Abstract
Akt and mammalian target of rapamycin (mTOR) are both activated after traumatic brain injury (TBI), however complex interplay between the two hampers deciphering their functional implications in vivo. We examined the effects of single and combination inhibitors of Akt/mTOR in a mouse controlled cortical impact (CCI) model. Following CCI, phospho-Akt-473 (p-Akt) and -S6 ribosomal protein (p-S6RP), a downstream substrate of mTOR, were increased in cortical and hippocampal brain homogenates (P<0.05 versus sham). At 24 hours, p-S6RP was detected in neurons and was robustly induced in microglia and astrocytes in injured hippocampus. In vivo activity of Akt and mTOR inhibitors administered separately was confirmed by reduced expression of p-GSK3β (P<0.01) or p-S6RP (P<0.05), respectively, after CCI. Importantly, administration of Akt and mTOR inhibitors together (but not of either alone) improved postinjury motor (P=0.02) and cognitive deficits (hidden platform trials, P=0.001; probe trials, P<0.05), decreased propidium iodide-positive cells in CA1 and CA3 (P<0.005), and unexpectedly increased p-GSK3β in hippocampus. Although the roles of Akt and mTOR in the pathogenesis of TBI remain to be fully elucidated, dual inhibition of Akt and mTOR may have therapeutic potential for TBI.
Keywords: Akt, cognition, mammalian target of rapamycin, mice, protein kinase B, traumatic brain injury
Introduction
The phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin (mTOR) pathway regulates key intracellular pathways involved in translation, transcription, metabolism, proliferation, and response to cellular injury (Wymann et al, 2003). Akt (protein kinase B) is a serine–threonine kinase that is activated by phosphorylation of Thr308 by phosphoinositide-dependent kinase-1 and of Ser473 by mTOR complex 2 (TORC2). Activation of Akt is dependent on membrane translocation mediated by binding to phosphatidylinositol 3,4,5-triphosphate, which is generated by phosphatidylinositol-3-kinase in response to RTK (receptor tyrosine kinase) activity (Franke and Cantley, 1997). Direct Akt substrates involved in cell stress include glycogen synthase kinase 3-β (GSK3β) and the FoxO transcription factors. Akt inhibits apoptosis by phosphorylating and inactivating proapoptotic factors such as Bad, caspase-9, and GSK3β (at Ser9) and activating nuclear factor-κB (Franke and Cantley, 1997; Song et al, 2008). Recent work suggests that Akt can also promote necrosis in some contexts in part by increasing oxidative stress (Komandirov et al, 2011; Maddika et al, 2008; Nogueira et al, 2008) and suppressing autophagy (Wu et al, 2009).
Mammalian target of rapamycin is a serine/threonine kinase that exists in the rapamycin-sensitive mTOR complex 1 (TORC1), which feedback inhibits Akt by phosphorylating and activating S6K, which phosphorylates and activates IRS1. Phospho-IRS1 dissociates from insulin-like growth factor-1/insulin receptors and prevents downstream AKT activation (Hollander et al, 2011). Conversely, TORC1 activity is increased by growth factor stimulation in an Akt-dependent manner and by amino acids independent of Akt. One of the major functions of TORC1 is stimulation of translation through phosphorylation and activation of S6 kinase (p70S6K), which in turn phosphorylates S6 ribosomal protein (S6RP). In addition, p70S6K shares some substrates with Akt, including Ser9 of GSK3b, adding further complexity to regulation of Akt/mTOR signaling (Frame and Cohen, 2001).
Akt has been implicated as a prosurvival factor in experimental stroke (Carloni et al, 2010; Endo et al, 2006; Noshita et al, 2001; Zhao et al, 2005), nerve transection (Namikawa et al, 2000), and spinal cord injury (Hu et al, 2010). Akt and mTOR signaling pathways are induced after experimental traumatic brain injury (TBI) in rodents but their functional significance remains incompletely characterized (Chen et al, 2007; Jenkins et al, 2002; Neary, 2005). Akt is activated and colocalizes with uninjured neurons after controlled cortical impact (CCI) in mice (Noshita et al, 2002); however, direct evidence for specific functional role(s) for Akt in TBI, as determined by pharmacological or genetic inhibition of Akt, is lacking. In contrast, a detrimental role for TORC1 is suggested by the observation that rapamycin administration reduced p70S6K phosphorylation, postinjury motor deficits, neuronal cell loss, and microglial activation after cerebral contusion in mice (Erlich et al, 2007). Here, we interrogated the functional significance of Akt and mTOR pathways with specific inhibitors of Akt and TORC1 alone and in combination using a mouse CCI model. We describe herein a marked beneficial effect of dual inhibition of Akt/mTOR on outcome associated with an unexpected increase in GSK3β Ser9 phosphorylation.
Materials and methods
All experiments were performed by investigators blinded to study group, and were approved by the Massachusetts General Hospital Institutional Review Board and complied with the NIH Guide for the Care and Use of Laboratory Animals. Mice were given free access to food and water and were housed in laminar flow racks in a temperature-controlled room with 12-hour day/night cycles.
