Abstract
Angiotensin II-mediated hypertension (HTN) is accompanied by a pro-inflammatory and pro-thrombotic state in the cerebral microvasculature. Whether comparable phenotypic changes are elicited in other models of HTN remains unclear. Using wild-type mice with deoxycorticosterone acetate (DOCA) salt-induced HTN and intravital microscopy, we observed significant increases in the adhesion of both leukocytes and platelets in cerebral venules, compared with uninephrectomized control mice, without an accompanying increase in blood–brain barrier permeability. The cell–cell interactions in hypertensive mice were more pronounced after ischemic stroke, but no difference in infarct size was detected. The blood cell recruitment was largely prevented in the following groups of DOCA salt mice: losartan (angiotensin II AT1 receptor blocker) treated, AT1 receptor knockout mice, tempol (a membrane-permeable oxygen radical scavenger) treated, and mito-TEMPO (a mitochondria-targeted antioxidant) treated. A similar pattern of protection was noted in mice subjected to ischemic stroke. The blunted cell recruitment responses were not accompanied by reductions in blood pressure (BP). These findings implicate mitochondria-derived oxygen radicals and angiotensin II in the cerebral inflammation associated with DOCA salt HTN and suggests that BP per se is not a critical determinant of the phenotypic changes that accompany HTN, even after ischemic stroke.
Keywords: angiotensin II, blood cells, cerebral venules, DOCA salt-induced hypertension, ROS, stroke
Introduction
Cerebrovascular disease (stroke) is the third leading cause of death in the United States, with ∼800,000 strokes occurring every year. Many strokes result from clot formation in cerebral vessels, which reduces blood perfusion to a discrete brain region that ultimately exhibits tissue necrosis. Early administration of fibrinolytic agents has proven successful in the restoration of brain perfusion and prevention of tissue injury after stroke. However, a negative consequence of ischemia followed by reperfusion of brain tissue is endothelial cell activation and the consequent recruitment and activation of leukocytes and platelets, which can lead to cerebral microvascular dysfunction and tissue injury. A variety of mediators have been implicated in the brain injury mediated by activated leukocytes and platelets, including reactive oxygen species (ROS), platelet activating factor, perforin, granzyme, regulated on activation normal T-cell expressed and secreted, and tumor necrosis factor-alpha (Herd and Page, 1994; Suzuki et al, 2001; Yilmaz and Granger, 2010).
Several controllable risk factors are known to render the brain more susceptible to ischemic stroke, including hypertension (HTN), diabetes, smoking, and obesity. Of these, HTN is considered the most prevalent risk factor, with over 60% of strokes worldwide attributed to suboptimal blood pressure (BP) control (systolic BP >115 mm Hg; WHO, 2002). Further support for the importance of HTN as a risk factor is provided by reports describing significant reductions in stroke risk in patients with well-controlled BP due to antihypertensive medication (Turnbull and Blood Pressure Lowering Treatment Trialists' Collaboration, 2003). While the mechanisms that underlie the increased incidence and severity of stroke in hypertensive patients remain poorly understood, recent evidence suggests that it may relate to the pro-inflammatory and pro-oxidative state that is assumed by the vasculature when BP is chronically elevated. The findings from two recent studies of the cerebral microvasculature in mice with angiotensin II-induced HTN are consistent with this hypothesis. The HTN produced by 2 weeks of angiotensin II infusion was accompanied by the accumulation of adherent leukocytes and platelets in cerebral venules, and an increased permeability of the blood–brain barrier (BBB; Vital et al, 2010; Zhang et al, 2010). A role for oxidative stress in these responses was proposed based on the observation that tempol, a membrane-permeable antioxidant, prevented both the leukocyte recruitment and BBB dysfunction associated with the angiotensin II-induced HTN (Zhang et al, 2010). Whether the cerebral microvascular responses noted in the angiotensin II model reflects the influence of BP per se or the cellular actions of the specific pressor agent (angiotensin II) remain unclear. Consequently, in the present study, a different mouse model of HTN (deoxycorticosterone (DOCA) salt), characterized by low renin/angiotensin II levels, was used to assess the responses of the cerebral microvasculature (leukocyte and platelet adhesion, BBB permeability) to HTN. Consideration was given to the contribution of ROS (oxidative stress), angiotensin II type 1 receptors, and BP to microvascular inflammation and tissue injury in DOCA salt HTN mice subjected to transient ischemic stroke.
