Abstract
Background
Many benefits are ascribed to vitamin D beyond its well-known effects on calcium and bone metabolism. Vitamin D in adequate amounts is apparently beneficial to muscle, lessening the risk of falls and fractures in the elderly. The elderly produce less vitamin D in their skin than younger persons do, and they also spend less time in the sun; they are therefore at greater risk of vitamin D deficiency.
Methods
We used gas chromatography with mass spectrometry coupling to measure the 25-OH-vitamin D level of 1578 elderly persons (72% women) who were consecutively admitted to an elderly care rehabilitation facility in Trier, Germany, from July 2009 to March 2011. Their mean and median age was 82 years.
Results
89% of the patients had 25-OH-vitamin D deficiency (defined as a level below 20 ng/mL), and 67% had a severe deficiency (below 10 ng/mL). Only 4% had levels in the target range (30–60 ng/mL); none had a level above 100 ng/mL.
Conclusion
Many of these patients were deficient in vitamin D. Persons of very advanced age need a better supply of vitamin D not only to keep their bones healthy, but also to lessen the risk of falls and fractures.
Data on vitamin D status among the elderly have been made available by many international studies (1). For example, in a British study from 2005 a lower vitamin D level was measured in persons aged over 65 years than in the general public. Patients cared for in care facilities had lower values than those who lived at home (2).
Earlier studies of the vitamin D status of the German population also frequently revealed low 25-OH-vitamin D levels. For instance, a study by the Robert Koch Institute (RKI) in 2008 in over 14 000 individuals aged between 1 and 79 years showed that overall 62% of boys, 64% of girls, 57% of men, and 58% of women had 25-OH-levels below 20 ng/mL (3). In the RKI’s study, vitamin D deficiency was particularly common in young people, particularly those from immigrant families, and in older women. Overall, 75% of elderly women aged between 65 and 79 years in this study had levels below 20 ng/mL, even in summer (4). A study of 200 elderly care facility residents in Germany in 2007/2008 showed substantial 25-OH-vitamin D deficiency, with values below 10 ng/mL, in 68.3% (e1). A 2007 study found a mean 25-OH-vitamin D level of 14 ng/mL in 205 patients in Bonn on admission to an acute geriatric department (e2).
Vitamin D deficiency increases the risk of osteoporosis, and severe vitamin D deficiency can cause osteomalacia. Osteomalacia is not easy to diagnose clinically or radiologically and therefore often goes undetected. However, a 2009 autopsy study of deceased individuals with clinically healthy bones found histopathological signs of osteomalacia with vitamin D levels below 30 ng/mL in 25% of all bone samples. By contrast, with vitamin D levels above 30 ng/mL there was no evidence of any bone alterations (5).
According to available meta-analyses from 2009, vitamin D deficiency can also increase the risk of falls and fractures (6, 7, e3).
Germany’s geographical location means that the body can only produce sufficient vitamin D in the skin from sunlight during the summer months; in autumn and winter, very little vitamin D synthesis occurs (8). Elderly persons usually spend less time in the sun than younger people, and in addition sun exposure should be limited due to the risk of skin cancer (9).
In order to examine the vitamin D supply of elderly patients in Germany, the vitamin D levels of patients of a geriatric rehabilitation facility were tested on admission. Also of interest was whether there was a difference between geriatric patients’ vitamin D supply in the darker and in the brighter half of the year. Vitamin D supply is becoming more and more important as a result of demographic changes and the resulting increase in the proportion of elderly people in the population.
Method
Between July 2009 and March 2011, the serum 25-OH-vitamin D levels of a total of 1578 patients consecutively admitted to the St. Irminen Geriatric Rehabilitation Facility in Trier, Germany, were measured following admission examinations. With only a few exceptions, all patients admitted during this period were tested (blood was generally taken on the working day following admission). 25-OH-vitamin D levels were measured using gas chromatography with mass spectrometry coupling; the AB Sciex QTRAPTM LC-MS/MS System for platforms 3200 and 4000; linear measurement accuracy 4 to 100 ng/mL 25-OH-vitamin D.
