H, Ellis TM, Guan Y, Peiris JSM, Webster RG. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10682–10687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Goto H, Yamamoto E, Tanaka H, Takeuchi M, Kuwayama M, Kawaoka Y, Otsuki K. Generation of a Highly Pathogenic Avian Influenza A Virus from an Avirulent Field Isolate by Passaging in Chickens. J Virol. 2001;75:4439–4443. doi: 10.1128/JVI.75.9.4439-4443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr JD, Gilliam WJ, Andres JW, Scott A. Tracing nitrate transport and environmental impact from intensive swine farming using delta nitrogen-15. Journal of Environmental Quality. 2001;30:1163. doi: 10.2134/jeq2001.3041163x. [DOI] [PubMed] [Google Scholar]
- Li KS, Guan Y, Wang J, Smith GJD, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- Long C, Yu-jun W, Dong-mei Z, Yuan-hua D. Heavy metals pollution in poultry and livestock feeds and manures under intensive farming in Jiangsu Province, China. Journal of Environmental Sciences. 2004;16 [PubMed] [Google Scholar]
- Matson PA, Parton WJ, Power AG, Swift MJ. Agricultural Intensification and Ecosystem Properties. Science. 1997;277:504–509. doi: 10.1126/science.277.5325.504. [DOI] [PubMed] [Google Scholar]
- Mazoyer M, Roudart L. Histoire des agricultures du monde: du néolithique à la crise contemporaine; Seuil, Paris.. 2002. [Google Scholar]
- Mennerat A, Nilsen F, Ebert D, Skorping A. Intensive Farming: Evolutionary Implications for Parasites and Pathogens. Evol Biol. 2010;37:59–67. doi: 10.1007/s11692-010-9089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh PQ, Stevenson MA, Schauer B, Morris RS, Quy TD. A description of the management of itinerant grazing ducks in the Mekong River Delta of Vietnam. Preventive Veterinary Medicine. 2010;94:101–107. doi: 10.1016/j.prevetmed.2009.11.011. [DOI] [PubMed] [Google Scholar]
- NaRanong V. Structural changes in Thailand’s poultry sector and its social implications. Thailand Development Research Institute; Bangkok, Thailand: 2007. [Google Scholar]
- Naylor R, Steinfeld H, Falcon W, Galloway J, Smil V, Bradford E, Alder J, Mooney H. Losing the Links Between Livestock and Land. Science. 2005;310:1621–1622. doi: 10.1126/science.1117856. [DOI] [PubMed] [Google Scholar]
- Nelson A. Travel time to major cities: A global map of Accessibility. Office for Official Publications of the European Communities; Luxembourg: 2008. [Google Scholar]
- Neumann K, Elbersen BS, Verburg PH, Staritsky I, Pérez-Soba M, Vries W, Rienks WA. Modelling the spatial distribution of livestock in Europe. Landscape Ecol. 2009;24:1207–1222. [Google Scholar]
- Otte J, Pfeiffer D, Soares-Magalhaes R, Burgos S, Roland-Holst D. Flock size and HPAI risk in Cambodia, Thailand, and Viet Nam. Food and Agricultural Organisation of the United Nations; 2008. [Google Scholar]
- Otte J, Pfeiffer D, Tiensin T, Price L, Silbergeld E. Research report. John Hopkins Bloomberg School of Public Health; 2006. Evidence-based policy for controlling HPAI in poultry: Bio-security revisited. [Google Scholar]
- Otte J, Roland-Holst D, Pfeiffer D, Soares-Magalhaes R, Rushton J, Graham J, Silbergeld E. Food and Agriculture Organization of the United Nations, Pro-Poor Livestock Policy Initiative Research Report. 2007. Industrial livestock production and global health risks. [Google Scholar]
- Paul M, Wongnarkpet S, Gasqui P, Poolkhet C, Thongratsakul S, Ducrot C, Roger F. Risk factors for highly pathogenic avian influenza (HPAI) H5N1 infection in backyard chicken farms, Thailand. Acta Tropica. 2011;118:209–216. doi: 10.1016/j.actatropica.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Poapongsakorn N, NaRanong V, Delgado C, Narrod C, Siriprapanukul P, Srianant N, Goolchai P, Ruangchan S, Methrsuraruk S, Jittreekhun T, et al. Policy, technical, and environmental determinants and implications of the scaling-up of swine, broiler, layer and milk production in Thailand. International Food Policy Research Institute; 2003. [Google Scholar]
