Abstract
Although platelet-derived growth factors (PDGFs) and receptors (PDGFRs) are abundantly expressed in the central nervous system, their functions largely remain elusive. We investigated the role of PDGFR-β in tissue responses and functional recovery after photothrombolic middle cerebral artery occlusion (MCAO). In the normal adult mouse brain, PDGFR-β was mainly localized in neurons and in pericyte/vascular smooth muscle cells (PC/vSMCs). From 3 to 28 days after MCAO, postnatally induced systemic PDGFR-β knockout mice (Esr-KO) exhibited the delayed recovery of body weight and behavior, and larger infarction volume than controls. In Esr-KO, PC/vSMC coverage was decreased and vascular leakage of infused fluorescent-labeled albumin was extensive within the ischemic lesion, but not in the uninjured cerebral cortex. Angiogenesis levels were comparable between Esr-KO and controls. In another PDGFR-β conditional KO mouse (Nestin-KO), PDGFR-β was deleted in neurons and astrocytes from embryonic day 10.5, but was preserved in PC/vSMCs. After MCAO, vascular leakage and infarction volume in Nestin-KO were worse than controls, but partly improved compared with Esr-KO. Astroglial scar formation in both Esr-KO and Nestin-KO was similarly reduced compared with controls after MCAO. These data suggested that PDGFR-β signaling is crucial for neuroprotection, endogenous tissue repair, and functional recovery after stroke by targeting neurons, PC/vSMCs, and astrocytes.
Keywords: blood–brain barrier, glial scar formation, middle cerebral artery occlusion, pericyte/vascular smooth muscle cell, platelet-derived growth factor receptor-β, tissue repair
Introduction
Stroke currently ranks first in adult disability worldwide and second in mortality in most regions (Strong et al, 2007). For the prevention and care of the disease, numbers of therapeutic strategies including neuroprotective agents have been developed (Warburton et al, 2011). In addition, owing to the discovery of adult neurogenesis in the mammalian central nervous system (CNS), regeneration-based treatments for CNS injury are expected as promising therapeutic strategies in which interactions among neurons, glial cells, and the vascular system should be elucidated and controlled appropriately (Okano et al, 2007).
Platelet-derived growth factor (PDGF) family members include PDGF-A, PDGF-B, PDGF-C, and PDGF-D, which are assembled as disulfide-linked homodimers or heterodimers. Two types of PDGF receptors, namely PDGFR-α and PDGFR-β, undergo overlapping, but distinctive, signal transduction in various cell types (Tallquist and Kazlauskas, 2004). Both PDGFs and PDGFRs are widely expressed in the CNS (Sasahara et al, 1991) and are upregulated in neurons, reactive astroglial cells, and pericyte/vascular smooth muscle cells (PC/vSMCs) after cerebral stroke in human and animal models (Iihara et al, 1996; Krupinski et al, 1997; Renner et al, 2003).
Both PDGF-B and PDGF-C protect neurons in several different animal models of neuronal injury including ischemia (Iihara et al, 1997; Tang et al, 2010). Using inducible knockout (KO) mutant, we have shown that PDGFR-β expressed in neurons, mediates neuroprotective signals against various insults both in vivo and in vitro experiments (Ishii et al, 2006; Zheng et al, 2010). Recent studies have shown that the PDGF-B/PDGFR-β signal axis is essential for the establishment of PC/vSMC population in developing the cerebral vasculature, and the congenital disturbance of this signal causes abnormal blood–brain barrier (BBB) functions (Armulik et al, 2010; Bell et al, 2010; Daneman et al, 2010). In addition, PDGF is a potent mitogen and chemoattractant for glial cells (Bressler et al, 1985). Thus, the PDGFR-β signal is supposed to promote integrated cellular responses among these cell types after cerebral stroke; however, its functional relevance largely remains elusive in CNS tissue remodeling. In contrast to these beneficial effects, PDGFR-α activation reportedly impairs BBB integrity during thrombolytic therapy of ischemic stroke (Su et al, 2008). Therefore, it is also necessary to determine the distinctive role of each PDGFR in stroke.
The null mutants of PDGF-B or PDGFR-β cause neonatal lethality owing to severe vascular abnormalities including kidney glomerulus (Lindahl et al, 1997, 1998). In contrast, postnatally induced systemic PDGFR-β KO in mouse does not show apparent adverse effects both on physiologic parameters and on the morphology of the kidney for a long term, but disturbs the adaptive responses of the stressed kidney glomerulus in our previous report (Nakagawa et al, 2011). These findings indicate that the role of PDGFR-β could be different between organogenesis and postnatal life, and raise the possibility that PDGFR-β may also have a role in the repair process after cerebral insult in the adult mouse brain. Accordingly, using two kinds of PDGFR-β KO mice (postnatally induced systemic KO and neuroepithelium-derived cells KO), we examined the role of PDGFR-β signal in CNS tissue responses to focal cerebral ischemia induced by photothrombotic middle cerebral artery occlusion (MCAO).
Materials and methods
All experimental animal procedures were conducted according to ‘the Institutional Animal Care and Use Committee at the University of Toyama' (University of Toyama, Sugitani, Toyama City, Japan). All of our study protocols were approved by the Ethics Committee of the University of Toyama before the study began, and we were given permission to carry out the study.
Conditional Knockout Mice
Mutant mice, in which exons 4 to 7 of PDGFR-β were flanked by 2 loxP sequences (PDGFR-βfloxed/floxed) (Gao et al, 2005), were cross-bred with chicken β-actin-promoter/CMV-enhancer-driven Cre-transgenic mice (Cre-ER+/−; CAGGCre-ER+/−; Jackson Laboratories, Bar Harbor, ME, USA) that systemically expressed a fusion protein consisting of Cre recombinase and a mutated form of the mouse estrogen receptor ligand-binding domain (Hayashi and McMahon, 2002). The gene construct was prepared as follows: a fragment encoding Cre-ER (Danielian et al, 1998) was cloned into the pCAGGS vector (Niwa et al, 1991) to generate pCAGGCre-ER. The ApaI site of pBS-Cre-ER was replaced by an EcoRI site using the oligo (CGAATTCGGGCC). An EcoRI fragment containing Cre-ER was then subcloned into the EcoRI site of pCAGGS. The orientation of the gene was confirmed by sequencing. The resulting male offspring expressing PDGFR-βfloxed/floxed/Cre-ER+/− and mice expressing PDGFR-βfloxed/floxed were subjected to oral tamoxifen administration (9 mg/40 g body weight; 5 consecutive days; Sigma-Aldrich, Louis, MO, USA) at 4 weeks of age; PDGFR-βfloxed/floxed/Cre-ER+/− mice were used as systematic PDGFR-β KO mice (Esr-KO), and PDGFR-βfloxed/floxed mice served as controls (Floxed), respectively.
In another line of PDGFR-β conditional KO mice, PDGFR-βfloxed/floxed mice were cross-bred with transgenic mice that expressed Cre under control of the nestin promoter/enhancer (nestin-Cre, Jackson Laboratories) (Ishii et al, 2006). In the resulting male offspring with the PDGFR-βfloxed/floxed/nestin-Cre+/− (Nestin-KO), the PDGFR-β gene was mostly deleted in the brain, but PDGFR-β was preserved in vascular PC/vSMCs (Ishii et al, 2006). PDGFR-βfloxed/floxed mice without tamoxifen administration (Floxed without tamoxifen) served as controls of Nestin-KO. All mice were housed at 25°C with a 12-hour light/12-hour dark cycle with free access to pellet chow and water.