72% of patients were women, 28% men, and the mean and median age was 82 years. Ages ranged from 46 to 105 years. No data were gathered on any vitamin D supplements taken before admission. Such data would have been very prone to errors, due to the common problem with medication histories relating to the vitamin D supplements that are freely available from pharmacies, drug stores, supermarkets, and online stores, and would therefore have provided very little information.
Most patients attended the rehabilitation facility after hospital treatment for typical geriatric illnesses (e.g. fractures, elective joint replacement, apoplexy, multiple morbidity). 95% of them had lived at home before acute hospital treatment and rehabilitation; 5% had previously been receiving care in a residential care home.
25-OH-vitamin D levels are usually stated in ng/mL or the SI unit nmol/L in the literature (for conversion: ng/mL × 2.5 = nmol/L). 1 µg vitamin D3 intake with food corresponds to 40 IU.
Unfortunately, as yet there is no universally accepted method for classifying vitamin D levels. Severe deficiency of below 10 ng/mL poses a risk of osteomalacia, and a level of at least 20 ng/mL is required for physiological bone metabolism (5, 10, e4). However, complete resolution of hyperparathyroidism secondary to vitamin D deficiency (10) and the bone alterations typical of osteomalacia (5) does not occur until levels above 30 ng/mL are reached. This region is therefore usually seen as “optimum.” However, in 2010 the US Institute of Medicine (IOM) declared even a 25-OH-vitamin D level above 20 ng/mL sufficient for bone health (11). I have therefore used both boundary values for evaluation. Toxicity reactions to high 25-OH-vitamin D levels usually occur only at levels above 150 ng/mL (1), and the maximum 25-OH-vitamin D level to prevent toxicity reactions has been established at 100 ng/mL, with an additional safety margin. Current knowledge indicates that an upper limit of 60 ng/mL should not be exceeded, as in various epidemiological studies adverse effects have been observed both with very low 25-OH-vitamin D values and with high values. A study with an increase in overall mortality yielded such a U-shaped relationship both with high 25-OH-vitamin D levels, above 60 ng/mL and with low levels, below 30 ng/mL (e5). The interval between 30 and 60 ng/mL 25-OH-vitamin D was therefore defined as “optimum” for this research (Table 1).
Table 1. Classification of 25-OH-vitamin D levels (according, 5, 10– 13, e3, e4).
| 25-OH-vitamin D (ng/mL) | |
| Toxic | >100 |
| Optimum | 31 to 60 |
| Suboptimum | 21 to 30 |
| Moderate deficiency | 11 to 20 |
| Severe deficiency | ≤ 10 |
Results
The mean 25-OH-vitamin D level for all 1578 patients was 10.2 ng/mL (range 1 to 77 ng/mL; median 8 ng/mL). There was no difference between the mean for women and for men. The median for women was 8 ng/mL, and for men 7 ng/mL. In the few patients under 60 years of age (n = 19), the mean was 13.84 ng/mL, and the corresponding value for those aged over 90 (n = 104) was 8.63 ng/mL. The largest groups were those consisting of patients aged between 71 and 80 years (n = 501), the mean for which was 10.48 ng/mL, and of patients aged between 81 and 90 years (n = 881), the mean for which was 10.36 ng/mL (Table 2).
Table 2. Distribution of 25-OH-vitamin D levels.