Materials and methods
Animals
Male C57BL/6 (wild-type) mice and AT1R knockout mice (C57BL/6 background) were obtained from Jackson Laboratories (Bar Harbor, ME, USA). The mice were housed under specific pathogen-free conditions and fed standard laboratory chow and water before entering the study. All of the experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center-Shreveport and performed according to the criteria outlined by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
After 2 days of acclimatization, under ketamine (150 mg/kg, IP)–xylazine (7.5 mg/kg, IP) anesthesia (∼100 μL/mouse), all mice underwent a left nephrectomy. After surgery, the mice were randomly assigned to either a control (Uni) or DOCA salt group (n=5 to 8/group). A slow release DOCA pellet (50 mg, 21-day release; Innovative Research of America, Sarasota, FL, USA) was inserted subcutaneously in the DOCA salt group and drinking water was replaced by a 1% saline/0.2% potassium chloride drinking solution. Mice in the control group received tap water. Treatments included: (1) the angiotensin II type 1 receptor antagonist losartan (Cozaar, Merck & Co., Whitehouse Station, NJ, USA), (2) the membrane permeable antioxidant 4-hydroxy-TEMPO (Tempol, Sigma-Aldrich, St Louis, MO, USA), in drinking water starting after DOCA (50 mg) pellet insertion, or (3) an Alzet minipump (Durect Corp., Cupertino, CA, USA) loaded with the mitochondria-targeted antioxidant mito-TEMPO (0.7 mg/kg/day, Enzo Life Sciences International, Plymouth Meeting, PA, USA), implanted 7 days after the DOCA pellet insertion.
In some experiments, we assessed the effects of treatment of DOCA salt hypertensive mice with losartan or mito-TEMPO treatment on the brain inflammation and injury responses to middle cerebral artery occlusion (20 minutes) and reperfusion (4 hours).
Losartan and Tempol Treatment
Losartan and tempol were dissolved in a 1% saline/0.2% potassium chloride drinking solution to achieve concentrations of 0.5 μmol/L and 1 mmol/L, respectively. The drinking water bottle in each mouse cage was wrapped in aluminum foil to prevent photo-degradation. Fresh losartan or tempol solutions were added to the drinking water bottle every other day.
Blood Pressure Measurement
Blood pressure was measured in non-anesthetized mice by tail plethysmography using the Hatteras Instruments system (model SC-1000, Cary, NC, USA). Mice were placed on a heated (40°C) platform and a cuff was placed around the tail and inflated for a period of 60 seconds to record systolic BP. The average of five successive measurements was used as the systolic BP for each animal.
Animal Preparation
Mice were anesthetized with intraperitoneal ketamine (150 mg/kg)–xylazine (7.5 mg/kg) anesthesia (∼100 μL/mouse). The left femoral vein was cannulated for intravenous administration of 6G-rhodamine, labeled platelets, and supplemental doses of anesthesia (15 mg/kg ketamine and 0.75 mg/kg xylazine, IV), given as needed. Body temperature was maintained at 36°C during the experiment with a homeothermic blanket and monitored with a rectal temperature probe. The head of each mouse was fixed on the acrylic frame before the cranial window was created. After skull fixation, a circular skin incision was made, and a craniectomy was created 3 mm lateral and 2 mm posterior to the bregma. The exposed brain tissue was immersed in artificial cerebrospinal fluid and covered with a glass slide. Cerebral vessels were observed through the dura mater.
Intravital Videomicroscopy
The procedures used to monitor blood cell–vessel wall interactions in murine cerebral venules are described elsewhere in detail (Ishikawa et al, 2005). Briefly, the cerebral microcirculation was visualized with an upright fluorescent microscope using a × 20 water immersion lens. Color images were captured with a three charge coupled device color video camera. Randomly selected segments of pial venules (20 to 70 μm diameter, 100 μm long) were chosen for observation. Approximately 100 × 106 platelets were isolated from a donor mouse, labeled (green) ex vivo with carboxyfluorescein diacetate succinimidyl ester (Ishikawa et al, 2007), and administered to recipient mice through the left femoral vein. This was followed by the continuous infusion of 0.02% rhodamine 6G, which fluorescently labeled (red) circulating leukocytes. Adherent leukocytes and platelets were defined as cells remaining stationary within venules for 30 seconds. Cell adhesion data are expressed as number of cells per millimeter squared of venular surface, calculated from venular diameter and length, assuming cylindrical geometry.