Genomic Polymerace Chain Reaction
The recombinant allele of PDGFR-β was detected by genomic PCR analysis (Ishii et al, 2006). Genomic DNA was extracted from various mouse brain tissues using the MagExtractor genome kit (Toyobo, Osaka, Japan). The PCR reaction was performed in a GeneAmp PCR System 9700 (PE Applied Biosystems, Carlsbad, CA, USA) using KOD Dash DNA polymerase (Toyobo). The primers were as follows: primer 1, 5′-TAGCCATGGAGTCATCTCTTCAGCCCTAAA-3′ primer 2, 5′-CCTGCATCAAGTAGCTCACAACTGCCTGTA-3′ primer 3, 5′-AGCAAGGTCGCGCAAGGGATAACAGC-3′.
Quantitative Real-Time Polymerace Chain Reaction
The efficiency of PDGFR-β gene deletion was evaluated by quantitative real-time PCR. Total RNA was isolated from the cerebral cortex using the RNeasy mini kit (Qiagen, Valencia, CA, USA), purified with the RNase-Free DNase Set (Qiagen), and used as a template for cDNA synthesis by Prime Script RT reagent kit (Takara, Shiga, Japan). PCR reactions were performed in a Thermal Cycler Dice Real-Time System Tp800 (Takara) using SYBR Premix EX Taq (Takara) according to the manufacturer's instructions as follows: 10 seconds at 95°C, 40 cycles of 5 seconds at 95°C, and 30 seconds at 60°C and then for the dissociation stage of 15 seconds at 95°C, 30 seconds at 60°C, and 15 seconds at 95°C. The sequences of primers were as follows: PDGFR-β: forward primer, 5′-AGGACAACCGTACCTTGGGTGACT-3′ reverse primer, 5′-CAGTTCTGACACGTACCGGGTCTC-3′ PDGFR-α: forward primer, 5′-CCATTCTAGTCAACGTGGGTACCAG-3′ reverse primer, 5′-TGCATCATTCCCGGACACA-3′ GAPDH (glyceraldehyde-3-phosphate dehydrogenase): forward primer, 5′-AAATGGTGAAGGTCGGTGTG-3′ reverse primer, 5′-TGAAGGGGTCG TTGATGG-3′. The relative gene expression was determined by the standard curve method, and fold changes of targeted genes were normalized by GAPDH mRNA, and relative to control values. Each sample was tested in triplicate.
Focal Cerebral Ischemia
Mice were subjected to permanent MCAO according to a modified method (Sugimori et al, 2004). Five- to six-month-old male mice were anesthetized with halothane (2% for induction, 1% for maintenance with a face mask; Takeda Pharmaceutical, Osaka, Japan) carried by a mixture of 70% nitrous oxide and 30% oxygen. Rectal temperature was maintained throughout the surgical procedure at 37°C±0.5°C using a homeothermic blanket (Harvard Apparatus, Holliston, MA, USA). The left middle cerebral artery (MCA) was exposed using a stereoscopic surgical microscope (Conan Medical, Hyogo, Japan). A 6-mW krypton laser with 568 nm (Melles Griot, Tokyo, Japan) was used to irradiate the distal MCA for 4 minutes, after intravenous administration of a photosensitizing rose Bengal dye solution (Wako, Osaka, Japan) at a dose of 20 mg/kg body weight over 90 seconds. After that, a secondary 4-minute laser irradiation was administrated to the proximal MCA. The left common carotid artery was then tightly ligated to induce a reproducible neocortical infarct (Brint et al, 1988). The temporalis muscle and skin were subsequently reconstructed; mice were subsequently maintained at a warm temperature and returned to the home cage until resuscitation. Sham controls were treated identically, excluding laser exposure and common carotid artery ligation.
Mouse Behavior
Following a previously described method (Orset et al, 2007), an open-field test was performed before and after MCAO. Each mouse was gently placed at the center of a cubic chamber (48 × 48 × 30 cm3). The mouse could move freely in the chamber for 15 minutes. The spontaneous locomotor activity (by the total distance covered) and exploration behavior (by the number of entries and time spent in the center zone) were assessed using an automated open-field system equipped with Scanet MV-10 computer software (Melquest, Toyama, Japan).
Infarction Volume Measurement
Under deep anesthesia with sodium pentobarbital (intraperitoneal injection, 50 mg/kg body weight; Dainippon Sumitomo Pharma, Osaka, Japan), mice were transcardially perfused with phosphate-buffered saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. Coronal sections (10-μm thick) were prepared with 200-μm intervals so that the entire ischemic lesion was covered. Hematoxylin–eosin staining was performed for measurement of areas of interest. Morphologic and morphometrical analyses were performed using a microscopy system (BX 50; Olympus, Tokyo, Japan) connected to a digital camera (DP70; Olympus). Measurements of areas of interest were conducted using MetaMorph software (Molecular Devices, Osaka, Japan). Lesion volumes were estimated using an indirect method to avoid effects of tissue swelling or shrinkage: 100% × (contralateral hemisphere volume−noninfarct ipsilateral hemisphere volume)/contralateral hemisphere volume (Swanson et al, 1990).
Immunohistochemistry and Immunofluorescence Staining in Paraffin-Embedded Tissue Sections
Paraffin-embedded, coronal tissue sections (10-μm thick) through the injured cortex were prepared according to the AMex method (Suzuki et al, 2002). In brief, transcardial perfusion with ice-cold phosphate-buffered saline was followed by perfusion and immersion in periodate-lysine-paraformaldehyde fixative containing 4% paraformaldehyde. After dehydrated in acetone at 4°C overnight, tissues were cleared in methyl benzoate for 1 hour and in xylene for 1 hour, respectively. These tissues were then embedded in paraffin. Deparaffinized coronal tissue sections were processed for antigen retrieval using target retrieval solution (pH 9.0; Dako, Cupertino, CA, USA) at 120°C for 4 minutes. Nonspecific immunoreactions were blocked at room temperature for 30 minutes using the Protein-Block kit (Dako). For immunostainings with mouse primary antibodies, sections were treated with Histofine Mouse Stain kit (Nichirei, Tokyo, Japan) for 1 hour before the Protein-Block kit. Sections were incubated at 4°C overnight with the following primary antibodies: goat polyclonal anti-PDGFR-β (1:100; R&D Systems, Minneapolis, MN, USA), mouse monoclonal anti-alfa smooth muscle actin (α-SMA; 1:100; Dako), rabbit polyclonal anti-laminin (1:1,000; Dako), and rabbit polyclonal anti-glial fibrillary acidic protein (GFAP; 1:1,000; Dako). Positive reactions were detected using the appropriate Histofine Simple Stain Mouse System (Nichirei) and were visualized following 3,3′-diaminobenzidine tetrahydrochloride (Dako) reaction. Nuclear counter stainings were conducted with hematoxylin. Images were captured using a light microscope (Olympus) and processed using Photoshop software (version 7.0, Adobe, San Jose, CA, USA).
To test the efficiency of PDGFR-β gene KO, single-immunofluorescence staining for PDGFR-β was used. Deparaffinized sections were processed for antigen retrieval, blocking, and incubated at 4°C overnight with goat polyclonal anti-PDGFR-β antibody (1:100; Neuromics, Edina, MN, USA). Staining was visualized by incubating with donkey anti-goat IgG conjugated with Alexa Fluor 488 (1:500; Molecular Probes, Eugene, OR, USA) at room temperature for 1 hour.
