Abstract
To study how various anesthetics affect the relationship between stimulus frequency and generated functional magnetic resonance imaging (fMRI) signals in the rat dentate gyrus, the perforant pathway was electrically stimulated with repetitive low frequency (i.e., 0.625, 1.25, 2.5, 5, and 10 Hz) stimulation trains under isoflurane/N2O, isoflurane, medetomidine, and α-chloralose. During stimulation, the blood oxygen level-dependent signal intensity (BOLD response) and local field potentials in the dentate gyrus were simultaneously recorded to prove whether the present anesthetic controls the generation of a BOLD response via targeting general hemodynamic parameters, by affecting mechanisms of neurovascular coupling, or by disrupting local signal processing. Using this combined electrophysiological/fMRI approach, we found that the threshold frequency (i.e., the minimal frequency required to trigger significant BOLD responses), the optimal frequency (i.e., the frequency that elicit the strongest BOLD response), and the spatial distribution of generated BOLD responses are specific for each anesthetic used. Concurrent with anesthetic-dependent characteristics of the BOLD response, we found the pattern of stimulus-induced neuronal activity in the dentate gyrus is also specific for each anesthetic. Consequently, the anesthetic-specific influence on local signaling processes is the underlying cause for the observation that an identical stimulus elicits different BOLD responses under various anesthetics.
Keywords: α-chloralose, electrical stimulation, fMRI, hippocampus, isoflurane, medetomidine
Introduction
Functional magnetic resonance imaging (fMRI) has proven to be an effective method of studying localized variations in neuronal activities induced by a number of more or less complex stimuli. However, fMRI visualize changes in neuronal activity only indirectly; namely by mapping hemodynamic parameters, such as changes in the blood oxygen level (i.e., BOLD response) or the local blood volume/flow. Although it is widely accepted that the measured variations of these hemodynamic parameters depend on changes in neuronal activities in these regions, the direct relationship between both is still not completely elucidated. With the introduction of a combined measurement of electrophysiological and fMRI responses (Logothetis et al, 2001), it became possible to study this relationship in detail. Furthermore, by concurrent stimulation of a defined fiber bundle projecting directly to the studied region, the input activity could also be adjusted to a specific amount, intensity, and/or pattern (Angenstein et al, 2007). Using these approaches, it became obvious that neither the input (defined by the stimulation protocol) nor the output activity (i.e., spiking of the principal cells) is directly related to the observed BOLD response in this area, but rather the amount of local processing in this area. The quality of the local processing is controlled by all participating neurons, that is, excitatory (principal) as well as inhibitory interneurons. Consequently, any pharmacological intervention affecting one of these components can interfere with the generated BOLD response in this region, even the input activity remains essentially the same (Harris et al, 2010).
Performing fMRI experiments with animals normally requires the immobilization of the animal during the scan to avoid motion artifacts. Therefore, in contrast to human studies, animals have to be sedated or even anesthetized. All sedatives and narcotics used affect the interplay of excitatory and inhibitory systems in favor of inhibition, and thus may confound the BOLD response.
Previous experiments using electrical stimulation of the perforant pathway have shown that identical stimulation protocols produces BOLD responses that vary considerably, depending on the applied narcosis (Angenstein et al, 2010). The differences were more distinct during low intensity stimulations, that is, when the applied stimuli did not elicit action potentials in principal cells. During this condition, only minor or no BOLD responses were observed when isoflurane was used as anesthetic, whereas under medetomidine and α-chloralose clear BOLD responses were generated. Consequently, the minimal stimulation intensity that is required to trigger significant BOLD responses depends on the applied anesthetic/sedative.
In addition to the stimulation intensity, there also exists a threshold for the lowest stimulation frequency required to elicit a significant BOLD response. Using isoflurane/N2O as anesthetic, the threshold was found to be around 5 Hz (Angenstein et al, 2007). Performing the same experiment under urethane also found that frequencies of 4 Hz or higher were able to induce significant BOLD responses in the hippocampal formation (Canals et al, 2008).
In addition to studies using direct electrical stimulation of a central fiber bundle, significant BOLD responses in the somatosensory cortex can be reliably generated by peripheral electrical stimulation of the rat forepaw. In this approach, very low stimulation frequencies trigger significant BOLD responses in the somatosensory cortex, namely 1 to 3 Hz under α-chloralose (Haasdijk et al, 2002; Huttunen et al, 2008; Keilholz et al, 2004), 1.5 to 3 Hz under isoflurane (Kim et al, 2010; Masamoto et al, 2007), and 3 Hz under medetomidine (Weber et al, 2006; Zhao et al, 2008) and urethane (Huttunen et al, 2008). The more interesting part about these studies is that under this stimulation condition, the frequencies that elicit the strongest BOLD response differ considerably from between 1 and 3 Hz for α-chloralose (Huttunen et al, 2008; Keilholz et al, 2004), and around 10 to 12 Hz for medetomidine, isoflurane, and urethane (Huttunen et al, 2008; Kim et al, 2010; Masamoto et al, 2007; Zhao et al, 2008). One reason for these differences could be that the anesthetics may not only interfere with local processes in the somatosensory cortex, but also with signal processing in upstream structures, such as the thalamus. To test whether varying BOLD responses under different anesthetics depends on local effects of the anesthetic, or are rather the result of an altered signal processing on the way to the appropriate region, we used an experimental approach in which a single brain structure, that is, the dentate gyrus, becomes only monosynaptically activated by electrical stimulation of the perforant pathway. This pathway projects directly to granular cells and interneurons in the dentate gyrus and, in addition, to other subregions of the hippocampal formation such as CA3, CA1, and the subiculum (Amaral and Lavenex, 2007). Granular cells, as principal cells in the dentate gyrus, only project to the CA3 field of the hippocampus. CA3 pyramidal cells, in turn, activate via Schaffer collaterals CA1 pyramidal cells and via commissural projections neurons in the CA3, CA2, and CA1 field of the contralateral hippocampus proper (Amaral and Lavenex, 2007). Most CA1 pyramidal cells project to the subiculum. The subiculum, as a major output structure of the hippocampal formation, is connected with a number of cortical and subcortical structures, including the entorhinal, limbic, prefrontal, and anterior cingulated cortex, as well as the amygdaloid complex, bed nucleus of stria terminalis, thalamus, mammillary nucleus, and nucleus accumbens (Witter, 2006). Consequently, anesthesia-induced variations in the BOLD response that depend on interferences with signal propagation should affect BOLD responses outside the dentate gyrus or hippocampus but not in the dentate gyrus. In contrast, if the anesthetics affect specifically local signaling processes and/or mechanisms of neurovascular coupling, then BOLD responses in the dentate gyrus during identical stimulation conditions should vary when different anesthetics are present. To prove, that anesthetics modify local signaling processing, concurrent electrophysiological recording can be used to monitor the elicited neuronal responses in the dentate gyrus. Local signal processing in a region can be roughly defined as relation between input and output activity. The input activity into the dentate gyrus is predefined by the chosen stimulation condition, whereas the output activity corresponds to the measured action potentials of the granular cells, that is, the population spikes. That means, local signal processing is changed whenever an identical stimulation protocol causes an altered population spike pattern. If anesthesia-dependent changes in the BOLD response within the dentate gyrus are not accompanied with changes in local signal processing, then the effect should mainly be mediated by an interference of the anesthetic with the neurovascular coupling.