| Overall data on 25-OH-vitamin D levels, July 2009 to March 2011 | ||||||
| n | Mean, ng/mL | Standard deviation | Median, ng/mL | Maximum ng/mL | Minimum ng/mL | |
| Total | 1578 (100%) | 10.2 | 8.66 | 8 | 77 | 1 |
| Women | 1131 (72%) | 10.2 | 8.74 | 7 | 77 | 1 |
| Men | 447 (28%) | 10.2 | 8.45 | 8 | 64 | 2 |
| Distribution of 25-OH-vitamin D levels by age, July 2009 to March 2011 | ||||||
| Age <60 years | 19 (1.2%) | 13.84 | ||||
| Age 61–70 years | 73 (4.6%) | 8.96 | ||||
| Age 71–80 years | 501 (31.7%) | 10.48 | ||||
| Age 81–90 years | 881 (55.8%) | 10.36 | ||||
| Age >90 years | 104 (6.6%) | 8.63 | ||||
| Seasonal data on 25-OH-vitamin D levels, July 2009 to March 2011 | ||||||
| n | Mean, ng/mL | Standard deviation | Median, ng/mL | Maximum ng/mL | Minimum ng/mL | |
| Summer ’09 (July to October) | 70 | 10.9 | 7.23 | 8 | 33 | 1 |
| Winter ’09/’10 (November to April) | 517 | 10.3 | 8.89 | 7 | 53 | 2 |
| Summer ’10 (May to October) | 598 | 10.7 | 8.95 | 8 | 64 | 2 |
| Winter ’10/’11 (November to March) | 393 | 9.29 | 8.06 | 7 | 77 | 2 |
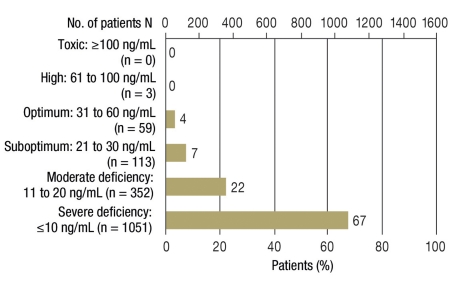
Overall, 89% of patients were deficient in 25-OH-vitamin D, with values below 20 ng/mL, and 96% of the measured values were below a limit of 30 ng/mL. Severe 25-OH-vitamin D deficiency below 10 ng/mL was identified in 67% of patients. Only 4% of all measured values lay in the target region of 30 to 60 ng/mL, three values (<1%) were between 60 and 100 ng/mL, and no individual measurements were in the toxic region of above 100 ng/mL. The highest measured value was 77 ng/mL (Figure).
Figure.
25-OH-vitamin D levels
In contrast to the variations seen between the brighter and the darker halves of the year in younger people (3), in our geriatric patients vitamin D levels were almost unaffected by the time of year. The maximum mean level over six months was 10.71 ng/mL in the brighter six months of 2010, and the minimum mean level was 9.29 ng/mL in the darker six months of 2010/2011 (Table 2).
Discussion
Vitamin D has well-known effects on bone and calcium metabolism, with a protective or therapeutic effect against osteoporosis and osteomalacia (5, 14).
However, vitamin D receptors are also found in the cells of many other organ systems. There is thus a large amount of epidemiological evidence of a possible positive effect of vitamin D on immune status (15, 16), cardiovascular diseases (17, 18), many tumor diseases (9, e6, e7), multiple sclerosis (19), and many other illnesses (1, e8, e9). As yet, though, there are no prospective, randomized, placebo-controlled, double-blind studies on this potential additional effect of vitamin D, besides its effect on the bones and muscles. Even the difficulty in quantifying individual levels of UV exposure and possible supplementary vitamin D intake make it hard to conduct such studies.
In addition to the effect on bone metabolism, in older patients the potential decrease in the risk of falls and fractures resulting from an adequate vitamin D supply is also important (6, 7, e3). This concerns not only direct effects on the bones but also effects on muscle strength and the neuromuscular system (20). For example, speed of walking increases when vitamin D supply is sufficient (21). According to two 2009 meta-analyses of the randomized controlled trials on the subject “falls and fractures” conducted up to that time, when vitamin D supply is adequate there is a 19% relative risk reduction for falling (overall risk of falling in the control group: 54.9%) (6), an 18% relative risk reduction for hip fracture (overall risk of hip fracture in the control group: 3.2%), and a 20% relative risk reduction for any non-vertebral fracture (overall risk of fracture in the control group: 5.4%) (e3). However, the meta-analyses showed a reduction in falls only for 25-OH-vitamin D levels above 24 ng/mL (6, 20), and a reduction in fractures only above 30 ng/mL (e3). The dosing of vitamin D supplements seems to play a role in its effect on the frequency of falls: According to a paper from 2010, a single dose of 500 000 IU/year actually led to an increase in the rate of falls and fractures in women aged over 70 years in the first few months following administration (22). The optimum doses and dosing intervals for reducing falls and fractures should be further clarified by future research.