Mouse Controlled Cortical Impact Model
The mouse CCI model was used as previously described (Bermpohl et al, 2006). Male C57Bl/6 mice (3 months of age; Jackson Laboratories, Bar Harbor, ME, USA) were anesthetized with 4% isoflurane, 70% N2O and balance O2 and placed in a stereotactic frame. Blow by anesthesia was maintained via a nose opening in the tubing leading from the anesthesia box and isoflurane was titrated to quiet respirations and lack of toe pinch response at a level that avoids hypotension (Khuman et al, 2010). A 5-mm craniotomy was performed over the left parietotemporal cortex and the bone flap removed. Controlled cortical impact was produced using a pneumatic cylinder with a 3-mm flat-tip impounder, velocity 6.0 m/s, and depth of 0.6 mm. Sham-injured mice received craniotomy without CCI. Following sham injury or CCI, the bone flap was discarded and the scalp sutured closed. Mice were returned to their cages to recover from anesthesia.
Administration of Rapamycin and Akt Inhibitor VIII
Rapamycin from Streptomyces hygroscopicus (Calbiochem, San Diego, CA, USA) and Akt inhibitor VIII (isozyme selective akti-1/2; Calbiochem) were administered singly or in combination in various concentrations into the left lateral ventricle (0.1 mm posterior 1 mm lateral, 2 mm deep to bregma) immediately before CCI. For all experiments, 4 μL total volume was given.
Assessment of Motor Function
Gross vestibulomotor function was assessed using a wire grip test on postinjury days 1 to 4. The wire grip test consisted of placing the mouse on a wire (45 cm long) suspended between two poles 45 cm high, and grading the ability of mice to traverse the wire over 60 seconds (Bermpohl et al, 2006).
Morris Water Maze
Spatial memory acquisition was assessed using the Morris Water Maze (MWM) as previously described on postinjury days 8 to 12 (Bermpohl et al, 2006). A white pool (83 cm diameter, 60 cm deep) was filled with water to 29 cm depth. A round, clear plexiglass platform 10 cm in diameter was positioned 1 cm below the surface of the water ∼10 cm from the southwest quadrant. Each mouse was subjected to no more than two hidden platform, probe, or visible platform trials per day over the 4-day period. For each trial, mice were randomized to one of four starting locations (north, south, east, and west) and placed in the pool facing the wall. Mice were given a maximum of 90 seconds to find and mount the platform. If the mouse failed to reach the platform by 90 seconds, it was placed on the platform by the experimenter and allowed to remain there for 10 seconds. Mice were dried and kept warm under heat lamps between trials. Performance in the MWM was quantitated by latency to the platform. Following five hidden platform trials, a probe trial was done in which mice were placed in the tank with the platform removed and latency in the target quadrant was measured (60 seconds maximum). Finally, two visible platform trials with the platform raised ½ cm above the water and clearly marked with tape were performed.
Assessment of Lesion Volume
Morphometric image analysis was used to determine lesion volume and brain tissue atrophy at 15 days after CCI. Mice were deeply anesthetized with isoflurane and decapitated, and the brains were removed and frozen in liquid nitrogen vapor. Coronal sections (20 μm) were cut at 0.5 mm from the anterior to the posterior brain, mounted on poly--lysine-coated slides, and stained with hematoxylin. The areas of the injured and noninjured hemispheres were determined using image analysis and the method of Cavalieri as previously described (Nikon NIS Elements, Melville, NY, USA) (Bermpohl et al, 2006). Lesion volume was the difference between noninjured and injured hemispheric brain tissue volume and was expressed in mm3.
Administration of Propidium Iodide and Assessment of Propidium Iodide-Positive Cell Counts
Propidium iodide (PI; 10 mg/mL; Sigma, St Louis, MO, USA) was diluted in phosphate-buffered saline (PBS) and 1 mg/kg was administered intraperitoneally in a total volume of not more than 100 μL 1 hour before kill. Mice were killed at 4 hours after CCI, the brains frozen in nitrogen vapor, and cryostat brain sections (12 μm) were cut at 150 to 200 μm intervals from anterior to posterior hippocampus. Cryostat sections were placed on poly--lysine slides and stored at −80 °C. For detection of PI-labeled cells, the brain sections were fixed in 100% ethanol for 10 minutes at room temperature, coverslipped with Permount (Biomeda, Foster City, CA, USA) and photographed on a Nikon Eclipse T300 fluorescence microscope (Tokyo, Japan) using excitation/emission filters 568/585. Propidium iodide-positive cells were quantitated in cortex and hippocampus in three brain sections separated by at least 150 to 20 μm as previously described (You et al, 2008).
Quantitative Real-Time Polymerase Chain Reaction
mRNA extraction and elution was performed using the protocols of GE Healthcare's illustra RNAspin Mini RNA Isolation Kit (Piscataway, NJ, USA) and previously reported methods (Khuman et al, 2010). Quantitative real-time polymerase chain reaction was performed using tumor necrosis factor-α as the target gene and 18S as the reference gene (Invitrogen, Carlsbad, CA, USA; Applied Biosystems, Carlsbad, CA, USA; Assay ID#: Hs00174128_m1).