To test how anesthetics affect the generation of a BOLD response, we compared the development of BOLD responses in the dentate gyrus to identical stimulation protocols under four frequently used anesthetic/sedative conditions, namely isoflurane/N2O, isoflurane, medetomidine, and α-chloralose. Stimulation-induced changes in neuronal activities were measured directly by electrophysiological recordings in the dentate gyrus and indirectly by BOLD-fMRI in the entire brain. Consequently, within the dentate gyrus, BOLD responses can be related to concurrent recorded electrophysiological data or neuronal activities, whereas BOLD responses outside the dentate gyrus indicate how signals become propagated.
Materials and methods
Animals and Surgical Procedure
For electrode implantation, 7- to 8-week-old male Wistar rats were anesthetized with pentobarbital (40 mg/kg intraperitoneally) and placed into a stereotactic frame. A bipolar stimulation electrode (114 μm in diameter, made from teflon-coated tungsten wire) was placed into the perforant pathway in the right hemisphere at the coordinates anterior-posterior (AP): −6.9, medial-lateral (ML): 4.1 mm from Bregma, dorsal-ventral (DV): 2.5 to 3.0 mm from the dural surface. A monopolar recording electrode (114 μm in diameter, made from teflon-coated tungsten wire) was lowered into the granular cell layer of the right dentate gyrus AP: −2.8 mm, ML: 1.8 mm from Bregma, DV: 2.8 to 3.2 mm from the dural surface. Monitoring the monosynaptic evoked field potentials during implantation controlled the correct placement, especially with regard to electrode depth. Grounding and indifferent electrodes (silver-wires) were set on the dura through the left side of the cranium, and fixed to the skull with dental cement and plastic screws. Following surgery, the animals were housed individually and given 7 days for recovery, with ad libitum food and water.
The experiments were approved by the animal care committee of the State Saxony-Anhalt (No. 203.h-42502-2-852 IfN).
Combined Functional Magnetic Resonance Imaging and Electrophysiological Measurements
Detailed descriptions of the experimental setup used for simultaneous fMRI and electrophysiological measurements during electrical stimulation of the perforant pathway can be found in Angenstein et al (2007). For the fMRI experiment, the anesthetized animals were connected to the stimulation and recording electrode after fixation of the head. Heating was provided from the ventral side and heart rate, breathing rate, and oxygen saturation was monitored during the entire experiment using an MRI-compatible pulse oxymeter (MouseOx, Starr Life Sciences Corp., Pittsburgh, PA, USA).
To test the influence of different anesthetics on electrophysiological and BOLD responses, different groups of rats were anesthetized with: (1) isoflurane/N2O (1.5% for induction and 1.1% to 1.3% during maintenance, in 50:50 N2O:O2; v:v); (2) isoflurane (1.5% for induction and 1.1% to 1.3% during maintenance, in 50:50 N2:O2; v:v); (3) medetomidine hydrochloride (Domitor, Pfizer GmbH, Karlsruhe, Germany; bolus of 50 μg/kg subcutaneously and after 15 minutes a continuous infusion of 100 μg/kg per hour subcutaneously); or (4) α-chloralose (Sigma-Aldrich, Hamburg, Germany; bolus 80 mg/kg intraperitoneally and after 30 minutes continuous infusion of 40 mg/kg per hour intraperitoneally). All experiments started with an isoflurane (1.5%) anesthesia that was switched to medetomidine or α-chloralose after the animals were prepared for the measurement. During all forms of narcosis, a mixture of N2 and O2 was given (50:50 N2:O2; v:v), except for the isoflurane/N2O group that received the same mixture of N2O and O2. All necessary adjustments for electrophysiological recordings such as determination of the stimulation intensities (see below) and the fMRI measurements, including the anatomical images, were performed in parallel and lasted about 45 minutes. Thus, the switch from isoflurane to medetomidine or α-chloralose was at least 45 minutes before beginning the fMRI experiments. The T1/2 elimination time of isoflurane is about 7.6 minutes (Chen et al, 1992), thus at the beginning of the fMRI experiment, the remaining isoflurane concentration should be negligible. The following fMRI measurement took 25 minutes; therefore, the total experiment lasted around 70 minutes. Animals receiving a low frequency stimulation protocol under one anesthesia were randomly selected for one further experiment using another anesthesia, with the exception of animals anesthetized by α-chloralose which were not reused. The time interval between the two experiments was at least 1 week.
Magnetic resonance imaging experiments were performed on a Bruker Biospec 47/20 scanner (Bruker Biospin GmbH, Ettlingen, Germany) at 4.7 T (free bore of 20 cm) equipped with a BGA09 (400 mT/m) gradient system. A 50-mm Litzcage small animal imaging system (DotyScientific Inc., Colombus, SC, USA) was used for RF excitation and signal reception. For anatomical images, eight horizontal T2-weighted spin-echo images were obtained simultaneously using a RARE sequence (rapid acquisition relaxation enhanced (Hennig et al, 1986)), using the following parameters: repetition time (TR)=4,000 milliseconds, echo time (TE)=15 milliseconds, slice thickness 1 mm, FOV 40 × 40 mm2, matrix 256 × 256, RARE factor 8, NEX 4. The total scanning time was 8 minutes 32 seconds. Functional MRI was performed using an EPI (echo planar imaging) sequence with the following parameters: TR=2,000 milliseconds, TE=24 milliseconds, slice thickness 1 mm, FOV 40 × 40 mm2, matrix 64 × 64, total scanning time per frame 2 seconds.
To determine the appropriate stimulation intensity for each individual fMRI experiment, the perforant pathway was first stimulated using single test pulses with increasing intensities (i.e., three test pulses at 10 seconds intervals for the following intensities: 50, 100, 200, 300, 400, 600 μA). The recordings were taken at 2 minutes intervals, with the exception of 600 μA, which were measured at 5 minutes intervals under the respective anesthesia. Using the input/output curve, the intensity required to elicit a population spike and the maximal population spike amplitude were determined. The intensity that elicited 50% of the maximal population spike amplitude was used for the following fMRI experiment (isoflurane/N2O: 287±26 μA; isoflurane: 250±35 μA; medetomidine: 304±20 μA; α-chloralose: 261±30 μA).
During the fMRI experiment, the perforant pathway was electrically stimulated with three subsequent stimulation blocks (see Figure 1). Each block contained seven identical stimulation trains. After 2 minutes, baseline one stimulation train was applied every minute. One stimulation train lasted 8 seconds (or four frames in the fMRI), followed by 52 seconds (or 26 frames in the fMRI) rest. The number of stimuli per train was adjusted to 5 (or 0.625 Hz), 10 (1.25 Hz), 20 (2.5 Hz), 40 (5 Hz), or 80 (10 Hz). Because experiments under α-chloralose were terminal, all five blocks were given in a single session.
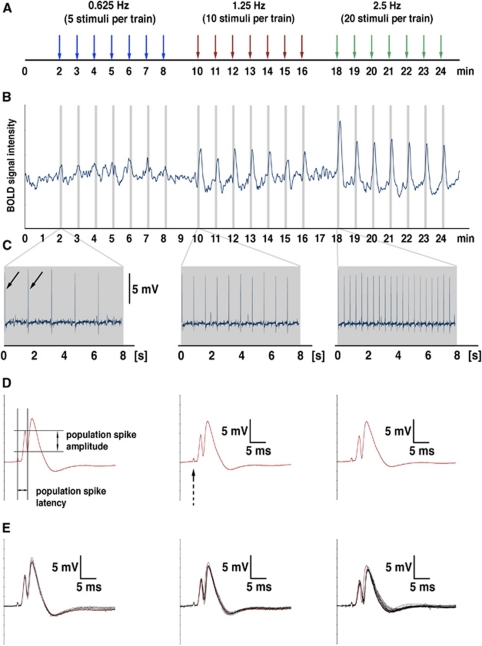
Figure 1.