To specifically identify PC/vSMC coverage and proliferation, double-immunofluorescence staining for α-SMA/laminin and Ki67 (a cellular proliferation marker)/α-SMA were used. Deparaffinized sections were processed for antigen retrieval, blocking, and incubated at 4°C overnight with the following primary antibodies: mouse monoclonal anti-α-SMA (1:100; Dako), rabbit polyclonal anti-laminin (1:1,000; Dako), and rat polyclonal anti-Ki67 (1:100; Dako). Stainings were visualized by incubating with donkey anti-mouse IgG conjugated with Alexa Fluor 488/594 (1:500; Molecular Probes), donkey anti-rabbit IgG conjugated with Alexa Fluor 594 (1:500; Molecular Probes), and donkey anti-rat IgG conjugated with Alexa Fluor 488 (1:500; Molecular Probes). All sections were mounted with the Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, USA) and imaged using a confocal microscope (TCS-SP5, Leica, Heidelberg, Germany). Montages were created using Photoshop software.
Western Blot
For western blot analysis, 2-mm thick, coronal tissue sections were harvested from the middle of the ischemic lesions. The cortical ischemic lesion was then dissected along the outer margin of the lesion and designated as the ipsilateral cortex. The cerebral cortex of the similar anatomic site was obtained from another side of the tissue section and designated as the contralateral cortex. All brain tissues were homogenized in RIPA buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 0.5% sodium deoxycholate, 1% NP-40, 0.1% SDS, 2 mmol/L Na3VO4, 500 mmol/L NaF, 58 μg/mL aprotinin, 10 μg/mL leupeptin, and 2 mmol/L PMSF) using the Multi-Beads Shocker system (Yasui-Kikai, Osaka, Japan) at 4°C. Protein (15 μg) samples were separated by SDS-PAGE using a gradient gel (5% to 20% ATTO, Tokyo, Japan), followed by electroblotting onto polyvinylidene difluoride membranes (ATTO) and incubation in a blocking buffer of 5% nonfat milk at room temperature for 1 hour. Membranes were incubated with the following primary antibodies: rabbit polyclonal anti-PDGFR-β (1:500, Upstate Biotechnology, Charlottesville, VA, USA), rabbit polyclonal anti-PDGFR-α (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-phospho-Tyr857 PDGFR-β (1:1,000, Santa Cruz Biotechnology), rabbit polyclonal anti-phospho-Tyr742 PDGFR-α (1:1,000, Novus Biologicals, Littleton, CO, USA), rabbit polyclonal anti-α-SMA (1:250, Abcam, Cambridge, MA, USA), rabbit polyclonal anti-laminin (1:250, Santa Cruz Biotechnology), goat polyclonal anti-GFAP (1:250, Santa Cruz Biotechnology), and mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (1:5,000, Chemicon, Temecula, CA, USA) at 4°C overnight. Immunoreactive bands were visualized by incubating with appropriate horseradish peroxidase-linked secondary antibodies, followed by enhanced chemiluminescence detection using enhanced chemiluminescence plus western blotting detection reagents (Amersham Biosciences, Little Chalfont, UK). The immunoreactive bands of targeted proteins were quantified using the VH Analyzer software (VH-H1A5, Keyence, Osaka, Japan), normalized with the GAPDH protein band, and then relative to sham values.
Fluorescein Isothiocyanate-Labeled Albumin Leakage and Immunofluorescence Staining in Frozen Tissue Sections
To assess BBB permeability after cerebral ischemia, fluorescein isothiocyanate (FITC)-labeled albumin (Sigma-Aldrich; 5 mg/mL in Ringer HEPES buffer: 147 mmol/L NaCl, 4 mmol/L KCl, 3 mmol/L CaCl2, 1.2 mmol/L MgCl2, 15 mmol/L Hepes, 5 mmol/L glucose, 1% bovine serum albumin; pH 7.4) was transcardially infused (Cavaglia et al, 2001). In brief, at 1, 3, 6, and 14 days after MCAO, mice were anesthetized with sodium pentobarbital. Heparin (100 Units/kg in 0.1 mol/L phosphate-buffered saline buffer) was intravenously injected (right jugular vein), followed by transcardial (left ventricle) perfusion with FITC-labeled albumin solution at a rate of 1 mL/min for 5 minutes. To avoid the effects of systemic blood pressure, the same amount of blood was simultaneously withdrawn through the right jugular vein. The brains were rapidly removed, fixed in 4% paraformaldehyde for 3 days, and immersed in 30% sucrose for 2 days. The brains were then embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA, USA). Five coronal tissue sections (30-μm thick) with 100-μm intervals were collected from the ischemic center of each brain using a cryostat (CM1900, Leica). Fluorescein isothiocyanate-albumin-infused brain sections were further immunostained. The floating sections were permeabilized in 0.3% Triton X-100 solution at room temperature for 1 hour, and then incubated with primary antibodies against α-SMA (1:100, mouse monoclonal antibody, Dako) and PDGFR-β (1:100, goat polyclonal antibody, Neuromics) in a blocking buffer of 10% normal donkey serum at 4°C for 2 days. Secondary antibodies were raised in appropriate hosts and conjugated with Alexa Fluor 594 and Alexa Fluor 633 (1:500, Molecular Probes). To determine glial scar formation, rabbit polyclonal anti-GFAP antibody (1:1,000; Dako) or rabbit polyclonal anti-vimentin antibody (1:100; Abcam) were incubated with floating sections without FITC-labeled albumin perfusion, followed by secondary antibody conjugated with Alexa Fluor 488 (1:500, Molecular Probes) or Alexa Fluor 594 (1:500, Molecular Probes), respectively. All sections were mounted on glass slides with the Vectashield mounting medium with 4′,6-diamidino-2-phenylindole and imaged using a confocal microscope (Leica). Montages were created using the Photoshop software.
Image Analysis and Quantification
Areas of PDGFR-β, α-SMA-positive stainings and areas of laminin-positive blood vessels were determined within randomly selected three squares of 0.16 mm2 per section in the ischemic border and core, respectively, using a Biorevo BZ-9000 microscope (Keyence) and BZ-II Analyzer software (Keyence). Three nonadjacent coronal sections per mouse were randomly selected and examined.
To determine the PC/vSMC coverage of blood vessels, the colocalization rates between α-SMA and laminin signals from microvessels were analyzed using LAS AF software (Leica). In each animal, four randomly selected squares of 0.0441 mm2 per section in the ischemic border of the cerebral cortex were analyzed in three nonadjacent coronal sections.
The percentage of Ki67-positive cells within α-SMA-positive cells was determined within three randomly selected 0.16 mm2 squares per section in the ischemic border of the cerebral cortex. Three nonadjacent coronal sections per mouse were randomly selected and examined.
To quantify FITC-albumin leakage, five frozen coronal tissue sections were prepared from the ischemic center of each mouse, and the lower-magnified entire view of the ischemic lesion was acquired throughout the entire depth of each section, i.e., eight images were acquired at every 3.75-μm depth using a confocal microscope (Leica; Supplementary Figure 1). On each acquired image, the fluorescence mean intensity was automatically read within three randomly selected 0.04 mm2 squares in the ischemic core, 8 to 10 fields covering the entire ischemic border, and 9 randomly selected cortical fields in the contralateral hemisphere using LAS AF software (Leica).
The astrocyte density in the glial scar at 14 days after MCAO was quantified using paraffin-embedded tissue sections stained for GFAP immunohistochemistry. The number of cell nucleus with cytoplasmic GFAP staining was counted in three consecutive 0.09 mm2 squares in the vicinity of the lesion per section, the squares of which are illustrated in Figure 6C, using a light microscope (Olympus) and analysis software (Olympus). In each animal, three nonadjacent sections were examined.
Statistics
Quantitative data were expressed as mean±s.e.m. Comparisons between two experimental groups were made using unpaired Student's t-test. For infarction volume and open-field test, two-way analysis of variance was used, followed by Fisher's protected least significant difference test for each group. P<0.05 was considered significant.
Results
In this study, MCAO was induced in 188 mice (Floxed, n=68; Esr-KO, n=73; Floxed without tamoxifen, n=24; Nestin-KO, n=23). Sham-operated mice were used as controls (Floxed, n=11; Esr-KO, n=11).