Western Blot Analyses
Western blotting was performed using left hemispheric tissue (cortex or hippocampus) from injured or sham-injured mice. In dual inhibitor experiments, we measured p-GSK3β in hippocampus to correlate GSK3β with hippocampal function as assessed in the MWM, and because diffusion of drugs injected ICV may be inconsistent to the ipsilateral cortex. The brain tissue was homogenized in RIPA buffer containing protease inhibitor (Roche Diagnostics, Indianapolis, IN, USA) and phosphatase inhibitor (Roche Diagnostics). Protein content was quantitated with a standard curve using bovine serum albumin and a colorimetric assay from Bio-Rad (Richmond, CA, USA). Samples were denatured by boiling in 2-mercaptoethanol and 30 μg of protein was loaded into each well of a 4% to 15% gradient or 10% Tris–HCl precast gel (Bio-Rad). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Millipore, Immobilon transfer membrane, Bedford, MA, USA). Membranes were blocked in 5% milk in Tris-buffered saline Tween 0.1% (TBST) and incubated in primary antibodies (all from Cell Signaling Technology, Beverly, MA, USA; 1:750 or 1:1,000 dilution) overnight at 4 °C. Membranes were washed in TBS-T and incubated in HRP (horseradish peroxidase)-conjugated secondary anti-rabbit-HRP conjugate (1:5,000 dilution) or anti-mouse-HRP antibodies (for B-actin, 1:5,000) (Cell Signaling Technology) for 1 to 2 hours and immunoblotted proteins were detected using the ECL Plus Western Blotting Detection system (GE Healthcare, Woburn, MA, USA). Relative optical densities for protein bands were determined using image analysis (Kodak Image Station, Rochester, NY, USA).
Immunohistochemistry
Mice were anesthetized and transcardially perfused with 30 mL PBS followed by 2% paraformaldehyde at 4 or 24 hours after sham injury or CCI. The brains were postfixed in 2% paraformaldehyde for 24 hours and cryoprotected in 30% sucrose for 24 hours then refrigerated at 4 °C in 0.01% sodium azide. The brains sections (40 μm) were cut on a microtome and incubated in 1:100 diluted antigen retrieval solution (Sigma, Allentown, PA, USA), washed three times, and incubated with primary antibodies from Cell Signaling (rabbit anti-phospho-S6, mouse anti-NeuN-Alexa Fluor 488 conjugate, rabbit anti-IBA-1, or rabbit anti-GFAP-Cy3 conjugate) (all antibodies from Cell Signaling except Iba-1 from Wako, Tokyo, Japan) overnight at 4 °C. Sections were washed three times in PBS and incubated with the corresponding secondary antibody-fluorophore conjugate for 1 hour (1:300). As a negative control, alternate sections were incubated with isotype-matched IgG. Sections were washed three times in PBS and mounted onto glass slides using Clearmount with Tris buffer (Electron Microscopy Sciences, Hatfield, PA, USA) and photomicrographs obtained with the following excitation–emission filters: 568/585 for Cy3 and 489/506 for Alexa Fluor 488.
Statistical Analyses
Data are mean±standard error of the mean. Morris Water Maze hidden and visible platform and motor data were analyzed by repeated measures analysis of variance (group × time). Probe trial and densitometry data were analyzed by t-test. Propidium iodide-positive cell count data were analyzed by rank sum.
Results
Akt and Mammalian Target of Rapamycin Signaling Is Induced After Controlled Cortical Impact
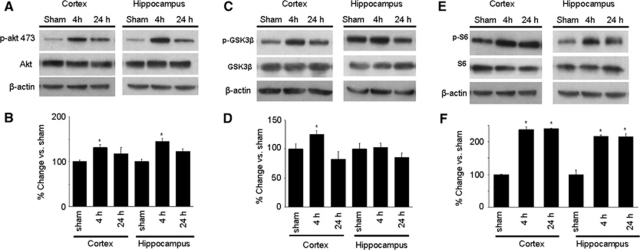
Figure 1A shows Western blot analysis of phospho-Akt and total Akt expression after sham injury or CCI. Phospho-Akt (p-Akt) and Akt were detectable in cortex and hippocampus of sham-injured mice. At 4 hours after CCI, p-Akt was significantly increased in both hippocampus and cortex (P<0.05 versus sham; Figures 1A and 1B). At 24 hours, p-Akt expression was similar to that of sham-injured mice. Expression of total Akt did not differ at any time in cortex or hippocampus (Figure 1A and densitometry data not shown). Similar to phospho-Akt, phospho-GSK3β, a direct substrate of Akt, was also induced by 4 hours after CCI (P<0.05 versus sham) and reduced at 24 hours in cortex (Figures 1C and 1D). However, in hippocampus, expression of phospho-GSK3β did not differ between sham and injured mice at 4 or 24 hours. No change was observed in total GSK3β (Figure 1C and densitometry data not shown). Figures 1E and 1F show changes in p-S6RP, a substrate of TORC1/p70S6K, after CCI. Phospho-S6RP was significantly increased in cortex and hippocampus at 4 and 24 hours after CCI (P<0.01 and 0.001 versus sham, respectively), whereas total S6RP did not change after CCI at either time point (Figure 1E and densitometric analyses not shown). Total mTOR expression did not change at 4 or 24 hours after CCI compared with sham levels (not shown). Overall, these data indicated an increase in both Akt and TORC1 activity in both cortex and hippocampus following CCI.
Figure 1.
Expression of phosphorylated akt (p-akt), S6RP (p-S6), and glycogen synthase kinase 3-β (GSK3β) (p-GSK3β) after sham injury or controlled cortical impact (CCI) (n=5/group). (A) Representative Western blots of p-Akt (ser473) and total Akt. (B) Densitometric quantitation of phosphorylated akt. *P<0.05 versus sham injured. (C) Representative Western blots of p-GSK3β and total GSK3β. (D) Densitometric quantitation of phosphorylated GSK3β. (E) Representative Western blots of p-S6 and total S6. (F) Densitometric quantitation of p-S6. *P<0.01 4 hours versus sham injured; *P<0.001 24 hours versus sham injured.