Example of one simultaneous measurement of blood oxygen level-dependent (BOLD) signals and electrophysiological responses in the rat dentate gyrus during electrical stimulation of the right perforant pathway. (A) The perforant pathway was electrically stimulated with three consecutive stimulation blocks, each containing seven identical stimulation trains. The duration of one stimulus train was 8 seconds, followed by 52 seconds rest. The stimulus frequency was always doubled in the subsequent train and, if not otherwise mentioned, three different frequencies (here 0.625, 1.25, and 2.5 Hz) were applied during one experiment. (B) Time course of BOLD signal intensities in the right dentate gyrus during the entire experiment. The time course represents the variations in BOLD signal intensities of all significantly activated voxels in this region. The gray bars indicate the stimulation periods (i.e., 8 seconds trains). (C) Time course of field potentials during the entire stimulation period (8 seconds, equivalent to the gray bars in B). The arrows indicate the first two elicited population spikes. (D) For each recorded population spike, the amplitude and latency was determined. The location of the stimulus artifact is indicated with dashed arrow. (E) For comparison all elicited population spike within one train are overlaid, the first population spike is highlighted in red. The color reproduction of this figure is available on the Journal of Cerebral Blood Flow and Metabolism journal online.
Electrophysiological responses were recorded using a sampling rate of 4,000 Hz, and band pass filtered from 1 Hz to 5 kHz using a differential amplifier (EX4-400, Science Products, Hofheim, Germany). The signal was converted using an analog-to-digital interface (power-CED: Cambridge Electronic Design, Cambridge, UK), and stored on a personal computer. Because the artifacts caused by the imaging system were small compared with the recorded field potentials, no further processing was necessary (see Figures 1C and 1D).
Data Processing and Analysis
The functional data were loaded and converted into BrainVoyager data format. A standard sequence of preprocessing steps implemented in the BrainVoyager QX software (Brain Innovation, Maastrich, The Netherlands), such as 3D motion correction and temporal filtering (Gaussian filter; full width at half maximum three data points) were applied to each data set. Functional activation was analyzed by correlation of the observed signal intensity changes in each voxel with the given stimulus protocol (see above; general linear model (multiple regression analysis): single subject), and based on this an appropriate activation map was generated. To account for the hemodynamic delay, the stimulus representing block design was modified by a double-γ hemodynamic response function (onset 0 seconds, time to response peak 5 seconds, time to undershoot peak 15 seconds). To exclude false-positive voxels, we considered only voxels with a significance level less than P=10−9 for the analysis of the size of the activated area (tmin=6). Event-related BOLD responses were calculated by measuring the signal intensities starting six frames (−12 seconds until 0 seconds) before stimulus onset (stimulus presentation was between 0 and 8 seconds, which corresponds to four frames) until 20 frames (8 to 48 seconds) after the stimulus end. The averaged signal intensities within the appropriate area in the first five frames (−12 seconds until −2 seconds) were set to 100%. The event-related averaged BOLD response represents the arithmetic mean±standard error of the mean (s.e.m.) of all individual BOLD responses within the appropriate stimulation block. To visualize the activation pattern during each of the three stimulation blocks, all fMRI data sets were aligned to a 3D standard rat brain using anatomical landmarks. These data sets were then further analyzed with a linear regression analysis (general linear model, multisubject analysis, implemented in BrainVoyager QX software). Only voxels with a significance level less than tmin=14 are marked. To quantify the magnitude of BOLD response, the area below the BOLD response curve was measured for each animal (starting with the stimulus onset t=0 up to 12 seconds after stimulus end, that is, 10 frames from the stimulus onset), then averaged for comparison (see Figure 7). To quantify the volume of the activated region according to the BOLD response, the number of significantly activated voxels within the appropriate region were counted for each animal then averaged for comparison. All averaged data represent arithmetic mean values±standard error of the mean (s.e.m.).
Results
To search for the minimal synchronized activity that is required to induce significant BOLD responses in the hippocampal formation, the right perforant pathway was electrically stimulated with three subsequent stimulation blocks. Each block contained seven identical stimulation trains, each 8 seconds long with 52 seconds rest in between. During the first block, each stimulus train contained 20 stimuli (i.e., 2.5 Hz), in the second block, each stimulation train contained 40 stimuli (i.e., 5 Hz), and in the third block, each train contained 80 stimuli (i.e., 10 Hz). Consequently, the number of stimuli was always doubled in the subsequent stimulation block. These experiments were performed under isoflurane/N2O, isoflurane, medetomidine, and α-chloralose.
Blood Oxygen Level-Dependent Responses Under Isoflurane/N2O
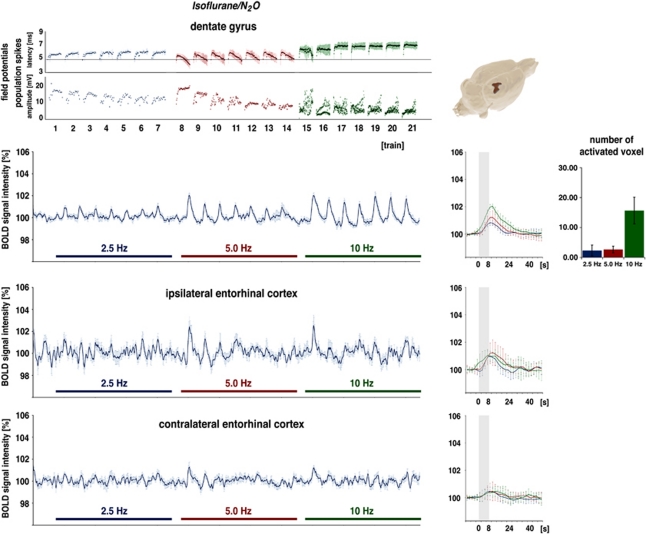
Under isoflurane/N2O, 2.5 Hz stimulation for 8 seconds elicited in one out of seven animals significant changes in BOLD signal intensities in the dentate gyrus. This resulted in a small but not significant stimulus-dependent variation in BOLD signal intensities when the BOLD time series were averaged for all animals (Figure 2). Doubling the stimulus number to 40 (or 5.0 Hz) within one stimulation train triggered a small but significant BOLD response in the dentate gyrus region in all animals. A further doubling of the stimulus number during the third block (i.e., 10 Hz or 80 stimuli) again triggered an increased BOLD response in the dentate gyrus but still no significant BOLD responses in the right and left entorhinal cortex regions. Simultaneous electrical recordings in the dentate gyrus revealed that during repetitive 2.5 Hz stimulations, the population spike latency increased slightly during each train. However, the population spike amplitude varied with no clear recurring pattern. In contrast, during repetitive 5 Hz stimulation trains, the population spike latency increased at the beginning of each train and returned then to the starting level, whereas the population spike amplitude increased during each train, but decreased on average during consecutive trains. The following 10 Hz stimulation trains caused variable responses during the first train, changing to a more consistent pattern during later stimulation trains that displayed alternating population spike amplitudes that on average decreased while response latencies increased.
Figure 2.