Platelet-Derived Growth Factor Receptor-β is Depleted in Pericyte/Vascular Smooth Muscle Cells and Neurons of Esr-KO Mice
We confirmed the efficiency of PDGFR-β gene deletion in adult mutant mice at genome, mRNA, and protein levels, respectively. Genomic PCR predominantly generated 410-bp bands corresponding to the deleted allele in the cerebral cortex, hippocampus, cerebellum, middle brain, and brain stem of adult Esr-KO mice (Figure 1A). The same PCR generated 329-bp bands corresponding to PDGFR-β-floxed allele in the brain tissues of Floxed mice (Figure 1A).
Figure 1.
Specific PDGFR-β knockout in the adult mouse brain. (A) PCR of genomic DNA isolated from brain tissues of Floxed and Esr-KO mice. The PCR products represented the floxed allele (329 bp) and the Cre-mediated recombined allele (410 bp) of PDGFR-β, respectively. Cx, cerebral cortex; Hi, hippocampus; Cb, cerebellum; Mb, middle brain; Bs, brain stem. (B) Western blot of PDGFR-β and PDGFR-α in brain tissues of Floxed and Esr-KO mice. (C) Real-time PCR measurement of PDGFR-β and PDGFR-α mRNA levels in the cerebral cortices of Floxed and Esr-KO mice. Each bar was normalized by GAPDH (n=3 per group). a2P<0.01 versus Floxed mice. NS: nonsignificant. (D) Body weight and brain weight of Floxed and Esr-KO mice (n=5 per group). (E) Macroscopic appearance of the Esr-KO brain was indistinguishable from that of the Floxed brain. (F) Immunofluorescence staining of PDGFR-β (green) in the cerebral cortices of Floxed and Esr-KO mice, counterstained with DAPI (blue). Many neurons and PC/vSMCs (indicated by arrows) were positively stained for PDGFR-β. Scale bars=50 μm. DAPI, 4′,6-diamidino-2-phenylindole; Esr-KO, PDGFR-β knockout mice; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PC/vSMC, pericyte/vascular smooth muscle cell; PDGFR, platelet-derived growth factor receptor.
In western blot, PDGFR-β was consistently detected in all brain tissues obtained from Floxed mice, but was decreased to undetectable levels in those tissues from Esr-KO mice (Figure 1B). Platelet-derived growth factor receptor-α was detected at similar levels between two groups in all tissues examined (Figure 1B).
Real-time PCR analysis showed that PDGFR-β mRNA expression was decreased by ∼85% in the cerebral cortices of adult Esr-KO compared with Floxed mice (Figure 1C, P<0.01). In contrast, PDGFR-α mRNA expression was expressed at comparable levels in two groups of mice (Figure 1C, P=0.976). These data confirmed efficient postnatal gene deletion in our Cre–loxP system together with our previous data (Nakagawa et al, 2011).
Body weight, brain weight, and macroscopic appearance of brains from Esr-KO mice were indistinguishable from those of Floxed mice before MCAO (Figures 1D and 1E).
To further characterize PDGFR-β depletion in the cerebral cortex, immunostaining of PDGFR-β was conducted. Immunofluorescence clearly showed that PDGFR-β was expressed in neurons and in PC/vSMCs of Floxed mice as reported previously (Ishii et al, 2006; Virgintino et al, 2007), and mostly disappeared in both cell types of adult Esr-KO mice (Figure 1F). Throughout the experiments, our immunostaining did not show PDGFR-β in astrocytes in the adult mouse brain, although cultured astrocytes isolated from the neonatal mouse brain showed abundant PDGFR-β expression that was phosphorylated after PDGF-BB stimulation (Supplementary Figure 2).
Platelet-Derived Growth Factor Receptor-β Deletion Increases Infarction Volume and Impairs Functional Recovery After Cerebral Ischemia
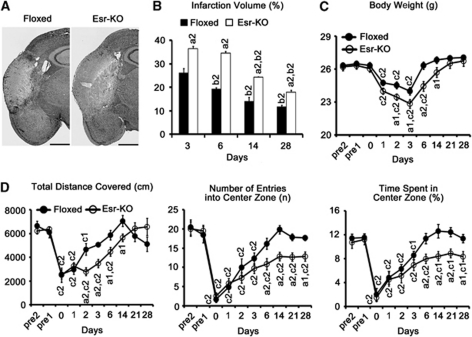
Middle cerebral artery occlusion induced reproducible and well-demarcated ischemic lesions in the cerebral cortices of Esr-KO and Floxed mice (Figure 2A).
Figure 2.
PDGFR-β deletion increases infarction volume and impairs functional recovery after cerebral ischemia. (A) Six-day lesions after MCAO (HE staining) in Floxed and Esr-KO mice. Scale bars=1 mm. (B) Infarction volume in Floxed (n=6) and Esr-KO (n=6) mice from 3 to 28 days after MCAO. (C) Body weight before and after MCAO. (D) Three parameters obtained in open-field tests in Floxed (n=6) and Esr-KO (n=7) mice before and after MCAO. a1P<0.05, a2P<0.01 versus Floxed mice at the same day after MCAO; b2P<0.01 versus the same genotypic mice at 3 days after MCAO; c1P<0.05, c2P<0.01 versus the same genotypic mice at 1 day before MCAO (pre1). HE, hematoxylin–eosin; MCAO, middle cerebral artery occlusion; PDGFR, platelet-derived growth factor receptor.
Infarction volume was significantly larger in Esr-KO than in Floxed mice at 3, 6, 14, and 28 days after MCAO (Figure 2B, P<0.01 group effect). Infarction volume in Floxed mice decreased in a linear manner from 3 to 28 days (Figure 2B, P<0.01 time effect versus Floxed at 3 days). In contrast, infarction volume of Esr-KO mice showed no significant difference from 3 to 6 days (P=0.259), but linearly decreased from 6 to 28 days (P<0.01 time effect, versus Esr-KO at 3 days).
The body weight of Esr-KO mice was significantly lost at 1 day and recovered to pre-MCAO level at 14 days after MCAO, which showed obviously delayed recovery compared with Floxed mice that recovered to pre-MCAO level at 6 days (Figure 2C, P<0.01 group effect).
To test functional recovery after cerebral ischemia, the open-field test was performed before and after MCAO. Before MCAO, three parameters of the open-field test: the total distance covered (indicating the spontaneous locomotor activity), the number of entries into the center zone, and the time spent in the center zone (indicating the exploration behavior) were at comparable levels in Floxed and Esr-KO mice (Figure 2D). After MCAO, recovery of the total distance covered was significantly delayed in Esr-KO than in Floxed mice (P<0.01 group effect); the remaining two parameters recovered to pre-MCAO levels in Floxed at 6 days, but not in Esr-KO mice even until 28 days (P<0.01 group effect).