Phospho-S6RP Is Induced in Glia After Controlled Cortical Impact
Phospho-S6RP was constitutively expressed in cortex of sham-operated mice in subsets of NeuN+ neurons, GFAP+ astrocytes, and IBA-1+ microglia; most cortical p-S6RP staining was observed near the surface of the brain (Supplementary Figure 1). The presence of p-S6RP was also noted in neurons in hippocampal CA3, CA1, and DG (dentate gyrus), whereas little or no staining was observed in glial cells in hippocampus of sham-injured mice (Supplementary Figure 1 and data not shown). Following CCI, p-S6RP was robustly induced in cortical and hippocampal microglia and astrocytes (Figure 2, upper and lower panels). No clear increase in the numbers of p-S6RP-positively stained neurons was observed at 24 hours after CCI (Figure 2, middle panels).
Figure 2.
Representative photomicrographs of phospho-S6RP expression in NeuN–, IBA-1–, and GFAP-positive cells at 24 hours after controlled cortical impact (CCI) (n=3 to 4/group). (Top panels) Representative photomicrographs showing robust induction of p-S6RP expression in large numbers of cortical IBA-1+ microglia. (Middle panels) Representative photomicrographs depicting p-S6RP expression in NeuN+ cells in CA3, similar to sham injury. (Lower panels) Representative photomicrographs showing robust induction of p-S6RP in GFAP+ astrocytes in CA3. Scale bar, 10 μm.
Effect of Single and Dual Inhibition of Akt and Mammalian Target of Rapamycin on Phosphorylation of Downstream Substrates After Controlled Cortical Impact
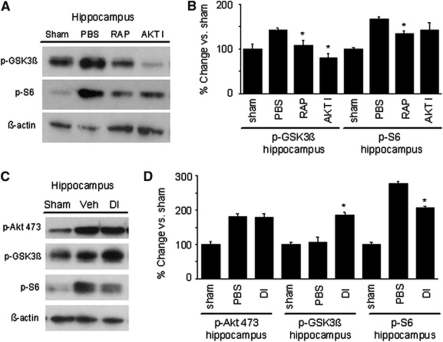
Considering robust increase in Akt and TORC1 activity following CCI, we next sought to determine the functional roles of these changes using corresponding inhibitors (Akt inhibitor viii and rapamycin, respectively). To confirm the activity of Akt and mTOR inhibitors in the brain, we assessed phosphorylation of the Akt substrate GSK3β and the mTOR substrate S6RP at 4 hours after CCI. Compared with vehicle-treated injured mice, Akt inhibitor alone did not change Akt phosphorylation (as expected) but reduced GSK3β phosphorylation. Rapamycin treatment also decreased p-GSK3β expression, which may be related to the reported p70S6K activity (Figures 3A and 3B). Conversely, rapamycin treatment alone reduced postinjury p-S6RP expression whereas the effect of Akt inhibitor was not statistically significant. Thus, single inhibitors showed good in vivo activity on their respective downstream substrates with Akt inhibitor more efficiently attenuating p-GSK3β and rapamycin p-S6RP. We next examined dual Akt/TORC1 inhibition. Curiously, while administration of Akt inhibitor and rapamycin together before CCI robustly decreased phospho-S6RP levels and did not alter p-Akt levels as could be expected, we observed an increase in GSK3β phosphorylation (Figures 3C and 3D).
Figure 3.
Effect of akt inhibitor viii (AKT I), rapamycin (RAP), or vehicle (phosphate-buffered saline, PBS) treatment on expression of phosphorylated akt (p-akt), S6RP (p-S6), and glycogen synthase kinase 3-β (GSK3β) (p-GSK3β) 4 hours after controlled cortical impact (CCI). Untreated sham-injured mice are shown for comparison to PBS groups. (A, B) Compared with PBS-treated mice, treatment of mice with single inhibitors AKT I or RAP each decreased p-GSK3β expression shown by Western blot in (A) and densitometric analysis in (B) (*P<0.05 versus PBS treated). In contrast, RAP but not AKT I significantly reduced p-S6RP induction in injured mice (*P<0.05 versus PBS treated). (C, D) Effect of combined treatment with RAP and AKT I (double inhibitor, DI) in expression of phosphorylated akt (p-akt), S6RP (p-S6), and GSK3β (p-GSK3β) in injured mice. DI treatment increased p-GSK3β and decreased p-S6RP expression (*P<0.05 versus PBS) (n=4/group).
Dual Inhibition of Akt and Mammalian Target of Rapamycin Reduces Propidium Iodide-Positive Cells in Hippocampus After Controlled Cortical Impact
We next assessed the effect of Akt and mTOR pathway inhibition on acute cellular injury and tissue damage 4 hours after CCI. Compared with vehicle-treated mice, treatment with combination Akt and mTOR inhibitors decreased numbers of PI-positive cells in ipsilateral CA1 and CA3 (P<0.05) but not DG or cortex (Figure 4), whereas treatment with Akt inhibitor or rapamycin alone did not reduce PI+ cells (CA1: Akt viii 24.0±3.1, RAP (rapamycin) 23.8±4.3, PBS 27.7±3.2, P=ns; CA3: Akt viii 48.5±5.0, RAP 44.4±8.0, PBS 39.8±4.5, P=ns; DG: Akt viii 226.9±8.4, RAP 223.0±8.6, PBS 219.9±5.4; cortex: Akt viii 90.4±7.2, RAP 92.3±5.6, PBS 103.9±4.2, P=ns, n=5 to 6/group). In addition, dual administration of Akt and mTOR inhibitors had no effect on the brain lesion size at 2 weeks after CCI (vehicle, 12.7±1.4 mm3; DI (double inhibitor), 13.4±1.8 mm3, P=0.77, n=11 to 12/group).