Electrophysiological responses and variations in blood oxygen level-dependent (BOLD) signal intensities during electrical stimulation of the perforant pathway with repetitive 2.5, 5, and 10 Hz stimulation trains under isoflurane/N2O. (Top) Recordings in the dentate gyrus region. Significant BOLD responses were first consistently observed during 5 Hz stimulation trains and the magnitude of BOLD responses increased during 10 Hz stimulation trains as seen in the event-related averages (right, blue line: 2.5 Hz stimulation trains, red line: 5.0 Hz stimulation trains, green line: 10 Hz stimulation trains, gray box: the location of the stimulation train). The number of significantly activated voxels during each stimulation condition is depicted as bars. (Right) 3D visualization of significant BOLD responses during the entire experiment. Only the dentate gyrus became significantly activated when the results of all seven animals were averaged. Simultaneous electrophysiological recordings revealed that during 2.5 Hz stimulation trains, the latency increased during each train, indicating that the first stimulus in each train was processed differently to the subsequent trains. A qualitatively different time course was observed during repetitive 5 Hz stimulation trains and 10 Hz stimulation trains. The strong variations in the population spike amplitude during 10 Hz stimulation trains is caused by the appearance of an alternating response, that is, a large population spike followed by an absent or minor population spike. (Bottom) Concurrently measured variations in BOLD signal intensities in the right and left entorhinal cortex regions. Stimulus-dependent changes in BOLD signal intensities in the right entorhinal cortex were only observed during the first 5 and 10 Hz stimulation trains, whereas BOLD signals in the left entorhinal cortex remained unaffected. The color reproduction of this figure is available on the Journal of Cerebral Blood Flow and Metabolism journal online.
Blood Oxygen Level-Dependent Responses Under Isoflurane
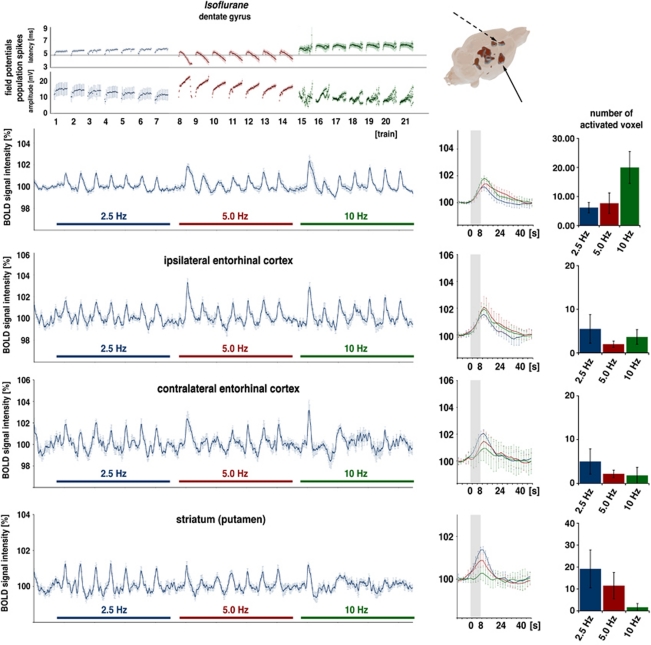
In a second series of experiments, N2O was omitted and replaced by N2, thus the animals were only anesthetized by 1.1% to 1.3% (v:v) isoflurane. The application of the stimulation protocol described previously under isoflurane/N2O resulted in a shift in the threshold frequency that was sufficient to induce significant BOLD responses in the dentate gyrus toward 2.5 Hz (Figure 3). In five out of seven animals, significant BOLD responses were observed in the dentate gyrus during 2.5 Hz stimulation trains. In addition to the dentate gyrus, significant BOLD responses were also generated in the right and left entorhinal cortex regions during 2.5 and 5 Hz stimulation trains. During 10 Hz stimulation trains, consistent BOLD responses were only observed in the right entorhinal cortex region, whereas after the first 10 Hz stimulation train, the BOLD responses in the contralateral entorhinal cortex region diminished. Thus, increasing the stimulation frequency resulted in enhanced BOLD responses in the dentate gyrus, but not in the entorhinal cortex regions. Similarly to the magnitude of the BOLD response, the volume of the activated region, defined by the number of significantly activated voxels, changed only in the dentate gyrus/hippocampus proper, but not in the two entorhinal cortex regions.
Figure 3.
Electrophysiological responses and variations in blood oxygen level-dependent (BOLD) signal intensities during electrical stimulation of the perforant pathway with repetitive 2.5, 5, and 10 Hz stimulation trains under isoflurane. (Top) Under this condition, stimulus-dependent variations in BOLD signal intensities in the dentate gyrus were observed during 2.5 Hz stimulation trains. Increasing the stimulation frequency caused an increase in the magnitude of the BOLD response in the dentate gyrus, as seen in the event-related average and the number of activated voxels (right). Under isoflurane, significant variations in BOLD signal intensities were not only observed in the right dentate gyrus but also in the right entorhinal cortex, striatum (solid arrow), and anterior cingulated cortex (dashed arrow). Concurrent electrophysiological recordings revealed different response patterns in the dentate gyrus, especially during 5 Hz stimulation trains; a clear augmentation of the population spike amplitude can be observed. (Bottom) BOLD time series in the right and left entorhinal cortex revealing stimulus-dependent variations in the right entorhinal cortex but not in the left side. In the striatum, significant BOLD responses are generated during 2.5 Hz stimulation trains and to a lesser extend during 5 Hz stimulation but not during 10 Hz stimulation trains.
During repetitive 2.5 Hz stimulation, a significant BOLD response was also detected in the right and left striatum. Doubling the stimulation frequency clearly reduced the BOLD response in this region and a second doubling of the stimulation frequency per train did not generate any detectable changes in BOLD signal intensities in the striatum.
Simultaneous electrophysiological recordings also revealed different response patterns from the granular cells during the second block, as observed under isoflurane/N2O. Although the population spike latencies developed similarly, the population spike augmentation within each train was much more distinctive (Figure 3).
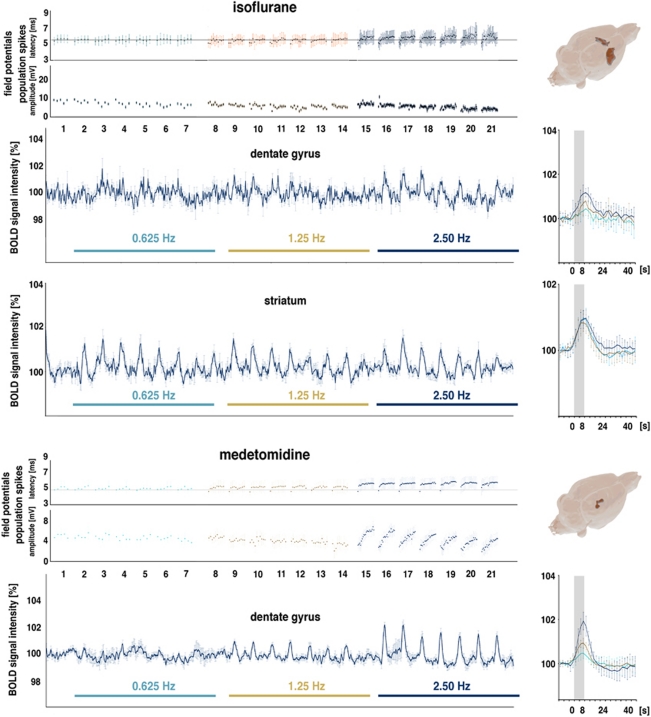
Owing to stimulation at 2.5 Hz triggering significant BOLD responses in the dentate gyrus, an additional experiment was performed where the stimulation frequency was reduced to 10 per train (i.e., 1.25 Hz) and 5 per train (i.e., 0.625 Hz). Both frequencies did not trigger significant BOLD responses in the dentate gyrus; therefore, the threshold frequency required to generate BOLD responses in the dentate gyrus amounts to 2.5 Hz (Figure 4). However, both stimulation frequencies triggered significant BOLD signal intensity changes in the striatum (Figure 4); thus, during very low stimulation frequencies significant changes in BOLD signal intensities were only detected in the striatum.