Platelet-Derived Growth Factor Receptor-β Deletion Decreases Pericyte/Vascular Smooth Muscle Cell Coverage and Proliferation in Lesions After Cerebral Ischemia
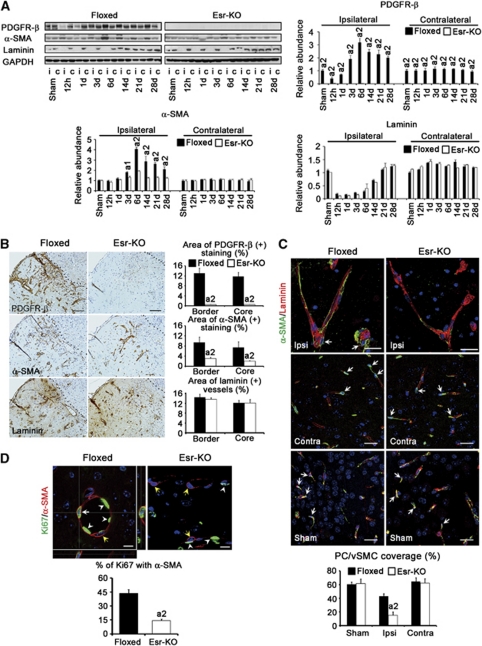
According to western blot, PDGFR-β protein levels decreased at 12 hours in Floxed lesions compared with the sham-operated group, subsequently increased from 1 to 6 days, and then sustained high levels until 28 days. In Esr-KO lesions, PDGFR-β levels were almost undetectable (Figure 3A). Levels of α-SMA were distinctly increased in Floxed lesions from 3 to 28 days compared with the sham-operated group, and were higher than those in Esr-KO lesions during this period (Figure 3A). After transient decreases, laminin levels were similarly restored in the 14-day lesions in the two groups (Figure 3A). In the contralateral side, there were no evident differences of α-SMA and laminin levels between the two groups, except apparently decreased PDGFR-β levels in Esr-KO mice.
Figure 3.
PDGFR-β deletion decreases PC/vSMC coverage and proliferation in ischemic lesions after cerebral ischemia. (A) Western blot analysis and quantification of PDGFR-β, α-SMA, and laminin protein expression in ipsilateral (i) and contralateral (c) cerebral cortices of Floxed and Esr-KO mice after MCAO and sham-operated mice (n=3 per group). Each bar was normalized by GAPDH. a1P<0.05, a2P<0.01 versus Floxed mice at the same day after MCAO. (B) Immunohistochemistry on serial sections and quantification of the areas of PDGFR-β, α-SMA-positive stainings, and areas of laminin-positive blood vessels in the ischemic border and ischemic core in Floxed (n=6) and Esr-KO (n=5) mice at 6 days after MCAO. Scale bar=100 μm. Dotted lines indicate the ischemic borders. a2P<0.01 versus Floxed mice. (C) PC/vSMC coverage, using double-immunofluorescence stainings for α-SMA (green) and laminin (red) in the ipsilateral (ipsi) and contralateral (contra) cerebral cortices of Floxed and Esr-KO mice at 6 days after MCAO and cerebral cortices of sham-operated mice (sham) (n=5 per group). Counterstained with DAPI (blue). Arrows indicate α-SMA-positive PC/vSMCs. Scale bars=25 μm. a2P<0.01 versus ipsilateral cerebral cortices of Floxed mice. (D) Double-immunofluorescence staining for Ki67 (green, a proliferation marker) and α-SMA (red) in the ischemic lesions of Floxed and Esr-KO mice at 6 days after MCAO. Counterstained with DAPI (blue). Orthogonal images were shown in panel D. White arrow indicate Ki67-positive PC/vSMCs with cytoplasmic staining for α-SMA staining. Yellow arrows indicate Ki67-negative PC/vSMCs with cytoplasmic staining for α-SMA staining. White arrowheads indicate Ki67-positive vascular endothelial cells. Scale bars=10 μm. Lower panel of panel D: percentage of Ki67-positive cells within α-SMA-positive PC/vSMCs (n=6 per group). a2P<0.01 versus Floxed mice. α-SMA, α-smooth muscle actin; DAPI, 4′,6-diamidino-2-phenylindole; Esr-KO, PDGFR-β knockout mice; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MCAO, middle cerebral artery occlusion; PC/vSMC, pericyte/vascular smooth muscle cell; PDGFR, platelet-derived growth factor receptor.
In the immunohistochemistry of 6-day ischemic lesions, many blood vessels were positively stained for PDGFR-β and α-SMA in Floxed mice compared with those in Esr-KO mice, and these increases were further quantitatively approved by morphometrical measurement (Figure 3B). In contrast, the features and morphometry of laminin staining, a marker of basement membrane of vascular endothelial cells, were similar between two groups of mice, indicating that angiogenesis occurred at similar extents between Esr-KO and Floxed mice. This notion was further confirmed by PCR-array study showing that the sets of genes related to angiogenesis were not significantly alternated in the lesions of Esr-KO mice compared with Floxed mice (Supplementary Table). In the contralateral side, α-SMA and laminin were similarly stained in the blood vessels, and these stainings were at comparable levels in morphometry between two groups, although PDGFR-β immunoreactivities were mostly undetectable in the contralateral cerebral cortices of Esr-KO mice (data not shown).
Pericyte/vascular smooth muscle cell coverage in the blood vessels in the ischemic lesion, which was measured by the percentage association between α-SMA and laminin stainings, was significantly lower in Esr-KO (14.9%±4.8%) than in Floxed mice (43.1%±3.4%) at 6 days after MCAO (Figure 3C). Pericyte/vascular smooth muscle cell coverage of Esr-KO was similar to that of Floxed mice in the contralateral cerebral cortices, and in the sham-operated ipsilateral cerebral cortices, respectively (Figure 3C).
The proliferation of PC/vSMCs was measured by the immunofluorescence of Ki67. In 6-day lesions, the percentage of Ki67-positive cells within α-SMA-positive PC/vSMCs was significantly lower in Esr-KO (14.2%±1.7%) than in Floxed mice (43.7%±3.9%) (Figure 3D).
Platelet-Derived Growth Factor Receptor-β Deletion Increases Vascular Permeability Correlating With the Loss of Pericyte/Vascular Smooth Muscle Cell Coverage After Cerebral Ischemia
The leakage of FITC-albumin tracer appeared in the ischemic border at 1 day after MCAO, and extended towards the ischemic core until 6 days in Floxed and Esr-KO mice (Figure 4A). When compared, the leakage was more intense in Esr-KO than in Floxed mice (Figure 4A), and this increase was found to be significant in the ischemic border of 3- and 6-day lesions, and in the ischemic core of the 6-day lesion by quantitative analyses (Figure 4D). In 14-day lesions, leakage was decreased and fluorescent tracer was primarily localized within a fine meshwork of blood vessels in Esr-KO and Floxed mice (Figure 4A). The sham-operated and contralateral cerebral cortices from two groups did not show abnormally increased leakage of FITC-albumin, respectively (Figures 4B and 4D). Leakage was consistently detected in the hypothalamus of each mouse where the BBB is physiologically absent, and this was used as an indicator to proof appropriate measurement of BBB leakage as described previously (Cavaglia et al, 2001).
Figure 4.
PDGFR-β deletion increases vascular permeability after cerebral ischemia. (A) Confocal microscopic images of the cerebral cortices perfused with FITC-labeled albumin in Floxed and Esr-KO mice at 1, 3, 6, and 14 days after MCAO. Scale bars=250 μm. Dotted lines indicate the ischemic borders. * and arrows indicate the ischemic core and FITC-labeled albumin leakage, respectively. (B) Cerebral cortices from sham-operated Floxed and Esr-KO mice. Scale bars=250 μm. (C) FITC-labeled albumin leakage in the hypothalamus of sham-operated Floxed mice. Inset, higher-magnified view of the squared region in panel C. Scale bars=250 μm. (D) Mean intensity of FITC-labeled albumin in the ischemic border, ischemic core, and contralateral side in Floxed and Esr-KO mice at 1, 3, and 6 days after MCAO and sham-operated mice (n=5 to 6 per group). a2P<0.01 versus Floxed mice at the same day after MCAO. FITC, fluorescein isothiocyanate; MCAO, middle cerebral artery occlusion; PDGFR, platelet-derived growth factor receptor.