Blood–Brain Barrier Dysfunction
Blood–brain barrier permeability was assessed using the Evans blue (EB) extravasation method (Uyama et al, 1988). A 2% solution of EB (Sigma-Aldrich) was injected (4 mL/kg) into the femoral vein. Twenty-four hours later, 0.4 mL of blood was obtained by cardiac puncture and the mouse was transcardially perfused with phosphate-buffered saline (100 mm Hg) for 5 minutes. The brain was removed and separated from the dura mater and cerebellum. The cerebrum was divided into two hemispheres, each of which was homogenized and sonicated in 1 mL of 50% trichloroacetic acid (Sigma-Aldrich) and centrifuged at 10,000 r.p.m. for 20 minutes. The supernatant was diluted with ethanol and the concentrations of EB in brain tissue and plasma were measured using a fluorescence spectrophotometer (FLUOstar Optima microplate reader; BMG LABTECH, Inc., Ortenberg, Germany). BBB permeability was determined by dividing tissue EB concentration (μg/g brain weight) by the plasma concentration (μg/g).
Brain Water Content
Brain was removed, stripped of the dura mater and cerebellum, and divided into two hemispheres. Each hemisphere was placed into a 60°C oven for 3 days to achieve complete desiccation. Water content was determined from (wet weight−dry weight)/wet weight and expressed as percent.
Plasma Cytokine Measurements
Heparinized blood (plasma) from control (normotensive) and DOCA salt-induced hypertensive mice either untreated or treated with losartan or tempol was drawn from the tail vein for cytokine measurements. A cytometric bead array (BD Biosciences, San Jose, CA, USA) was used to measure plasma IFN-γ, tumor necrosis factor-α, MCP-1, IL-6, IL-12p70, and IL-10 concentration (pg/ml plasma), with samples analyzed on a fluorescence-activated cell sorter Caliber.
Middle Cerebral Artery Occlusion
The effects of ischemic stroke on cerebral microvascular inflammation and brain injury in DOCA salt hypertensive mice were evaluated in untreated mice and mice receiving either losartan or mito-TEMPO (same dose as described before) were anesthetized by intraperitoneal injection of a solution containing ketamine (150 mg/kg) and xylazine (7.5 mg/kg). Transient focal cerebral ischemia was induced by middle cerebral artery occlusion using the previously described intraluminal filament method (Arumugam et al, 2004). Briefly, the blunted tip of a 6–0-nylon monofilament was advanced to the level of the carotid bifurcation via the internal carotid artery until light resistance was felt. The distance from the nylon thread tip to the bifurcation of the internal and external carotid arteries was around 10 mm. The monofilament was removed after 20 minutes of occlusion. In the sham group, these arteries were visualized but filament was not inserted. Animal body temperature was kept at 36°C during the middle cerebral artery occlusion surgery and for 25 minutes of reperfusion. After 4 hours reperfusion, leukocyte and platelet adhesion in cerebral pial venules (protocol previously described) and infarct area were measured.
Detection and Quantification of Cerebral Infarction
At the end of a 4-hour reperfusion period, mice were anesthetized by an intraperitoneal injection of a solution containing ketamine (150 mg/kg) and xylazine (7.5 mg/kg). The brain was then flushed by transcardial perfusion with a 1 × phosphate-buffered saline solution, removed and 2 mm coronal sections were cut and stained with 2% 2,3,5-triphenyltetrazolium chloride, as previously described (Lin et al, 1993). The total and infarcted areas of each brain section were quantified on digitized images with a computerized image analysis program (Image J). Infarct volume was expressed as a percentage of ipsilateral hemisphere.