Figure 4.
Electrophysiological responses and variations in blood oxygen level-dependent (BOLD) signal intensities during electrical stimulation of the perforant pathway with repetitive, and 2.5 Hz stimulation trains under isoflurane or medetomidine. (Top) Under isoflurane, BOLD responses in the dentate gyrus were only generated during 2.5 Hz stimulation trains. In contrast, in the striatum, significant BOLD signal responses were detected during all stimulation trains. Consequently, 3D visualization of activated voxels highlights only the striatum as an activated region. Recorded electrophysiological responses indicate that during 1.25 Hz stimulation, the population spike latency increased during each train. (Bottom) Under medetomidine, stimulus-induced variations in BOLD signal intensities observed during 1.25 Hz stimulation trains. 3D visualization of activated voxels depicts only a restricted region in the dentate gyrus. Concurrent electrophysiological recordings also indicate that during 1.25 Hz stimulation trains, a variation in the processing of successive stimuli took place. Thus, population spike latencies always increase after the first stimulus, as seen also during the 2.5-Hz stimulation trains; however, augmentation of the population spike amplitude was absent.
Electrical stimulation of the perforant pathway with 1.25 Hz stimulation trains induced an increase in the population spike latencies during the train, but no clear variations in the population spike amplitude. In contrast, during 0.625 Hz stimulation trains, the elicited population spikes remained similar (Figure 4).
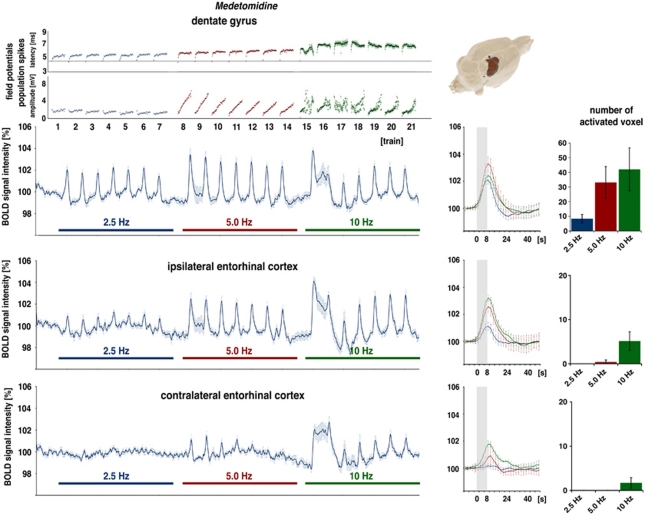
Blood Oxygen Level-Dependent Responses Under Medetomidine
In a third set of experiments, the same stimulation protocol was applied under medetomidine. Again, stimulation of the perforant pathway with 2.5 Hz (or 20 stimuli in 8 seconds) triggered significant BOLD responses in the dentate gyrus (Figure 5) in all seven of the animals tested. Doubling the stimulus number in each train (to 40 stimuli) during the second stimulation block increased significantly the magnitude of BOLD responses in the dentate gyrus and generated stable, significant BOLD responses in the right entorhinal cortex region and, at the beginning, in the contralateral entorhinal cortex area. A second doubling of the stimulus number per train (to 80 stimuli) did not further increase the magnitude of BOLD response in the dentate gyrus, but rather reduced the magnitude to a level observed during the first block. During this stimulation condition, significant BOLD responses were also observed in the right and left entorhinal cortex regions. In contrast to the dentate gyrus, the average magnitude of BOLD responses increased with higher stimulation frequencies in the two entorhinal cortex regions (Figure 5). To determine the threshold frequency, the stimulus number per train was again halved to 10 (i.e., 1.25 Hz) and to 5 (i.e., 0.625 Hz). During the first block, the 0.625-Hz stimulation trains did not cause significant changes in BOLD signal intensities, whereas 1.25 Hz stimulation trains applied during the second train generated significant BOLD responses in the dentate gyrus in four out of six animals; thus, the threshold frequency under medetomidine is around 1.25 Hz (Figure 4). In clear contrast to stimulations under isoflurane, BOLD signal intensities in the striatum were not affected by any chosen frequency under medetomidine.
Figure 5.
Electrophysiological responses and variations in blood oxygen level-dependent (BOLD) signal intensities during electrical stimulation of the perforant pathway with repetitive 2.5, 5, and 10 Hz stimulation trains under medetomidine. (Top) Clear BOLD responses in the dentate gyrus observed during 2.5 Hz stimulation trains. Doubling the stimulation frequency to 5.0 Hz in each train increased the magnitude of the BOLD response, whereas another doubling to 10 Hz reduced the magnitude of BOLD responses to a level seen at 2.5 Hz. Electrophysiological responses in the dentate gyrus are similar to that seen under isoflurane (see Figure 3) except that the population spike latencies developed differently during 5.0 Hz stimulation trains. (Bottom) BOLD time series in the right and left entorhinal cortex display a significant stimulus-dependent signal change in the right entorhinal cortex during all trains, and in the left entorhinal cortex only during the 10-Hz stimulation trains.
The concurrent electrophysiological recordings showed similar response patterns as observed under isoflurane/N2. Noticeable differences were detected during 5 Hz stimulation trains, though under isoflurane, the latency decreased after an initial increase; however, under medetomidine, the latency clearly increased after the first stimulus and remained at this level (Figure 5).
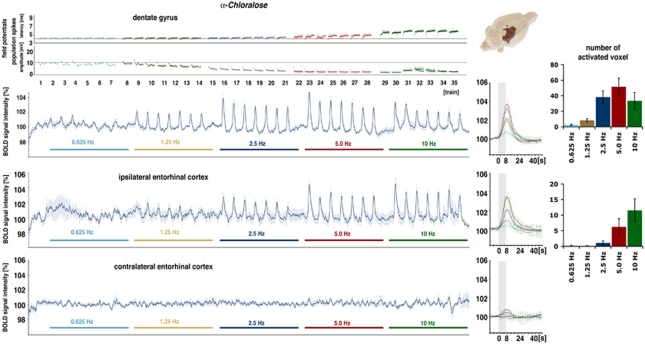
Blood Oxygen Level-Dependent Responses Under α-Chloralose
In a fourth set of experiments, the same stimulation protocol was applied under α-chloralose. Because α-chloralose can only be used for terminal experiments, the rats were stimulated in one experiment with five subsequent blocks starting from 0.625 Hz up to 10 Hz. Repetitive stimulation of the perforant pathway with 0.625 Hz trains elicited significant BOLD responses in the dentate gyrus from three out of nine animals. Doubling the stimulation frequency to 1.25 Hz triggered significant BOLD responses in eight out of nine animals, and a second doubling to 2.5 Hz induced significant BOLD responses in all animals. A further doubling of the stimulation frequency to 5 Hz caused a slight increase in the BOLD response, and during subsequent 10 Hz stimulation trains, the BOLD response declined or even collapsed at the end, as seen in three animals. In addition to the dentate gyrus, stimulus-related BOLD signal intensity changes were also observed in the right entorhinal cortex region during stimulation frequencies >1.25 Hz. In the left entorhinal cortex region, BOLD signal intensities were not affected by electrical perforant pathway stimulations in the range from 0.625 up to 10 Hz (Figure 6). Similarly to the results under medetomidine, no stimulus-related variations in BOLD signal intensities were observed in the striatum under α-chloralose during any of the applied stimulation conditions.