We further tested the hypothesis that PDGFR-β knockdown exacerbates BBB permeability owing to the loss of PC/vSMC coverage after MCAO. In 6-day lesions of Floxed mice, BBB leakage of fluorescent tracer was slight, and FITC-albumin containing blood vessels was consistently associated with PC/vSMCs, as determined by double-immunofluorescence staining of α-SMA and PDGFR-β in frozen-tissue sections from FITC-albumin-infused brains (Figure 5). In contrast, in 6-day lesions of Esr-KO mice, BBB leakage was extensive, and FITC-albumin containing blood vessels were frequently unassociated with α-SMA- and PDGFR-β-positive PC/vSMCs (Figure 5). Thus, the loss of PC/vSMCs and increased BBB leakage were closely localized, and the causal relationship was suggested between the two phenomena.
Figure 5.
Increased vascular permeability correlates with the loss of PC/vSMCs owing to PDGFR-β deletion after cerebral ischemia. Confocal microscopic images of FITC-labeled albumin (green), α-SMA (red), and PDGFR-β (blue) stainings in the ischemic border in Floxed and Esr-KO mice at 6 days after MCAO. Scale bars=100 μm. α-SMA, α-smooth muscle actin; FITC, fluorescein isothiocyanate; MCAO, middle cerebral artery occlusion; PC/vSMC, pericyte/vascular smooth muscle cell; PDGFR, platelet-derived growth factor receptor.
Platelet-Derived Growth Factor Receptor-β Deletion Disturbs Glial Response to Ischemic Injury
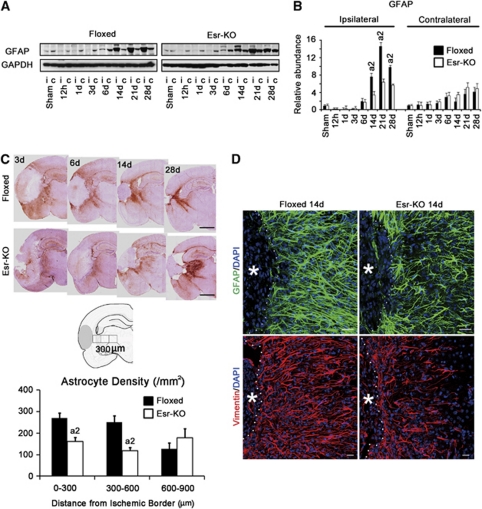
According to western blot analysis, GFAP levels in the ipsilateral cerebral cortices were increased from 14 days and after in 2 groups and were significantly lower in Esr-KO than in Floxed mice (Figures 6A and 6B). Glial fibrillary acidic protein levels were comparable in the contralateral cortices between the two groups.
Figure 6.
PDGFR-β deletion disrupts glial scar formation after cerebral ischemia. (A) Western blot analysis and (B) quantification of GFAP level in the ipsilateral (i) and contralateral cortices (c) of Floxed and Esr-KO mice after MCAO and sham-operated mice (n=3 per group). Each bar was normalized by GAPDH. Upper panel of (C) GFAP immunohistochemistry in Floxed and Esr-KO mice after MCAO. Scale bar=1 mm. Middle panel of panel C: schematic diagram of astrocyte density measurements. Lower panel of panel C: astrocyte density at different distances from the ischemic border in Floxed (n=6) and Esr-KO (n=7) mice at 14 days after MCAO. a2P<0.01 versus Floxed mice at the same distance from ischemic border. (D) Immunofluorescence stainings of GFAP in upper panel (green) and vimentin in lower panel (red), in Floxed and Esr-KO mice at 14 days after MCAO, counterstained with DAPI (blue). * indicates the ischemic core. Dotted lines indicate the ischemic border. Scale bar=20 μm. DAPI, 4′,6-diamidino-2-phenylindole; Esr-KO, PDGFR-β knockout mice; FITC, fluorescein isothiocyanate; GFAP, glial fibrillary acidic protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MCAO, middle cerebral artery occlusion; PC/vSMC, pericyte/vascular smooth muscle cell; PDGFR, platelet-derived growth factor receptor.
In immunohistochemistry, many GFAP-positive astrocytes accumulated in the ischemic border of Floxed mice at 3, 6, 14, and 28 days after MCAO, but these responses of astrocytes were much less in Esr-KO mice (Figure 6C, upper). Quantitative analysis of astrocyte density in the ischemic border was significantly lower in Esr-KO than in Floxed mice at 14 days after MCAO (Figure 6C, lower). In immunofluorescence staining, the density of GFAP- or vimentin-positive astrocytes in the ischemic border was less in Esr-KO than in Floxed mice at 14 days (Figure 6D).
Platelet-Derived Growth Factor Receptor-β Expression in Pericyte/Vascular Smooth Muscle Cells Increases Vascular Maturation and Tissue Responses After Cerebral Ischemia
Our previous study clearly showed that PDGFR-β was mostly depleted in neurons, but was well preserved in PC/vSMCs in Nestin-KO mice (Ishii et al, 2006), although nestin is reportedly expressed in both cell types (Alliot et al, 1999). Accordingly, we induced MCAO in Nestin-KO and tried to further examine the role of PDGFR-β expressed in different cell types.
As expected, PDGFR-β-positive blood vessels were frequently seen in 6-day ischemic lesions in Nestin-KO and Floxed mice, but were few in Esr-KO mice (Supplementary Figure 3). Blood vessels, as detected by laminin, were distributed to similar extents in all mouse lines.
Infarction volume significantly increased in Nestin-KO mice at 3 and 6 days after MCAO compared with Floxed mice without tamoxifen (Floxed (−) TM, Figure 7A), which might suggest the neuroprotective role of PDGFR-β. However, when compared with Esr-KO mice, infarction volume in Nestin-KO mice was similar at 3 days (Figures 2B and 7A, 36.4%±1.09% in Esr-KO; 33.4%±1.29% in Nestin-KO; P=0.11), and was significantly smaller at 6 days (Figures 2B and 7A, 34.6%±0.67% in Esr-KO; 26.0%±0.85% in Nestin-KO; P<0.01).
Figure 7.
Infarction volume and BBB leakage are worse, and glial scar formation is disrupted in Nestin-KO mice after MCAO. (A) Infarction volume in Floxed mice without tamoxifen (Floxed (−) TM; n=6) and Nestin-KO mice (n=6) at 3 and 6 days after MCAO. a1P<0.05, a2P<0.01 versus Floxed (−) TM at the same day after MCAO. (B and C) Quantification and microscopic images of FITC-labeled albumin leakage in Floxed (−) TM (n=3) and Nestin-KO mice (n=3) at 6 days. a2P<0.01 versus Floxed (−) TM. Scale bars=250 μm. Dotted lines indicate the ischemic borders. * and arrows indicate the ischemic cores and FITC-labeled albumin leakage at ischemic border, respectively. Left panel of (D) GFAP immunohistochemistry in Floxed (−) TM and Nestin-KO mice at 14 days after MCAO. Scale bar=1 mm. Right panel of panel D: astrocyte density at different distances from the ischemic border in Floxed (−) TM (n=5) and Nestin-KO (n=4) mice at 14 days after MCAO. a2P<0.01 versus Floxed (−) TM at the same distance from ischemic border. (E) Immunofluorescence stainings of GFAP in the upper panel (green) and vimentin in lower panel (red), in Floxed (−) TM and Nestin-KO mice at 14 days after MCAO, counterstained with DAPI (blue). * indicates the ischemic core. Dotted lines indicate the ischemic border. Scale bar=20 μm. BBB, blood–brain barrier; DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; GFAP, glial fibrillary acidic protein; MCAO, middle cerebral artery occlusion.