As a rule, elderly people spend less time in the sun, and older skin has significantly less capacity to synthesize vitamin D from sunlight than the skin of younger people (23). Increased vitamin D production from sunlight is therefore not a realistic option for most geriatric patients. This is also demonstrated by the data showing that, unlike younger people with equivalent sun exposure during the summer, in the elderly there is clearly almost no increase in vitamin D levels during the summer months. Food contains only low concentrations of vitamin D, so an adequate vitamin D supply from food alone is almost impossible (24).
The study showed significant vitamin D deficiency; 89% of patients had 25-OH-vitamin D levels below 20 ng/mL. However, this is not a representative study of Germany’s elderly population, as data were obtained from patients of a geriatric rehabilitation facility in Trier with multiple morbidities, most of whom had previously received acute hospital treatment.
In an earlier study by the RKI in 1996, values below 20 ng/mL were obtained in 75% of women aged between 65 and 79 years (3). Another study of care facility residents found levels below 10 ng/mL in 68.3% of individuals (e1); the corresponding figure for patients in the present study was 67% with levels below 10 ng/mL. In acute geriatric patients, the mean level measured in another study was 14 ng/mL (e2), only slightly above the 10.2 ng/mL of the patients in the present study.
In view of how common vitamin D deficiency is in the elderly, the question arises of whether or not this has any clinical significance. The effects of vitamin D on bones and muscles are indisputable, and problems such as muscle weakness and pain in the musculoskeletal system, which are often seen as age-related, may also be partly caused by the frequent vitamin D deficiency in old age. This possible relationship should also be further investigated in the future, by studies with the potential to yield useful information.
Approximately 95% of the patients investigated in this study still lived at home before hospital admission, and only a small number were receiving care in a care home before their acute treatment and rehabilitation. This means that, in contrast to earlier assumptions, not only care home residents but also, according to these data, elderly patients admitted to geriatric rehabilitation facilities following acute hospital treatment comprise a high-risk group for vitamin D deficiency. Vitamin D levels may also have fallen somewhat prior to admission to the rehabilitation facility, as a result of patients’ acute hospital treatment and associated lack of sun exposure.
At the facility involved in the study, patients deficient in vitamin D received a load dose, usually 20 000 IU, over ten days, and regular 20 000 IU vitamin D supplements were then recommended, between once a week and once a month depending on baseline vitamin D levels. The dose needed for an adequate increase in vitamin D levels was significantly higher than the 400 IU/day vitamin D for the elderly recommended by the German Nutrition Society (DGE, Deutsche Gesellschaft für Ernährung), for example. The 2009 guidelines of Germany’s Osteology Umbrella Organization (Dachverband Osteologie, www.dv-osteologie.de) recommend a daily 2000 IU vitamin D supplement for patients with osteoporosis. In 2010 the US Institute of Medicine (IOM) recommended a daily intake of 800 IU vitamin D for persons aged over 70 years whose 25-OH-vitamin D levels have not been measured. The maximum daily dose given for this age group is 4000 IU (11).
A daily dose of 100 IU vitamin D results in a long-term increase in vitamin D levels of only approximately 1 ng/mL. This means that the daily intake of 400 IU currently recommended by the DGE would result in an increase of only approximately 4 ng/mL; with a mean like the value of 10.2 ng/mL found in this study, the target region of 30 to 60 ng/mL would remain some way off, and even the 20 ng/mL minimum level recommended by the IOM in 2010 would be hard to achieve.