Figure 6.
Electrophysiological responses and variations in blood oxygen level-dependent (BOLD) signal intensities during electrical stimulation of the perforant pathway with five stimulation blocks under α-chloralose. In the dentate gyrus, clear BOLD responses were observed during 1.25 Hz stimulation trains. Doubling the stimulation frequency increased the magnitude of the BOLD response. Another doubling of stimulation frequency to 5 Hz did not further enlarge the BOLD response and a further doubling of the stimulation frequency caused a reduced BOLD response. Corresponding electrophysiological recordings show a frequency-dependent increase in the population spike latency with a concurrent decrease in the population spike amplitude. 3D visualization of activated voxels points to a restricted activation of the right dentate gyrus and entorhinal cortex. (Bottom) BOLD time series in the right and left entorhinal cortex regions. In the right entorhinal cortex, stimulus-dependent variations in the BOLD signal were observed, though no variations were detected in the left entorhinal cortex.
Concurrent electrophysiological recordings revealed that by starting with repetitive 1.25 Hz stimulation trains, the responses of the granular cells in the dentate gyrus within each train became very similar during each train. In every train, the first stimulus elicited the highest population spike amplitude with the shortest latency. Whereas the latency of the first response during all following trains remained almost the same, the population spike amplitude decreased continuously with each additional train. Thus, during the final 10 Hz stimulation trains, population spikes were only observed in two of the nine animals. Dependent on the stimulation frequency, a stronger increase of the latency within each train was observed; thus, the higher the stimulation frequency the longer the latency becomes.
In summary, identical stimulation protocols, and by that identical variations in input activity to the hippocampal formation, induced variable neural activation patterns that depend crucially on the applied anesthetic/sedative. As a result, the generated BOLD responses were specific for each anesthesia condition. The most obvious spatial difference in generated BOLD responses was observed in the striatum. In this region, BOLD responses were only observed during stimulation of the perforant pathway in the δ waves range (1 to 4 Hz) when isoflurane was used as the anesthetic, but not under any other anesthesia/sedation conditions. Furthermore, in the left entorhinal cortex region, reliable BOLD responses were only detected when medetomidine was used as sedative; under isoflurane BOLD responses were only seen in three out of seven of the animals, whereas under α-chloralose, significant BOLD responses were never observed in this region.
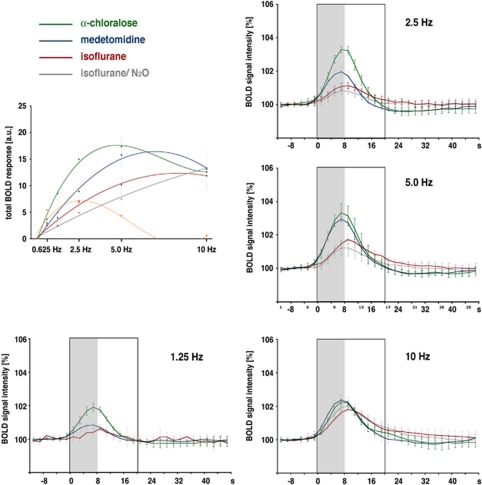
In the dentate gyrus region, identical stimulation protocols induced gradual differences with respect to the frequency threshold and BOLD response magnitude and linearity. Under medetomidine and α-chloralose, the BOLD responses in the hippocampal formation were generated during 1.25 Hz stimulation trains, whereas the threshold under isoflurane was around 2.5 Hz and slightly higher under isoflurane/N2O. Besides the varying frequency thresholds, the variation of the elicited BOLD responses differed substantially between the four groups. Under isoflurane, the magnitude of the BOLD response increased during all subsequent stimulation blocks, that is, up to 10 Hz. In contrast, under medetomidine and α-chloralose, frequency-dependent increases in BOLD responses were not detectable above 5 Hz (Figure 7).
Figure 7.
Summary of the generated blood oxygen level-dependent (BOLD) responses under various forms of anesthesia. To visualize the effect of the used anesthesia, the averaged BOLD response during identical stimulation frequencies are plotted. Besides anesthesia-dependent variations in the magnitude of the BOLD response at individual stimulation frequencies, a different time course of the BOLD response was also observed. The shape of the BOLD response curve is similar under α-chloralose and medetomidine, but different under isoflurane. To compare the relation between stimulation frequency and the generated BOLD response, the BOLD response was quantified by calculating the area below the curve in the range between stimulus onset and 12 seconds after cessation of the stimulus (indicated with the black frame, gray box indicates the location of the stimulus) and expressed in arbitrary units (a.u.). For each data set, the best fit function was calculated (green: α-chloralose (y=0.0154x3−0.5975x2+6.839x−6.8517 (R2=0.993); blue: medetomidine (y=−0.135x2+3.2879x−3.6095 (R2=0.9871); red: isoflurane (y=−0.0692x2+1.9787x−1,7893 (R2=0.981); gray: isoflurane/N2O (y=−0.0182x2+1.1441x−0.9942 (R2=0.9942)). For comparison, the relation between the stimulation frequency and BOLD response in the striatum is shown in orange (y=0.0197x3−0.5864x2+4.4912x−3.0957 (R2=0.8882)). The color reproduction of this figure is available on the Journal of Cerebral Blood Flow and Metabolism journal online.
Discussion
This study was aimed at determining the threshold of synchronous neuronal activity necessary to generate significant BOLD responses in the rat hippocampus under various forms of anesthesia/sedation. To exclude any confounding effects of upstream signal processing/propagation, the major direct input into the dentate gyrus, the perforant pathway, was electrically stimulated with a defined pattern, that is, 5, 10, 20, 40, or 80 stimuli per train. The main results of the present study are (1) identical input activity into the dentate gyrus elicited a neural activation pattern that is specific for each applied anesthesia and (2) these variations in neural responses under various anesthetics, in turn, generate spatial and temporal BOLD response patterns that are characteristic for each individual anesthetic. These anesthetic-dependent characteristics are (1) the minimal frequency that triggers a significant BOLD response; (2) the optimal frequency, that is, the frequency that elicit the maximal BOLD response; and (3) signal propagations to and/or activity in other regions of the brain vary.
The result that the chosen anesthetic affects the BOLD response to similar stimulation conditions is not so surprising, considering all the results obtained during peripheral electrical forepaw stimulation in rats. In this experimental approach, as already summarized by Kim et al (2010), the optimal stimulation frequency required to generate a maximal BOLD response varies under different anesthetics. However, it is often difficult to compare different studies because the stimulation parameters (especially duration and intensity) vary considerably between different studies. Thus, it was found that even under α-chloralose, the optimal frequency required to generate maximal BOLD responses in the sensory cortex varies between 1.5 Hz (Brinker et al, 1999; Gyngell et al, 1996; Huttunen et al, 2008; Sanganahalli et al, 2008) and 10 Hz (Van Camp et al, 2006).