Figure 4.
Effect of combined akt inhibitor viii and rapamycin treatment on propidium iodide-positive (PI+) cells at 4 hours after controlled cortical impact (CCI). (A) Representative photomicrographs showing PI+ cells in CA1, CA3, and cortex after CCI in double inhibitor (DI; black bars)- or vehicle (VEH; white bars)-treated mice (n=5 to 6/group, magnification × 200). (B) Quantitation of PI+ cells at 4 hours after CCI. *P=0.03 rank sum versus VEH-treated animals. No differences between groups were observed in cortex or dentate gyrus (DG).
Combined Administration of Akt and Mammalian Target of Rapamycin Inhibitors Improves Motor Function After Controlled Cortical Impact
Compared with preinjury motor testing, CCI produced significant deficits in wire grip testing (P<0.001 for group versus sham injury) that resolved over time in vehicle and drug treatment groups (P<0.0001 for time). Treatment of sham-injured mice with DI did not alter motor function compared with vehicle-treated sham-injured mice (Supplementary Figure 2). Pretreatment of injured mice with combined Akt and mTOR inhibitors (but not single inhibitors alone) improved postinjury wire grip test performance (P=0.02 for group; Figure 5).
Figure 5.
Reduced motor deficits after controlled cortical impact (CCI) in double inhibitor (DI)-treated mice. Vestibulomotor function was improved in DI-treated mice (n=12) compared with vehicle (VEH) treatment (n=16 VEH, P=0.0016 for group) and compared with rapamycin (RAP) alone (P=0.04 for group). No effect was observed with Rap (n=12) or akt inhibitor viii (AKT I, n=20) alone versus VEH.
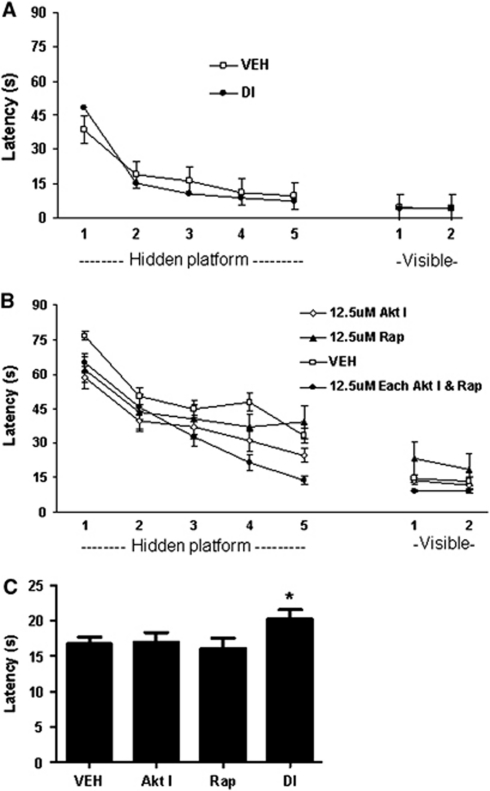
Combined Administration of Akt and Mammalian Target of Rapamycin Inhibitors Improves Postinjury Spatial Memory Acquisition After Controlled Cortical Impact
To assess the effect of Akt and mTOR inhibition on postinjury cognitive function, we first administered Akt inhibitor and rapamycin (12.5 μmol/L each) to sham-injured mice and showed no affects on spatial learning compared with vehicle treatment (Figure 6A; P=0.78 for group in hidden platform trials, P=0.72 for probe trials: vehicle 22.5±1.2 seconds, DI 23.8±2.3seconds). Following CCI, administration of Akt inhibitor or rapamycin alone at 12.5 μmol/L had no effect on hidden platform or probe trials (Figure 6B). We next carried out dose-response experiments in injured mice using 12.5 μmol/L Akt Inhibitor VIII and a range of concentrations of rapamycin together. Administration of Akt (12.5 μmol/L) and rapamycin (12.5 μmol/L) together yielded strong beneficial effects on hidden platform latencies (P=0.0011 for group; Figure 6B) and probe trials (P<0.05 versus vehicle; Figure 6C) whereas combination treatment with 12.5 μmol/L Akt inhibitor and 1.25 or 125 μmol/L of rapamycin had no effect on MWM performance (Supplementary Figure 2). No differences in visible platform trials were noted among any of the injured groups.
Figure 6.
Effect of akt inhibitor viii (AKT I), rapamycin (RAP), vehicle (VEH) (phosphate-buffered saline, PBS), or combined treatment with RAP/AKT I (double inhibitor, DI) on Morris water maze (MWM) performance after sham injury or controlled cortical impact (CCI). (A) MWM performance did not differ between DI and VEH-treated, sham-injured mice (n=6/group). Following CCI, DI treatment significantly improved hidden platform performance (B) and probe trial scores (C) versus VEH (P=0.0011 for group, repeated measures analysis of variance (ANOVA), n=18/group). No significant differences were observed after CCI between single inhibitors (Akt inhibitor viii, n=20; RAP, n=12) and VEH groups in hidden platform (B) or probe trial (C) performance.