In the ischemic border and core of 6-day lesions, FITC-labeled albumin leakage was significantly extensive in Nestin-KO compared with Floxed (−) TM (Figures 7B and 7C, ischemic border: 69.1±1.9 in Nestin-KO, 36.5±4.5 in Floxed (−) TM, P<0.01; ischemic core: 81.8±1.7 in Nestin-KO, 45.3±6.6 in Floxed (−) TM, n=3 per group, P<0.01), but was significantly less in Nestin-KO than in Esr-KO mice (Figures 4A, 4D, 7B, and 7C and Supplementary Figure 1, ischemic border: 90±8.7 in Esr-KO, P<0.05; ischemic core: 104.9±14.7 in Esr-KO, P<0.05). Thus, infarction volume and BBB leakage were improved in Nestin-KO than in Esr-KO mice, indicating that PDGFR-β in PC/vSMCs partly contributed to the tissue remodeling after stroke.
Immunohistochemistry of GFAP showed that astrocyte density in the ischemic border of Nestin-KO (Figure 7D, 0 to 300 μm: 166±41; 300 to 600 μm: 138±36) was significantly lower than Floxed (−) TM (Figure 7D, 0 to 300 μm: 287±51, P<0.01; 300 to 600 μm: 261±31, P<0.01), but was similar to Esr-KO mice (Figure 6C, 0 to 300 μm: 160±20, P=0.885; 300 to 600 μm: 119±16, P=0.627). In immunofluorescence staining, the density of GFAP- or vimentin-positive astrocytes in the ischemic border was less in Nestin-KO than in Floxed (−) TM at 14 days (Figure 7E).
Discussion
We induced systemic PDGFR-β deletion, and conducted comprehensive analyses on the postnatal role of PDGFR-β in the acute phase of neuroprotection and tissue remodeling after focal cerebral ischemia. To the best of our knowledge, this study first reported that after MCAO, PDGFR-β conditional KO mice showed increased infarction volume and impaired functional recovery compared with control mice throughout the experiments. In these KO mice, disturbed coordinated responses of the different cell types, including neurons, glial cells, and blood vessel-associated cells were observed.
In non-ischemic Esr-KO mice, PDGFR-β expression was substantially decreased in western blot and real-time PCR analysis compared with Floxed mice. In accordance with these, the positive immunoreaction products detected in neurons and PC/vSMCs in Floxed mice mostly disappeared in Esr-KO mice in immunofluorescence staining. Platelet-derived growth factor receptor-β has been repeatedly shown to be expressed in neurons and PC/vSMCs (Smits et al, 1991; Virgintino et al, 2007). Furthermore, PDGFR-β is widely expressed in cerebral cortical neurons, and is involved in the dopamine receptor-mediated regulation of N-methyl--aspartate current in these cells (Beazely et al, 2006). Taken together, we concluded that PDGFR-β was mainly expressed in neurons and PC/vSMCs in the mouse brain and mostly disappeared after tamoxifen-induced gene recombination in Esr-KO mice.
In sham-operated or nonischemic cerebral cortices of Esr-KO mice, PC/vSMCs were well preserved in immunostaining and western blot for α-SMA, even though these cells were negative for PDGFR-β staining. Furthermore, vascular structures were preserved, and abnormal FITC-albumin leakage was not detected in these regions. In contrast, congenital disturbance of the PDGF-B/PDGFR-β signal axis results in the decreased numbers of PC/vSMCs and abnormal BBB leakage (Armulik et al, 2010; Bell et al, 2010). These findings are compatible with a notion that the PDGF-B/PDGFR-β signal axis is essential for the development/organogenesis, but not for the maintenance of PC/vSMC populations and BBB function in the normal adult brain. Along this line, the congenital deficits of PDGF-B/PDGFR-β signal severely disturb the organogenesis of kidney glomerulus (Lindahl et al, 1998); however, postnatal deletion of PDGFR-β does not exert adverse effects on structure and physiologic functions of the glomerulus (Nakagawa et al, 2011). The PDGF-C/PDGFR-α signal may be one of the candidates to compensate for the loss of PDGFR-β in adult cerebral PC/vSMCs, because PDGF-CC activates PDGFR-α and promotes the proliferation, survival, and migration of retinal pericytes (Hou et al, 2010).
At 3 days after MCAO, the ischemic lesions were larger in Esr-KO than in Floxed mice. Nestin-KO mice showed large 3-day lesions to the similar extent as did Esr-KO mice. These data from two different KO mice, in which PDGFR-β was commonly depleted in neurons, collaboratively indicated the neuroprotective effects of PDGFR-β that was expressed in neurons in acute cerebral ischemia. In this line, PDGF-BB and PDGF-CC pretreatments suppress lesion formation after ischemic insults (Iihara et al, 1997; Tang et al, 2010). It is a possibility that PDGFR-β protects neurons from ischemia by suppressing glutamate-induced excitotoxicity and oxidative stress, because neuron death induced by these stresses is augmented after PDGFR-β depletion (Ishii et al, 2006; Zheng et al, 2010), and because these stresses commonly underlie neuronal cell death in various CNS injuries, including ischemia and trauma (Arundine and Tymianski, 2004).
In our study, PDGFR-β deletion did not affect angiogenesis and the angiogenic gene expression in ischemic lesions. In contrast, in early angiogenesis in the ischemic Esr-KO lesion, the number of PC/vSMCs was severely decreased and BBB leakage was extensive, which was shown by the increased extravasation of FITC-labeled albumin. These phenotypes detected in Esr-KO mice were significantly rescued in Nestin-KO mice in which PDGFR-β expression was preserved in PC/vSMCs. Collectively, it was suggested that PDGFR-β signals are necessary for PC/vSMC recruitment and for acquisition of BBB functions in early angiogenesis in the injured adult mouse brain. These findings were similar but were transient and milder compared with the recent reports that show congenital disturbance of the PDGF-B/PDGFR-β signal axis results in the severely decreased PC/vSMCs population and life-long abnormality of BBB functions in the cerebral vascular system (Armulik et al, 2010; Bell et al, 2010; Daneman et al, 2010). Further studies are required to explore signal pathways that may compensate for the disturbed PDGF-B/PDGFR-β signal in PC/vSMCs in adult CNS.
In this study, infarction volume was well correlated with the degree of BBB leakage as shown by the following. In Esr-KO mice, 6-day lesions exhibited extensive BBB leakage and remained as large as 3-day lesions. The lesion volume in Nestin-KO mice was similar to that in Esr-KO mice at 3 days when BBB leakage was low in both strains, and was smaller than that in Esr-KO mice at 6 days when BBB leakage was less in Nestin-KO mice than in Esr-KO mice. Compared with Floxed mice, lesion volume and BBB leakage were exacerbated in Nestin-KO mice at 6 days. Blood–brain barrier dysfunction results in brain edema and detrimental clinical outcomes after stroke (Sandoval and Witt, 2008). Accordingly, PDGFR-β signal is believed to contribute to better clinical outcome by restoring BBB functions in early angiogenesis within the lesions after cerebral ischemia.
Pericyte/vascular smooth muscle cells induce expression of occludin, a tight junction protein that regulates BBB permeability, in cultured cerebral vascular endothelial cells (Hori et al, 2004). In addition, BBB tight junction proteins, such as ZO-1, occludin, and claudin-5, are reduced in pericyte-deficient mice (Bell et al, 2010). These results suggest that altered BBB-specific gene expression could underlie increased BBB leakage in Esr-KO mice, in which there were few PC/vSMCs in newly formed blood vessels.