In the present study, the stimulation protocol was identical for all anesthetics used, and the intensity was adjusted to a value that elicited 50% of a maximal population spike amplitude. Because the stimulation condition was kept almost identical in all experiments, the observed variations in the BOLD responses should only depend on the applied anesthetic. In general, the anesthetic could affect the BOLD responses in the following ways: (1) by an effect on the vascular system, such as vasodilatation/constriction and by that modifying the range of blood flow causing blood volume changes; (2) by interfering with mitochondrial function and energy metabolism (Szewczyk and Wojtczak, 2002); (3) by perturbations of the neurovascular coupling; and/or (4) modifications of signal processing in local neuronal circuits in the dentate gyrus. If a systemic effect on the vascular system would be the cause for the observed differences in the elicited BOLD responses under varying anesthetics, then the threshold intensity and the maximal BOLD response could differ; however, the optimal BOLD response should remain the same. Furthermore, the activation pattern outside the hippocampus could vary in a quantitative way, but not qualitatively and, more importantly, the recorded electrophysiological responses should be similar. If the effect on the anesthetics would be primarily mediated by an interference with mitochondrial functions and/or mechanisms of the neurovascular coupling, then variations in the threshold intensity, maximal BOLD response, and optimal BOLD response would be feasible, if one considers various mediators for the neurovascular coupling. However, the elicited electrophysiological responses should still remain the same. In the third option, an effect on the activity and/or properties of the local neuronal circuits, the main characteristic would be an altered signal processing of identical input activity.
In the hippocampus, we observed the strongest BOLD response during 5 Hz stimulation trains both under α-chloralose and medetomidine and during 10 Hz under isoflurane (with or without N2O). Calculating the best fit function for the relation between stimulation frequency and induced BOLD response, points to a best frequency just below 5 Hz for α-chloralose, above 5 Hz for medetomidine, around 10 Hz for isoflurane, and above 10 Hz for isoflurane/N2O (Figure 7). Thus, the relationship between the BOLD response and numerical input activity is specific for every narcosis used, and a linear range between the two parameters exists, if at all, only over a very small range, indicating that a general influence of the anesthetics on the vascular system is not the main cause for the observed differences in generated BOLD responses.
According to the simultaneously recorded electrophysiological responses, the onset of functional reorganizations in local neuronal networks begins during the 1.25-Hz stimulation trains under all measured conditions. In each subsequent 1.25 Hz train, the population spike latency increased. Furthermore, over the entire block, the average population spike amplitude per train became smaller as more trains were applied; consequently, during this short (i.e., 8 seconds) low frequency stimulation, the previous trains affect the responses in the following trains. Under α-chloralose, an even more consistent pattern was induced, in which the first stimulus in each train elicited a population spike with the highest amplitude and shortest latency per train; a pattern that became more pronounced during the following 2.5 and 5 Hz stimulation trains. In contrast, under medetomidine and isoflurane, there was no depression of the population spike amplitude, but an augmentation was observed in each train. The electrophysiological responses under medetomidine and isoflurane also differ, especially during 5 Hz stimulation trains. Whereas under medetomidine, a consistent increase in the population spike latency occurs, though under isoflurane, the latency only increased for some stimuli and decreases afterwards. Again, the elicited neuronal response pattern, induced by an identical stimulation protocol was characteristic for each used anesthetic. Therefore, the most likely cause for the observed differences in the BOLD response during the presence of various anesthetics is an anesthetic-dependent effect on local signal processing. An important role of signal processing for the magnitude of the BOLD response becomes obvious by the fact that no significant BOLD responses were observed when no clear variations were detected in the population spike latency/amplitude within one train. Therefore, simple propagation of the signal through the dentate gyrus seems insufficient to generate a substantial BOLD response. The measured parameters of neuronal activity, population spike amplitude and latency, or more precisely their activity-dependent decrease or increase are, however, no clear predictors for the magnitude of the BOLD response. Whereas, as already mentioned, BOLD responses were only observed when the population spike latencies of successive stimuli increased during one train, though the magnitude of the BOLD response does not depend on the extent of the latency change. Under all anesthetics, the latency during 10 Hz stimulation trains was always longer, compared with the previous 5 Hz stimulation trains. Nevertheless, the BOLD response declined under α-chloralose and medetomidine, but not under isoflurane. As a result, the magnitude of the BOLD response became almost identical during 10 Hz stimulation under all conditions. Previous work by Angenstein et al (2010) shows similar results where low intensity stimulation, that is, stimulation intensities that did not elicited population spikes caused strong BOLD responses under α-chloralose and medetomidine but only minor BOLD responses under isoflurane. The same stimulation protocol, however, with a high-stimulation intensity, generated similar BOLD responses in the dentate gyrus under all three anesthetics. It is possible that under isoflurane, mechanism(s) become activated and inhibit the intensity of the BOLD response, which is subsequently activated by a high-stimulus intensity, or, as in the present study, by a higher-stimulation frequency.
All anesthetics affect the balance between excitation and inhibition toward inhibition. The main mechanisms are (1) an enhancement or potentiation of the inhibitory GABAergic system (e.g., as allosteric modulator of postsynaptic GABAergic receptors (e.g., α-chloralose, isoflurane)); (2) inhibition of excitatory glutamatergic transmission (via postsynaptic N-methyl--aspartate (NMDA) receptors (e.g., isoflurane, N2O) or inhibition of glutamate release (e.g., medetomidine, isoflurane, N2O); and/or (3) by activation of two-pore-domain K+ channel (K2P) subunits (e.g., isoflurane, nitrous oxide, and α-chloralose). There exists diverse overlapping of these mechanisms being induced by the used anesthetics, but only isoflurane and N2O inhibit the NMDA receptor. Consequently, it would be tempting to speculate, that NMDA-dependent mechanisms are especially involved in the different BOLD characteristics observed between α-chloralose and medetomidine on the one hand, and isoflurane on the other. This assumption is supported by numerous studies, mainly demonstrating a dilatation of brain arterioles after topical application of NMDA (summarized in Busija et al, 2007) and an fMRI study pointing to an essential role of NMDA-mediated mechanisms for the generation of a BOLD response (Gsell et al, 2006). However, the present study contradicts the simple relationship between the amount of NMDA receptor activation and magnitude of the BOLD response. Thus, the main effect on the intensity of the BOLD response between NMDA receptor inhibiting (i.e., isoflurane, N2O) and NMDA receptor independent anesthetics (i.e., α-chloralose, medetomidine) was observed during low intensity (subthreshold; Angenstein et al, 2010) and low frequency (below 10 Hz) stimulation. Under these two conditions, NMDA receptor inhibition was paralleled with lower BOLD responses. This supports the hypothesis that NMDA-dependent mechanism(s) facilitate the BOLD response. However, during higher intensity or higher frequency stimulation, both conditions activate NMDA receptors; the BOLD responses increased when anesthetics that inhibit NMDA receptors were used, but on the other hand, the BOLD responses decreased though NMDA receptors were not affected. Under this condition, NMDA receptor activation seems to impair the BOLD response. Further studies may identify alternative NMDA-dependent mechanisms controlling the generation of BOLD responses.