Naturally, contraindications of vitamin D administration such as pre-existing hypercalcemia, primary hyperparathyroidism, sarcoidosis, and others must be taken into account. In the facility involved in this study, routine electrolyte testing did not reveal any hypercalcemia or other significant side effects caused by vitamin D during the patients’ hospital stays, which lasted an average of 22 days. However, as a safety precaution, calcium levels should be tested at regular intervals during treatment with vitamin D supplements. These vitamin D supplements are very cost-effective: Even administration of 20 000 IU every ten days (corresponding to a daily dose of 2000 IU) gives rise to an annual treatment cost of only some 15 euros at current prices.
Determining 25-OH-vitamin D levels, however, is relatively expensive. Outpatients must usually pay for this themselves (current price according to the German medical fees schedule [GOÄ, Gebührenordnung für Ärzte]: 27.98 euros). As more than 90% of all geriatric patients in this study had 25-OH-vitamin D levels below 30 ng/mL, and no measured levels were in the toxic range, the option of administering vitamin D to this group of patients even without first determining vitamin D levels should be considered. Whether there is a difference in the effects of an initial load dose following measurement of vitamin D levels and administration of vitamin D, for example, at the dose of 800 IU/day currently recommended by the IOM, on bone health, for example, or on the rate of falls and fracture rates, should be further clarified by future studies.
Conclusion
Vitamin D deficiency is very common among Germany’s elderly population. In 96% of the patients of a geriatric rehabilitation facility, the values measured were below the target region of 30 to 60 ng/mL 25-OH-vitamin D. This alarming figure is particularly important for health policy because of demographic changes. Adequate vitamin D levels are required for effective bone metabolism, and there is also a large amount of evidence that a good vitamin D supply in elderly patients can also reduce the frequency of falls and fractures. An adequate vitamin D supply is therefore very desirable for the elderly population. As it is currently almost impossible to obtain sufficient vitamin D intake from food alone in Germany, vitamin D supplements should be used to boost vitamin D supply, particularly for the elderly, in addition to the natural source of vitamin D, which is moderate sun exposure. The most recent recommendations currently available date from 2010 and were issued by the US IOM. They recommend a daily intake of 800 IU vitamin D for those aged over 70 years, and a daily maximum dose for this age group of 4000 IU.
Key Messages.
Vitamin D plays a substantial role not only in bone metabolism: There are also vitamin D receptors in the muscles and many other organs and tissues of the body.
An adequate vitamin D supply cannot be obtained from food alone; in addition, vitamin D synthesis in the skin is seldom sufficient to achieve adequate vitamin D levels in elderly patients.
96% of the patients of a German geriatric rehabilitation facility were found to have 25-OH-vitamin D levels below the target region of 30 to 60 ng/mL. 67% had very low levels, below 10 ng/mL.
Those aged over 70 years are therefore advised to take a daily supplement of 800 IU vitamin D, according to the recommendations of the Institute of Medicine.
Measurement of 25-OH-vitamin D levels followed by higher doses for a short period should be considered in order to restore adequate vitamin D levels swiftly when they are very low.