In addition to PC/vSMCs, astrocytes are the primary constituents of BBB structure, and are involved in BBB repair after injury (Bush et al, 1999). Astroglial scar formation was disturbed in both Nestin-KO and Esr-KO, and these facts indicated that PDGFR-β deletion resulted in the dysfunction of astrocytes. Severe PC/vSMC dysfunctions are not expected in Nestin-KO mice, because many PC/vSMCs with PDGFR-β expression repopulated in newly formed blood vessels of Nestin-KO as observed in control mice. Accordingly, disturbed astrocyte functions are, at least partly, likely to correspond to increased BBB leakage in Nestin-KO and in Esr-KO. Disturbed glial scar formation results in enlargement of the ischemic lesions (Li et al, 2008). Taken all together, it is suggested that PDGFR-β in astrocytes has a role in CNS tissue responses to injury such as restoration of disturbed BBB and gliosis. In support of these, PDGF is a potent chemoattractant and mitogen of astrocytes (Bressler et al, 1985), and we detected PDGFR-β expression in cultured astrocytes isolated from the neonatal mouse brain. However, we could not detect PDGFR-β in astrocytes in our immunostaining in vivo, and the mechanisms underlying disturbed astrocyte function and its relevance in the present mouse models remain to be elucidated.
In addition to the neuroprotective effects, this study showed that the PDGFR-β signal is involved in the functional recovery and local tissue responses after focal cerebral ischemia. Therefore, our data gave novel perspective that enhancement of PDGFR-β signal may potentially minimize CNS injury, improve clinical outcomes, and may be a novel therapeutic candidate for ischemic stroke, by targeting neurons, PC/vSMCs and astrocytes.
Acknowledgments
The authors thank Yoichi Kurashige for technological assistance, and Masako Tonami for preparing the manuscript (University of Toyama, Japan).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by the Grants-in-Aid for Scientific Research (No. 20390108; No. 20590381) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Core Research for Evolutional Science and Technology, Japan Science and Technology Agency (CREST, JST).
Supplementary Material
References
- Alliot F, Rutin J, Leenen PJ, Pessac B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J Neurosci Res. 1999;58:367–378. [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beazely MA, Tong A, Wei WL, Van Tol H, Sidhu B, MacDonald JF. D2-class dopamine receptor inhibition of NMDA currents in prefrontal cortical neurons is platelet-derived growth factor receptor-dependent. J Neurochem. 2006;98:1657–1663. doi: 10.1111/j.1471-4159.2006.04064.x. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler JP, Grotendorst GR, Levitov C, Hjelmeland LM. Chemotaxis of rat brain astrocytes to platelet derived growth factor. Brain Res. 1985;344:249–254. doi: 10.1016/0006-8993(85)90802-9. [DOI] [PubMed] [Google Scholar]
- Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. Focal brain ischemia in the rat: methods for reproducible neocortical infarction using tandem occlusion of the distal middle cerebral and ipsilateral common carotid arteries. J Cereb Blood Flow Metab. 1988;8:474–485. doi: 10.1038/jcbfm.1988.88. [DOI] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Cavaglia M, Dombrowski SM, Drazba J, Vasanji A, Bokesch PM, Janigro D. Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 2001;910:81–93. doi: 10.1016/s0006-8993(01)02637-3. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Gao Z, Sasaoka T, Fujimori T, Oya T, Ishii Y, Sabit H, Kawaguchi M, Kurotaki Y, Naito M, Wada T, Ishizawa S, Kobayashi M, Nabeshima Y, Sasahara M. Deletion of the PDGFR-β gene affects key fibroblast functions important for wound healing. J Biol Chem. 2005;280:9375–9389. doi: 10.1074/jbc.M413081200. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- Hou X, Kumar A, Lee C, Wang B, Arjunan P, Dong L, Maminishkis A, Tang Z, Li Y, Zhang F, Zhang SZ, Wardega P, Chakrabarty S, Liu B, Wu Z, Colosi P, Fariss RN, Lennartsson J, Nussenblatt R, Gutkind JS, Cao Y, Li X. PDGF-CC blockade inhibits pathological angiogenesis by acting on multiple cellular and molecular targets. Proc Natl Acad Sci USA. 2010;107:12216–12221. doi: 10.1073/pnas.1004143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iihara K, Hashimoto N, Tsukahara T, Sakata M, Yanamoto H, Taniguchi T. Platelet-derived growth factor-BB, but not -AA, prevents delayed neuronal death after forebrain ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1097–1106. doi: 10.1097/00004647-199710000-00012. [DOI] [PubMed] [Google Scholar]
- Iihara K, Sasahara M, Hashimoto N, Hazama F. Induction of platelet-derived growth factor beta-receptor in focal ischemia of rat brain. J Cereb Blood Flow Metab. 1996;16:941–949. doi: 10.1097/00004647-199609000-00018. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Oya T, Zheng L, Gao Z, Kawaguchi M, Sabit H, Matsushima T, Tokunaga A, Ishizawa S, Hori E, Nabeshima Y, Sasaoka T, Fujimori T, Mori H, Sasahara M. Mouse brains deficient in neuronal PDGF receptor-beta develop normally but are vulnerable to injury. J Neurochem. 2006;98:588–600. doi: 10.1111/j.1471-4159.2006.03922.x. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, Kaluza J. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28:564–573. doi: 10.1161/01.str.28.3.564. [DOI] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Izumino K, Ishii Y, Oya T, Hamashima T, Jie S, Ishizawa S, Tomoda F, Fujimori T, Nabeshima Y, Inoue H, Sasahara M. Roles of PDGF receptor-beta in the structure and function of postnatal kidney glomerulus. Nephrol Dial Transplant. 2011;26:458–468. doi: 10.1093/ndt/gfq468. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. Regeneration of the CNS using endogenous repair mechanism. J Neurochem. 2007;102:1459–1465. doi: 10.1111/j.1471-4159.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, Agin V, Vivien D. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- Renner O, Tsimpas A, Kostin S, Valable S, Petit E, Schaper W, Marti HH. Time- and cell type-specific induction of platelet-derived growth factor receptor-β during cerebral ischemia. Brain Res Mol Brain Res. 2003;113:44–51. doi: 10.1016/s0169-328x(03)00085-8. [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Sasahara M, Fries JW, Raines EW, Gown AM, Westrum LE, Frosch MP, Bonthron DT, Ross R, Collins T. PDGF B-chain in neurons of the central nervous system, posterior pituitary, and in a transgenic model. Cell. 1991;64:217–227. doi: 10.1016/0092-8674(91)90223-l. [DOI] [PubMed] [Google Scholar]
- Smits A, Kato M, Westermark B, Nistér M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci USA. 1991;88:8159–8163. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6:182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimori H, Yao H, Ooboshi H, Ibayashi S, Iida M. Krypton laser-induced photothrombotic distal middle cerebral artery occlusion without craniectomy in mice. Brain Res Brain Res Protoc. 2004;13:189–196. doi: 10.1016/j.brainresprot.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Katsuyama K, Adachi K, Ogawa Y, Yorozu K, Fujii E, Misawa Y, Sugimoto T. Combination of fixation using PLP fixative and embedding in paraffin by the AMeX method is useful for histochemical studies in assessment of immunotoxicity. J Toxicol Sci. 2002;27:165–172. doi: 10.2131/jts.27.165. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Tang Z, Arjunan P, Lee C, Li Y, Kumar A, Hou X, Wang B, Wardega P, Zhang F, Dong L, Zhang Y, Zhang SZ, Ding H, Fariss RN, Becker KG, Lennartsson J, Nagai N, Cao Y, Li X. Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation. J Exp Med. 2010;207:867–880. doi: 10.1084/jem.20091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- Warburton E, Alawneh JA, Clatworthy PL, Morris RS. Stroke management. Clin Evid (Online) 2011;pii:0201. [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Ishii Y, Tokunaga A, Hamashima T, Shen J, Zhao QL, Ishizawa S, Fujimori T, Nabeshima Y, Mori H, Kondo T, Sasahara M. Neuroprotective effects of PDGF against oxidative stress and the signaling pathway involved. J Neurosci Res. 2010;88:1273–1284. doi: 10.1002/jnr.22302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.