- Pritchard B. Geographies of the Firm and Transnational Agro-food Corporations in East Asia. Singapore Journal of Tropical Geography. 2000;21:246–262. [Google Scholar]
- Prosser DJ, Wu J, Ellis EC, Gale F, Van Boeckel TP, Wint W, Robinson T, Xiao X, Gilbert M. Modelling the distribution of chickens, ducks, and geese in China. Agriculture, Ecosystems & Environment. 2011;141:381–389. doi: 10.1016/j.agee.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TP, Thornton PK, Franceschini G, Kruska RL, Chiozza F, Notenbaert A, Cecchi G, Herrero M, Epprecht M, Fritz S, You L, Conchedda G, See L. Global livestock production systems. Food and Agriculture Organization of the United Nations (FAO) and International Livestock Research Institute (ILRI); 2011. [Google Scholar]
- Robinson TP, Franceschini G, Wint W. The Food and Agriculture Organization’s Gridded Livestock of the World. Veter Ital. 2007;43:745–751. [PubMed] [Google Scholar]
- Rushton J, Viscarra R, Guerne Bleich E, McLeod A. Impact of Avian Influenza Outbreaks in the Poultry Sectors of Five South East Asian Countries (Cambodia, Indonesia, Lao PDR, Thailand, Viet Nam) Outbreak Costs, Responses and Potential Long Term Control. World’s Poultry Science Journal. 2005;61:491–514. [Google Scholar]
- Siebert S, Döll P, Feick S, Hoogeveen J, Frenken K. Global map of irrigation areas version 4.0.1. University of Frankfurt (Main); Germany, and FAO, Rome, Italy: 2007. [Google Scholar]
- Slingenbergh J, Gilbert M, Balogh K, Wint W. Ecological sources of zoonotic diseases. Revue Scientifique et Technique-Office International des Epizooties. 2004;23:467–484. doi: 10.20506/rst.23.2.1492. [DOI] [PubMed] [Google Scholar]
- Songserm T, Jam-on R, Sae-Heng N, Meemak N, Hulse-Post DJ, Sturm-Ramirez KM, Webster RG. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerg Infect Dis. 2006;12:575–581. doi: 10.3201/eid1204.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld H, Gerber P. Livestock’s long shadow. Food and Agriculture Organization of the United Nations; 2006. [Google Scholar]
- Steinfeld H. The livestock revolution--a global veterinary mission. Veterinary Parasitology. 2004;125:19–41. doi: 10.1016/j.vetpar.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Thornton PK, Kruska RL, Henninger N, Kristjanson PM, Reid RS, Atieno F, Odero AN, Nedgwa T. Mapping Poverty and Livestock in the Developing World. International Livestock Research Institute; Nairobi, Kenya: 2002. [Google Scholar]
- UN. World Urbanization Prospects: The 2009 Revision. United Nations, Department of Economic and Social Affairs, Population Division; 2010. [Google Scholar]
- Van Boeckel TP, Prosser D, Franceschini G, Biradar C, Wint W, Robinson T, Gilbert M. Modelling the distribution of domestic ducks in Monsoon Asia. Agriculture, Ecosystems & Environment. 2011;141:373–380. doi: 10.1016/j.agee.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG. HUMAN ALTERATION OF THE GLOBAL NITROGEN CYCLE: SOURCES AND CONSEQUENCES. Ecological Applications. 1997;7:737–750. [Google Scholar]
- Weiss RA, McMichael AJ. Social and environmental risk factors in the emergence of infectious diseases. Nature Medicine. 2004;10:70. doi: 10.1038/nm1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wint W, Robinson TP. Gridded livestock of the world 2007. Food and Agriculture Organisation of the United Nations, Animal Production and Health Division; 2007. p. 131. [Google Scholar]
- Xiao X, Boles S, Frolking S, Li C, Babu JY, Salas W, Moore B., III Mapping paddy rice agriculture in South and Southeast Asia using multi-temporal MODIS images. Remote Sensing of Environment. 2006;100:95–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.