Statistical Analysis
All data were expressed as mean±s.e. Statistical difference between the different groups was determined by an one-way analysis of variance with the Tukey post hoc test. An unpaired t-test was used to compare responses between two groups as needed. All analyses were performed using Prism 4 software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was set at P<0.05.
Results
Roles of Angiotensin II and Reactive Oxygen Species in the Elevated Blood Pressure Response to Deoxycorticosterone Acetate Salt
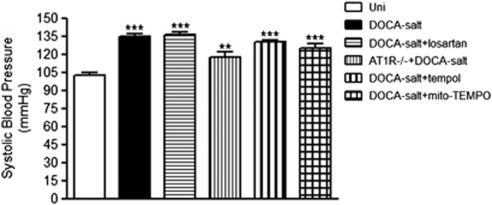
The indirect tail cuff method was used to measure systolic pressure in DOCA salt wild-type mice treated with losartan, tempol, or mito-TEMPO and in AT1R knockout mice. Systolic BP was increased an average 30% at 3 weeks after implantation of the DOCA pellet, compared with the uninephrectomized (Uni) control mice (Figure 1). The DOCA salt-induced BP elevation was not significantly changed in any experimental group tested. Thus, angiotensin II and ROS are not involved on the DOCA salt-induced increase in BP.
Figure 1.
Systolic blood pressures measured in control (Uni, n=9), deoxycorticosterone acetate (DOCA) salt (n=11), DOCA salt mice treated with losartan (n=12), AT1R knockout (−/−) mice+DOCA salt (n=8), DOCA salt mice treated with tempol (n=12), and DOCA salt mice treated with mito-TEMPO (n=6). **P<0.01 and ***P<0.001 relative to the control group.
Dependence of Deoxycorticosterone Acetate Salt-Induced Leukocyte Adhesion in Cerebral Venules on Angiotensin II and Reactive Oxygen Species
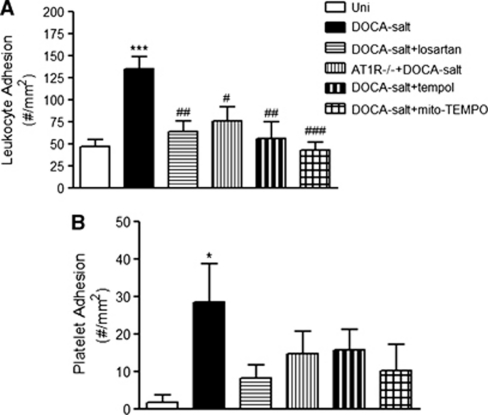
Intravital fluorescence microscopic examination of cerebral venules in DOCA salt hypertensive mice revealed a significant increase in the number of firmly adherent leukocytes (135±14 cells/mm2), compared with the adhesion response noted in the Uni control group (47±7 cells/mm2). Treatment of DOCA salt hypertensive mice with losartan (64±11 cells/mm2), tempol (57±18 cells/mm2), or mito-TEMPO (43±9 cells/mm2) significantly blunted the recruitment of adherent leukocytes in cerebral venules (Figure 2A). The number of adherent leukocytes was also significantly reduced in angiotensin II AT1 receptor-deficient hypertensive mice (76±16 cells/mm2; Figure 2A). The number of adherent leukocytes in venules of all treated groups did not differ (P>0.05) from the values detected in the control (Uni) group.
Figure 2.
Effects of losartan, tempol, mito-TEMPO, and AT1R deficiency on the leukocyte (A) and platelet (B) adhesion responses to deoxycorticosterone acetate (DOCA) salt hypertension. Uninephrectomized (Uni) controls (n=8), DOCA salt (n=9), DOCA salt+losartan (n=8), AT1R knockout (−/−) mice+DOCA salt (n=7), DOCA salt+tempol (n=7), and DOCA salt+mito-TEMPO (n=6) were tested. *P<0.05 and ***P<0.001 versus Uni, #P<0.05, ##P<0.01 and ###P<0.001 versus DOCA salt.