No effect of Akt/Mammalian Target of Rapamycin Inhibitors on Brain Inflammation After Controlled Cortical Impact
Because p-S6RP was robustly increased in glial cells after injury, we determined the antiinflammatory effects of Akt inhibitor and rapamycin by assessing microgliosis and astrocytosis and quantitation of the brain tumor necrosis factor mRNA. No difference in microgliosis or astrocytosis was observed at 48 hours (the peak of glial inflammation in our CCI model) (Figure 7). Likewise, no difference in the brain tumor necrosis factor-α mRNA expression was observed at 6 hours after CCI in vehicle and dual inhibitor groups (mean normalized expression: injured, vehicle treated 1.32 × 10−5+3.3 × 10−6; injured, combination inhibitor treated 1.7 × 10−5+1.5 × 10−6).
Figure 7.
Representative photomicrographs showing microglial and astrocyte activation at 48 hours after controlled cortical impact (CCI) in mice administered akt inhibitor viii and rapamycin together (double inhibitor, DI; n=3) versus vehicle (VEH; n=3). Treatment with DI had no apparent effect on microgliosis or astrocytosis after CCI.
Discussion
In agreement with previous studies in rodents, p-Akt-473, p-GSK3β, and p-S6RP were transiently increased in cortex, and p-Akt-473 and p-S6RP in hippocampus between 4 and 24 hours after CCI, suggesting activation of Akt and mTOR signaling after TBI (Chen et al, 2007; Erlich et al, 2007; Noshita et al, 2001). However, administration of either inhibitors of Akt or mTOR alone, while expectedly reducing phosphorylation of downstream substrates, did not alter functional outcome after CCI. Strikingly, combination treatment with Akt/mTOR inhibitors improved postinjury motor and cognitive function and reduced the numbers of cells with plasmalemma damage in CA3 and CA1, brain regions involved in spatial learning and memory in mice. To our knowledge, these are the first data suggesting a possible treatment strategy for TBI by dual inhibition of Akt/mTOR.
Importantly, our approach in documenting inhibition of Akt and mTOR activity in vivo is similar to that of other investigators using ICV or intravenous inhibitors (Erlich et al, 2007; Pignataro et al, 2010). Moreover, rapamycin was administered ICV at the doses similar to those in non-central nervous system injury models (Ropelle et al, 2008). Overall, although additional (off target) effects of Akt inhibitor and rapamycin are possible, our data suggest that activity of the intended targets Akt and mTOR was inhibited by Akt inhibitor viii and rapamycin, respectively, in the injured brain.
We found that partial inhibition of Akt did not worsen histopathological or functional outcome after CCI. Similarly, intracerebroventricular administration of an Akt inhibitor, as well as Akt1 gene knockout, did not increase infarct size in experimental stroke models (Li et al, 2008). Our findings are consistent with the notion that Akt inhibition alone does not necessarily worsen outcome after brain injury, similar to a previous study in which blocking insulin-like growth factor-1 receptor signaling also did not worsen functional outcome in mice with mild TBI (Rubovitch et al, 2010). One reason for this could be that the partial inhibition of Akt achieved in the current study is inadequate to observe a negative impact on outcome after CCI. Akt inhibitor viii targets Akt1 and Akt2 in the nmol/L range but Akt3 in the μmol/L range. Thus, inadequate inhibition of Akt3 might compensate for loss of function of Akt1/2. Alternatively, surpassing the threshold of Akt activation required to observe a beneficial impact (e.g., insulin-like growth factor-1 treatment) may be easier to achieve, and explain why activating Akt is neuroprotective in brain injury (D'Ercole et al, 1996) whereas Akt inhibition does not worsen outcome after CCI (current study) and other TBI models (Rubovitch et al, 2010).
A novel finding in the current study is that p-S6RP was strongly induced by CCI in microglia and astrocytes in injured hippocampus. Phosphorylation of S6RP was previously described exclusively in neurons after TBI in rats, and was hypothesized to increase neuronal translational capacity (Chen et al, 2007). We also found neuronal expression of p-S6RP before and after CCI. Our study extends the possibility that Akt/mTOR signaling may impact functional recovery by injury-specific translation or transcription in glial cells, despite the finding that combined Akt/mTOR inhibitors did not appreciably reduce morphological changes of microglia and astrocytes after CCI. TORC1 signaling might lead to gene expression and protein synthesis of proinflammatory cytokines, chemokines, inducible nitric oxide synthase, and other regulatory intermediates of the inflammatory response of glia to TBI. In this regard, Akt/mTOR inhibition might be more important in glia than in neurons (where traditionally Akt has been considered protective). Astrocytes and microglia have been hypothesized to mediate detrimental effects after TBI, and TORC1 inhibition was protective in a closed-head TBI model (Hailer, 2008; You et al, 2008; Erlich et al, 2007). The lack of similar effects of rapamycin in our CCI model may be related to a higher injury severity and less sensitive motor tests employed in the current study (Erlich et al, 2007).