One unexpected finding is the extremely divers spatial variations in BOLD response under different anesthetics. The perforant pathway directly targets neurons in the dentate gyrus, subiculum, and the hippocampus proper, that is, CA3, CA2, CA1 (Amaral and Lavenex, 2007). Whereas granular cells project exclusively to the CA3 region, principal neurons in the hippocampus proper have more divergent projections. CA3 pyramidal cells send projections to CA3, CA2, and CA1 region of the ipsilateral and contralateral hippocampus (via commissural fibers) and lateral septal nucleus (Amaral and Lavenex, 2007). CA1 pyramidal cells mainly target the adjacent subiculum and the entorhinal cortex and to a much lesser extend a number of cortical areas (Cenquizca and Swanson, 2007). The subiculum, in turn, projects to a number of structures, that is, strong connections to the entorhinal, perirhinal postrhinal corticies and, in addition, to the orbitofrontal, pre- and infralimbic, agranular insular, and cingulated corticies. Furthermore, the subiculum has projections to subcortical structures, such as nucleus accumbens, amygdaloid complex, medial mammillary nucleus, thalamus (nc. reunions), and hypothalamus (summarized in Witter, 2006). Consequently, direct activation of subicular neurons along with indirect activation via the hippocampal trisynaptic pathway could activate these regions and, by that, trigger BOLD responses. In all experiments, BOLD responses were measured outside of the hippocampal formation, namely in the striatum; however, these were only observed under isoflurane. In contrast to the BOLD signals in the hippocampus, the strongest BOLD responses in the striatum were found at stimulation frequency of 2.5 Hz or lower, that means at a frequency below or around the minimal frequencies required to the generate significant BOLD responses in the hippocampus (Figures 3 and 7). One could argue that very low frequencies are easily propagated through the hippocampus; whereas during increased local processing of higher frequencies, an altered output activity occurred, which was expounded by the stronger BOLD response. However, induced BOLD responses in the striatum remain puzzling, because, as mentioned, no strong direct connections between the hippocampus and the striatum are known. Further work is needed to clarify how activation of the hippocampus in the δ wave range (i.e., 1 to 4 Hz) induces synchronized activity especially in the striatum. The observation of diverse BOLD activation pattern under different anesthetics indicates an interference of the present anesthetic with the signal propagation to appropriate target regions.
In conclusion, the results presented clearly indicate that the differences in the characteristics of elicited BOLD responses under various forms of anesthesia depend crucially on the effect of the particular anesthetic on local signal processing. This finding substantiates previous conclusions about the role of different anesthetics for the generation of a BOLD response in the somatosensory cortex during peripheral stimulations. It also implies that observed variations in BOLD responses to identical stimuli are good indicators for an altered processing of the incoming stimuli, and by that a change in output activity from the appropriate region. Therefore, fMRI is not only able to identify regions that become activated during a certain stimulus, but is also a powerful tool to verify time, activity, or experience-dependent variations in local processing of incoming stimuli.
Acknowledgments
The authors thank Dr Jonathan Lovell for critical reading of the manuscript.
The authors declare no conflict of interest.
Footnotes
FA is supported by the Deutsche Forschungsgemeinschaft (DFG) AN200-6.
References
- Amaral D, Lavenex P.2007Hippocampal neuroanatomy The Hippocampus Book(Andersen P, Morris R, Amaral D et al, eds),Oxford: Oxford University Press; 37–114. [Google Scholar]
- Angenstein F, Kammerer E, Niessen HG, Frey JU, Scheich H, Frey S. Frequency-dependent activation pattern in the rat hippocampus, a simultaneous electrophysiological and fMRI study. Neuroimage. 2007;38:150–163. doi: 10.1016/j.neuroimage.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Angenstein F, Krautwald K, Scheich H. The current functional state of local neuronal circuits controls the magnitude of a BOLD response to incoming stimuli. Neuroimage. 2010;50:1364–1375. doi: 10.1016/j.neuroimage.2010.01.070. [DOI] [PubMed] [Google Scholar]
- Brinker G, Bock C, Busch E, Krep H, Hossmann KA, Hoehn-Berlage M. Simultaneous recording of evoked potentials and T2*-weighted MR images during somatosensory stimulation of rat. Magn Reson Med. 1999;41:469–473. doi: 10.1002/(sici)1522-2594(199903)41:3<469::aid-mrm7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Busija DW, Bari F, Domoki F, Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals S, Beyerlein M, Murayama Y, Logothetis NK. Electric stimulation fMRI of the perforant pathway to the rat hippocampus. Magn Reson Imaging. 2008;26:978–986. doi: 10.1016/j.mri.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Olsen JI, Stolk JA, Schweizer MP, Sha M, Ueda I. An in vivo 19F NMR study of isoflurane elimination as a function of age in rat brain. NMR Biomed. 1992;5:121–126. doi: 10.1002/nbm.1940050304. [DOI] [PubMed] [Google Scholar]
- Gsell W, Burke M, Wiedermann D, Bonvento G, Silva AC, Dauphin F, Buhrle C, Hoehn M, Schwindt W. Differential effects of NMDA and AMPA glutamate receptors on functional magnetic resonance imaging signals and evoked neuronal activity during forepaw stimulation of the rat. J Neurosci. 2006;26:8409–8416. doi: 10.1523/JNEUROSCI.4615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyngell ML, Bock C, Schmitz B, Hoehn-Berlage M, Hossmann KA. Variation of functional MRI signal in response to frequency of somatosensory stimulation in alpha-chloralose anesthetized rats. Magn Reson Med. 1996;36:13–15. doi: 10.1002/mrm.1910360104. [DOI] [PubMed] [Google Scholar]
- Haasdijk ED, Vlug A, Mulder MT, Jaarsma D. Increased apolipoprotein E expression correlates with the onset of neuronal degeneration in the spinal cord of G93A-SOD1 mice. Neurosci Lett. 2002;335:29–33. doi: 10.1016/s0304-3940(02)01159-x. [DOI] [PubMed] [Google Scholar]
- Harris S, Jones M, Zheng Y, Berwick J. Does neural input or processing play a greater role in the magnitude of neuroimaging signals. Front Neuroenergetics. 2010;2:15. doi: 10.3389/fnene.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med. 1986;3:823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- Huttunen JK, Grohn O, Penttonen M. Coupling between simultaneously recorded BOLD response and neuronal activity in the rat somatosensory cortex. Neuroimage. 2008;39:775–785. doi: 10.1016/j.neuroimage.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP. Functional MRI of the rodent somatosensory pathway using multislice echo planar imaging. Magn Reson Med. 2004;52:89–99. doi: 10.1002/mrm.20114. [DOI] [PubMed] [Google Scholar]
- Kim T, Masamoto K, Fukuda M, Vazquez A, Kim SG. Frequency-dependent neural activity, CBF, and BOLD fMRI to somatosensory stimuli in isoflurane-anesthetized rats. Neuroimage. 2010;52:224–233. doi: 10.1016/j.neuroimage.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Sanganahalli BG, Herman P, Hyder F. Frequency-dependent tactile responses in rat brain measured by functional MRI. NMR Biomed. 2008;21:410–416. doi: 10.1002/nbm.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk A, Wojtczak L. Mitochondria as a pharmacological target. Pharmacol Rev. 2002;54:101–127. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- Van Camp N, Verhoye M, Van der Linden A. Stimulation of the rat somatosensory cortex at different frequencies and pulse widths. NMR Biomed. 2006;19:10–17. doi: 10.1002/nbm.986. [DOI] [PubMed] [Google Scholar]
- Weber R, Ramos-Cabrer P, Wiedermann D, van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage. 2006;29:1303–1310. doi: 10.1016/j.neuroimage.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Witter MP. Connections of the subiculum of the rat: topography in relation to columnar and laminar organization. Behav Brain Res. 2006;174:251–264. doi: 10.1016/j.bbr.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage. 2008;39:248–260. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]