Contributions of Angiotensin II and Reactive Oxygen Species to the Deoxycorticosterone Acetate Salt-Induced Recruitment of Adherent Platelets
The number of adherent platelets in cerebral venules was significantly increased in mice with DOCA salt HTN (29±10 cells/mm2), compared with the normotensive Uni control group (2±2 cells/mm2). Treatment with losartan (8±4 cells/mm2), tempol (16±5 cells/mm2), and mito-TEMPO (10±7 cells/mm2) attenuated the platelet recruitment response to DOCA salt, reducing the adhesion values to levels seen in the control Uni group (Figure 2B). In a similar way, number of adherent platelets was not significantly increased in hypertensive AT1R knockout mice (15±6 cells/mm2).
Influence of Deoxycorticosterone Acetate Salt Hypertension on Blood–Brain Barrier Integrity and Brain Water Content
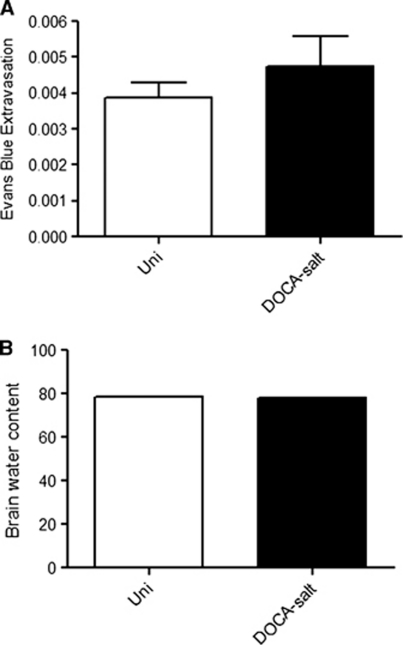
Blood–brain barrier integrity was monitored in normotensive (Uni) and DOCA salt hypertensive mice using the EB extravasation method. No significant differences in BBB permeability were noted between the hypertensive (0.0047±0.0008) and normotensive (0.0039±0.0004) mice (Figure 3A). A similar pattern was noted for brain water content, with no significant differences between the normotensive control (Uni) (78.46±0.05%) and DOCA salt hypertensive (77.89±0.32%) mice (Figure 3B).
Figure 3.
Effects of deoxycorticosterone acetate (DOCA) salt hypertension on blood–brain barrier (BBB) permeability (Evans blue (EB) extravasation) (A) and brain water content (B). Uninephrectomized (Uni) controls (n=5) and DOCA salt (n=5). No significant differences were noted between groups.
Influence of Deoxycorticosterone Acetate Salt Hypertension on Plasma Cytokines Concentration
Plasma concentrations of IFN-γ, tumor necrosis factor-α, MCP-1, IL-6, IL-12p70, and IL-10 were not significantly altered by DOCA salt HTN, and hypertensive mice treated with either losartan or tempo did not reveal any alterations in plasma levels of these cytokines.
Dependence of Leukocyte Adhesion in Cerebral Venules on Angiotensin II and Reactive Oxygen Species in Deoxycorticosterone Acetate Salt-Induced Hypertension After Ischemic Stroke
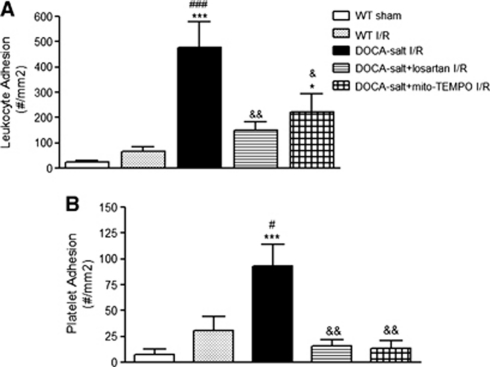
In DOCA salt hypertensive mice subjected to focal ischemia–reperfusion, we observed a large increase in the number of adherent leukocytes in cerebral venules (Figure 4A), compared with normotensive mice exposed to the same insult. Treatment with either losartan or mito-Tempo significantly blunted the exaggerated leukocyte recruitment response in cerebral venules of DOCA salt hypertensive mice.
Figure 4.