Akt and mTOR inhibition may be beneficial after TBI because of inhibitory effects on gliosis, whereas Akt inhibition may be detrimental to survival in neurons. Our data cannot distinguish these possibilities, making interpretation of the signaling results somewhat challenging. Genetic inhibition of TORC1 component(s) in glial cells, or expression of mutant isoforms of TORC1 components driven by glia-specific promoters, could be used to shed light on cell-specific functions of TORC1 in future studies.
We used in vivo propidium iodide as a sensitive marker of fatal cellular injury after CCI (Bermpohl et al, 2006; Whalen et al, 2008). Combined administration of Akt and mTOR inhibitors decreased PI+ cells in CA3/CA1. This observation may be due to the effects of rapamycin alone (Erlich et al, 2007) or to effects on GSK3β inhibition. Alternatively, the data may be explained in part by Akt inhibition. Although prevailing wisdom holds that Akt is antiapoptotic in central nervous system injury paradigms (Carloni et al, 2010), Akt may function to induce necrosis by rendering cells more sensitive to oxidant injury (Nogueira et al, 2008). Specific inhibitors of Akt inhibit photodynamic-induced necrosis in cultured neurons (Komandirov et al, 2011), and drugs that promote activation and nuclear translocation of Akt have been used to induce death in cancer cells (Maddika et al, 2008; Nogueira et al, 2008). Superoxide production and plasmalemma damage are detectable in hippocampal neurons and other vulnerable brain regions as early as 30 minutes after CCI (Lewen et al, 2001; Whalen et al, 2008), lending support to the idea that Akt may influence oxidative stress-induced necrosis in CA3/CA1 after CCI. Although our data do not conclusively demonstrate a detrimental role for Akt, the possibility that Akt may contribute to necrosis in the CCI model is intriguing and worthy of further investigation.
The most striking new discovery of our study is that dual inhibition of both Akt and TORC1 leads to significant protection following TBI. The precise mechanism of this effect remains to be fully explored. While this result does not appear to be attributable to changes in Akt phosphorylation (as p-Ser473 levels were not affected), we observed a significant increase in p-GSK3β in injured hippocampus. This result was particularly unexpected as single inhibition of either kinase decreased, rather than increased GSK3β phosphorylation in the brain. One possibility is that DI treatment yielded off-target effects leading to increased GSK3β phosphorylation. Another possibility is that inhibition of both Akt and TORC1 activity results in the changes in the mechanism of GSK3β regulation, which does not occur upon single inhibition of either kinase. In cancer cells, inhibitors of Akt or mTOR promoted unexpected activation of upstream mechanisms such as Akt itself (in the case of rapamycin) and RTKs (in the case of Akt inhibitors) as part of negative feedback regulation (Chandarlapaty et al, 2011; Fan et al, 2006). As a result, dual inhibition of Akt/mTOR or Akt/RTKs was required to block proliferation (Chandarlapaty et al, 2011; Fan et al, 2006). Reciprocal regulation of Akt and MAPK pathways is also well established (Guan et al, 2000; Moelling et al, 2002). In particular, Erk kinase and its downstream target, p90RSK, have been shown to also regulate Ser9 phosphorylation of GSK3β (Ding et al, 2005). Examining the status of these putative mechanisms in the brain following Akt/TORC1 inhibition is an important direction of future studies.
Several lines of evidence suggest that inhibition of GSK3β might contribute to improved functional outcome in the current study. GSK3β is implicated in the pathogenesis of cognitive decline and neurodegeneration in Alzheimer's disease models (Hooper et al, 2008). In rat and mouse hippocampal slices treated with amyloid β, overactivation of GSK3β potently inhibits synaptic long-term potentiation, a physiological correlate of learning and memory, that is restored by specific inhibitors of GSK3β (Jo et al, 2011). Mice treated with lithium, a specific inhibitor of GSK3β, had improved MWM performance after CCI compared with vehicle with an effect size similar to that observed in the current study (Zhu et al, 2010). Interestingly, mTOR is required for normal learning and memory processes and inhibition or dysregulation of mTOR causes learning deficits in normal mice (Qi et al, 2010). Data from the current study are consistent with an injury-specific beneficial effect of Akt/mTOR inhibitors as combined therapy did not affect MWM performance in sham-injured mice tested 8 to 10 days after administration of compounds.
We conclude that combination therapy targeting Akt and mTOR produces beneficial effects on functional recovery associated with GSK3β inhibition after CCI. Further studies examining the effect of combination Akt/mTOR inhibitors on related signaling pathways are needed to identify novel potential targets for treatment strategies in patients with contusion TBI.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by grants from NINDS 5RO1NS047447 and 5RO1NS064545 (MJW).