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
Footnotes
Conflict of interest statement
The author gives lectures for MerckSerono.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Hirani V, Primatesta P. Vitamin D concentrations among people aged 65 years and over living in private households and institutions in England: population survey. Age Ageing. 2005;34:485–491. doi: 10.1093/ageing/afi153. [DOI] [PubMed] [Google Scholar]
- 3.Hintzpeter B, Mensink GB, Thierfelder W, et al. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–1090. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 4.Hintzpeter B, Scheidt-Nave C, Müller MJ, et al. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. J Nutr. 2008;138:1482–1490. doi: 10.1093/jn/138.8.1482. [DOI] [PubMed] [Google Scholar]
- 5.Priemel M, von Domarus C, Klatte TO, et al. Bone mineralization defects and vitamin D deficiency: Histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. Journal of Bone and Mineral Research. 2010;2(Issue 25):305–312. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339 doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolland MJ, Grey A, Reid IR. Vitamin D and falls. Time for a moratorium on vitamin D meta-analyses? BMJ. 2009;339 doi: 10.1136/bmj.b4394. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. Environment factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61(628S-45S) doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 9.Zeeb H, Greinert R. The role of vitamin D in cancer prevention—does UV protection conflict with the need to raise low levels of vitamin D? Dtsch Arztebl Int. 2010;107(37):638–643. doi: 10.3238/arztebl.2010.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lips P. Vitamin D deficiency and secondary hyperparathyreodism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrine Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 11.Ross AC, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. Epub 2009, Jun 19. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 14.Zittermann A. The estimated benefits of Vitamin D for Germany. Mol Nutr Food Res. 2010;54:1–8. doi: 10.1002/mnfr.200900494. [DOI] [PubMed] [Google Scholar]
- 15.Li-Ng M, Aloia NF, Pollack S, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137:1396–1404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 16.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as a supplemental treatment for tuberculosis. Am J Respir Crit Care Med. 2009;179:843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer M, Begerow B, Minne HW, et al. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 18.Kilkkinen A, Knekt P, Aro A, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170:1032–1039. doi: 10.1093/aje/kwp227. [DOI] [PubMed] [Google Scholar]
- 19.Ascherio A, Munger KL, Simon C. Vitamin D and multiple sclerosis Lancet Neurol. 2010;9:599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari H, Steahelin HB. Vitamin D: Update bone and muscle effects. Aktuel Ernahrungsmed. 2010;35:18–22. [Google Scholar]
- 21.Annweiler C, Schott AM, Montero-Odasso M, et al. Cross-sectional association between serum vitamin D concentration and walking speed measured at usual and fast pace among older women: the EPIDOS study. J Bone Miner Res. 2010;25:1858–1866. doi: 10.1002/jbmr.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders KM, et al. Annual high-dose oral vitamin D and falls and fractures in older woman. JAMA. 2010;303:1185–1122. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 23.Trémezaygues L, Reichrath J. Zur Bedeutung des Vitamin Stoffwechsels in der humanen Haut. Hautarzt. 2010;61:478–486. doi: 10.1007/s00105-009-1893-z. [DOI] [PubMed] [Google Scholar]
- 24.Max Rubner-Institut. Nationale Verzehrsstudie II Ergebnisbericht, Teil 2. Karlsruhe. www.was-esse-ich.de/uploads/media/NVSII_Abschlussbericht_Teil_2.pdf. 2008 [Google Scholar]
- e1.Kaiser R, et al. Ernährungszustand und Funktionalität von Pflegeheimbewohnern - eine Longitudinalstudie - www.geriatrie-nuernberg.de/index.php?id=90e [Google Scholar]
- e2.Saeglitz C. Mangelernährung bei geriatrischen Patienten im Krankenhaus - Prävalenz, mögliche Ursachen, übliche Therapie und prognostische Bedeutung. http://hss.ulb.uni-bonn.de/2007/1150/1150.pdf [Google Scholar]
- e3.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral Vitamin D and dose dependency. A meta-analysis of randomized controlled trials. Arch Intern Med. 2009;116:551–560. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- e4.Bernecker PM. Osteomalazie und Rachitis. Wiener Med Wochenschr. 2004;154:102–106. doi: 10.1007/s10354-004-0054-3. [DOI] [PubMed] [Google Scholar]
- e5.Melamed Ml, Michos ED, Post W, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Garland C F, French CB, Baggerly LL, Heaney RP. Vitamin D supplement doses and serum 25-Hydroxyvitamin D in the range associated with cancer prevention. Anticancer Research. 2011;31:617–622. [PubMed] [Google Scholar]
- e7.Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340 doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Hutchinson MS, Grimnes G, Joakimsen RM, et al. Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population. The Tromsø study. Eur J Endocrinol. 2010;162:935–942. doi: 10.1530/EJE-09-1041. [DOI] [PubMed] [Google Scholar]
- e9.Ginde AA, Scragg R, Schwartz RS, et al. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older US. adults. J Am Geriatr Soc. 2009;57:1595–1603. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]