Effects of losartan and mito-TEMPO on the leukocyte (A) and platelet (B) adhesion responses during deoxycorticosterone acetate (DOCA) salt hypertension and after 20 minutes of cerebral ischemia and 4 hours reperfusion (I/R). In the sham group, the middle cerebral artery was visualized but not occluded. The following groups were tested: wild type, sham (WT) (n=10), wild type, I/R (WT) (n=7), DOCA salt, I/R (n=5), DOCA salt+losartan, I/R (n=4), and DOCA salt+mito-TEMPO, I/R (n=4). *P<0.05 and ***P<0.001 versus WT, sham, #P<0.05 and ###P<0.001 versus WT, I/R, &P<0.05 and &&P<0.01 versus DOCA salt, I/R.
Dependence of Platelet Adhesion in Cerebral Venules on Angiotensin II and Reactive Oxygen Species in Deoxycorticosterone Acetate Salt-Induced Hypertension after Ischemic Stroke
Focal cerebral ischemia–reperfusion (I/R) in DOCA salt hypertensive elicited a profound recruitment of adherent platelets in cerebral venules, compared with normotensive mice exposed to the same I/R protocol. The I/R-induced platelet recruitment response in DOCA salt mice was dependent on both angiotensin II type 1 receptors and ROS since both losartan and mito-TEMPO effectively reduced the number of adherent platelets (Figure 4B).
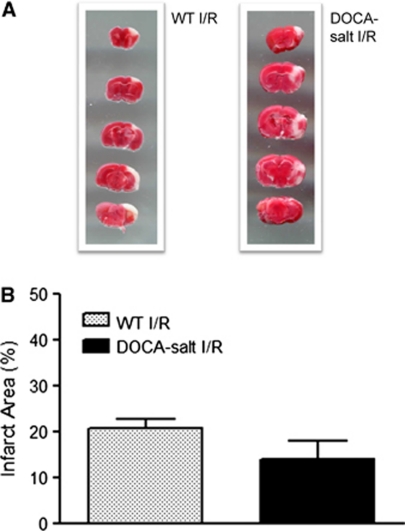
Influence of Deoxycorticosterone Acetate Salt Hypertension on Infarct Volume in Mice Exposed to Ischemic Stroke
DOCA salt HTN did not significantly alter the infarct volume (14±4%) in mice subjected to focal I/R, compared with normotensive mice (21±2% Figure 5).
Figure 5.
Representative picture (A) and graph (B) of the infarct area obtained in wild-type and deoxycorticosterone acetate (DOCA) salt hypertensive mice exposed to 20 minutes ischemia and 4 hours reperfusion. Wild type (WT) (n=7) and DOCA salt (n=6) were used. No significant differences were noted between groups.
Discussion
This study provides the first evidence of an enhanced recruitment of adherent leukocytes and platelets in the cerebral microvasculature in a mouse model of HTN that is not associated with elevated blood levels of renin/angiotensin II. As reported previously for angiotensin II-dependent models of HTN, the pro-inflammatory and pro-thrombotic phenotype associated with the low renin/angiotensin II DOCA salt model is dependent on activation of the angiotensin II type 1 receptor and on the production of ROS by mitochondria. The importance of angiotensin II and ROS as mediators of blood cell recruitment in cerebral microvessels of DOCA salt hypertensive mice is also evident after exposure of the brain to focal ischemia–reperfusion. Our findings also indicate that the phenotypic changes in the cerebral microvasculature induced by the DOCA salt model do not appear to be directly linked to elevated systemic BP.
The HTN elicited in the DOCA salt model has been attributed to both neural and humoral factors, with changing relative contributions of each factor during different phases of HTN development (Yemane et al, 2010). In mice made hypertensive with DOCA salt for 2 to 6 weeks, the neural and humoral factors make a similar contribution to the elevated BP (Yemane et al, 2010). Plasma cathecolamine levels are elevated in DOCA salt hypertensive rats as a result of increased plasma sodium concentration, which in turn enhances sympathetic tone (Reid et al, 1975). The major humoral components of the increased BP in the DOCA salt model are vasopressin (Crofton et al, 1979) and endothelin-1 (Bird et al, 1995), with a limited (or no) contribution by the renin–angiotensin system (Möhring et al, 1976). The absence of a role for angiotensin II in the HTN elicited in the DOCA salt model is supported by our observation that losartan treatment did not lower BP, as reported by others (Somers et al, 2000). The findings of our study also agree with others who have reported an absence of BP reduction in DOCA salt hypertensive animals treated with reagents that scavenges ROS, such as tempol and mito-TEMPO (our study) or heparin-binding superoxide dismutase (Somers et al, 2000).