Supplementary Material
References
- Bermpohl D, You Z, Korsmeyer SJ, Moskowitz MA, Whalen MJ. Traumatic brain injury in mice deficient in Bid: effects on histopathology and functional outcome. J Cereb Blood Flow Metab. 2006;26:625–633. doi: 10.1038/sj.jcbfm.9600258. [DOI] [PubMed] [Google Scholar]
- Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy. 2010;6:366–377. doi: 10.4161/auto.6.3.11261. [DOI] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Atkins CM, Liu CL, Alonso OF, Dietrich WD, Hu BR. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2007;27:939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- D'Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol. 1996;13:227–255. doi: 10.1007/BF02740625. [DOI] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Nishi T, Chan PH. Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1479–1489. doi: 10.1038/sj.jcbfm.9600303. [DOI] [PubMed] [Google Scholar]
- Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Cantley LC. Apoptosis. A Bad kinase makes good. Nature. 1997;390:116–117. doi: 10.1038/36442. [DOI] [PubMed] [Google Scholar]
- Guan KL, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011;11:289–301. doi: 10.1038/nrc3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LY, Sun ZG, Wen YM, Cheng GZ, Wang SL, Zhao HB, Zhang XR. ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats. Neuroscience. 2010;169:1046–1062. doi: 10.1016/j.neuroscience.2010.05.046. [DOI] [PubMed] [Google Scholar]
- Jenkins LW, Peters GW, Dixon CE, Zhang X, Clark RS, Skinner JC, Marion DW, Adelson PD, Kochanek PM. Conventional and functional proteomics using large format two-dimensional gel electrophoresis 24 hours after controlled cortical impact in postnatal day 17 rats. J Neurotrauma. 2002;19:715–740. doi: 10.1089/08977150260139101. [DOI] [PubMed] [Google Scholar]
- Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, Dickinson B, Scullion S, Sheng M, Collingridge G, Cho K. Abeta(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nat Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- Khuman J, Meehan WP, III, Zhu X, Qiu J, Hoffmann U, Zhang J, Giovannone E, Lo EH, Whalen MJ. Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J Cereb Blood Flow Metab. 2010;31:778–789. doi: 10.1038/jcbfm.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komandirov MA, Knyazeva EA, Fedorenko YP, Rudkovskii MV, Stetsurin DA, Uzdensky AB.2011On the role of phosphatidylinositol 3-kinase, protein kinase b/akt, and glycogen synthase kinase-3beta in photodynamic injury of crayfish neurons and glial cells J Mol Neurosciin press) [DOI] [PubMed]
- Lewen A, Sugawara T, Gasche Y, Fujimura M, Chan PH. Oxidative cellular damage and the reduction of APE/Ref-1 expression after experimental traumatic brain injury. Neurobiol Dis. 2001;8:380–390. doi: 10.1006/nbdi.2001.0396. [DOI] [PubMed] [Google Scholar]
- Li J, Lang J, Zheng Z, McCullough LD. Akt 1 gene deletion and stroke. J Neurol Sci. 2008;269:105–112. doi: 10.1016/j.jns.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddika S, Ande SR, Wiechec E, Hansen LL, Wesselborg S, Los M. Akt-mediated phosphorylation of CDK2 regulates its dual role in cell cycle progression and apoptosis. J Cell Sci. 2008;121:979–988. doi: 10.1242/jcs.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt cross-talk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, Miwa A, Okado H, Kiyama H. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20:2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT. Protein kinase signaling cascades in CNS trauma. IUBMB Life. 2005;57:711–718. doi: 10.1080/15216540500319143. [DOI] [PubMed] [Google Scholar]
- Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshita N, Lewen A, Sugawara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1442–1450. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Noshita N, Lewen A, Sugawara T, Chan PH. Akt phosphorylation and neuronal survival after traumatic brain injury in mice. Neurobiol Dis. 2002;9:294–304. doi: 10.1006/nbdi.2002.0482. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Esposito E, Cuomo O, Sirabella R, Boscia F, Guida N, Di Renzo G, Annunziato L. The NCX3 isoform of the Na+/Ca2+ exchanger contributes to neuroprotection elicited by ischemic postconditioning. J Cereb Blood Flow Metab. 2010;31:362–370. doi: 10.1038/jcbfm.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Mizuno M, Yonezawa K, Nawa H, Takei N. Activation of mammalian target of rapamycin signaling in spatial learning. Neurosci Res. 2010;68:88–93. doi: 10.1016/j.neures.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Ropelle ER, Fernandes MF, Flores MB, Ueno M, Rocco S, Marin R, Cintra DE, Velloso LA, Franchini KG, Saad MJ, Carvalheira JB. Central exercise action increases the AMPK and mTOR response to leptin. PLoS One. 2008;3:e3856. doi: 10.1371/journal.pone.0003856. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rubovitch V, Edut S, Sarfstein R, Werner H, Pick CG. The intricate involvement of the insulin-like growth factor receptor signaling in mild traumatic brain injury in mice. Neurobiol Dis. 2010;38:299–303. doi: 10.1016/j.nbd.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Song YS, Narasimhan P, Kim GS, Jung JE, Park EH, Chan PH. The role of Akt signaling in oxidative stress mediates NF-kappaB activation in mild transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1917–1926. doi: 10.1038/jcbfm.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen MJ, Dalkara T, You Z, Qiu J, Bermpohl D, Mehta N, Suter B, Bhide PG, Lo EH, Ericsson M, Moskowitz MA. Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:490–505. doi: 10.1038/sj.jcbfm.9600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Tan HL, Huang Q, Ong CN, Shen HM. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy. 2009;5:824–834. doi: 10.4161/auto.9099. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Bjorklof K, Calvez R, Finan P, Thomast M, Trifilieff A, Barbier M, Altruda F, Hirsch E, Laffargue M. Phosphoinositide 3-kinase gamma: a key modulator in inflammation and allergy. Biochem Soc Trans. 2003;31:275–280. doi: 10.1042/bst0310275. [DOI] [PubMed] [Google Scholar]
- You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD, Moskowitz MA, Whalen MJ. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZF, Wang QG, Han BJ, William CP. Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res Bull. 2010;83:272–277. doi: 10.1016/j.brainresbull.2010.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.