Despite the absence of an effect of losartan, tempol, or mito-TEMPO treatment on the DOCA salt-induced BP elevation, we showed that the same drug treatments largely prevent the recruitment of adherent leukocytes and platelets in cerebral venules. These observations implicate both angiotensin II (and its type 1 receptor) and oxidative stress in the induction of a pro-inflammatory and pro-thrombotic phenotype in the cerebral microvasculature of DOCA salt mice. Our findings with tempol and mito-tempo (a mitochondria-targeted antioxidant), both of which offered similar protection against the blood cell recruitment responses, suggest the ROS mediating these responses are largely derived from mitochondria. The differential effects of these treatments (losartan, tempol, and mito-TEMPO) on BP and the cerebral microvascular inflammatory response elicited by DOCA salt suggest that the HTN and cerebral inflammation are not inter-dependent responses. Previous work on the angiotensin II infusion model of HTN similarly suggests little or no role for BP in inducing the adhesion of both leukocytes and platelets in cerebral venules (Vital et al, 2010).
It was recently reported that pressor doses of exogenous angiotensin II, when infused over a period of 2 weeks, can induce both leukocyte and platelet adhesion in cerebral venules of mice (Vital et al, 2010), and that the induced inflammatory phenotype is dependent on ROS (Zhang et al, 2010). However, there are no published reports that address whether angiotensin II generated within brain tissue also exerts an influence on the leukocyte and platelet adhesion in the cerebral microvasculature. Although the DOCA salt model is associated with a suppressed systemic renin–angiotensin system, there is evidence for activation of the brain renin–angiotensin system in this model of HTN (Basso et al, 1981; Fournie-Zaluski et al, 2004). Therefore, our finding that losartan treatment largely prevented the recruitment of leukocytes and platelets in cerebral venules is consistent with a role for locally produced angiotensin II. Local generation of angiotensin II has also been implicated in the cardiac tissue response to DOCA salt HTN, as evidenced by the demonstration that losartan prevents the development of cardiac hypertrophy in DOCA salt mice (Wang et al, 2002). However, the role for locally produced angiotensin II in the DOCA salt HTN model appears to be tissue specific since losartan does not alter the impaired endothelium-dependent vascular relaxation (in aorta rings) that accompanies this condition (Somers et al, 2000).
Our finding that tempol and mito-TEMPO are as effective as losartan in preventing the DOCA salt-induced blood cell recruitment in cerebral venules suggests that angiotensin II-mediated activation of AT-1 receptors is a likely cause of the mitochondria dependent-oxidative stress in this model. This is consistent with a report by Dai et al (2011) that describes mitochondrial ROS production by neonatal cardiomyocytes in response to angiotensin II exposure and that the mitochondria-targeted antioxidant peptide SS-31 ameliorates angiotensin II-induced cardiac hypertrophy, diastolic dysfunction, and fibrosis, in the absence of a BP-lowering effect. The same group (Dai and Rabinovitch, 2011) also showed resistance to cardiac hypertrophy, fibrosis and mitochondrial damage, biogenesis, and autophagy induced by angiotensin II in mice that overexpress catalase targeted to mitochondria, but not in that overexpress peroxisomal targeted catalase (the natural site of catalase). These observations are consistent with our proposal that angiotensin II-induced mitochondria ROS generation is an important initiating event in the induction of the pro-inflammatory and pro-thrombotic phenotype observed in cerebral venules of DOCA salt hypertensive mice.
The responses of BBB permeability differ between the angiotensin II infusion and DOCA salt models of HTN. We (Vital et al, 2010) and others (Zhang et al, 2010) have shown an increased BBB permeability in the chronic angiotensin II infusion model, while the present study and a previously published study (Werber and Fitch-Burke, 1988) report either a slight but insignificant increase (present study) or no change (Werber and Fitch-Burke, 1988) in the DOCA salt model. This difference is noted despite the achievement of comparable elevations in BP in the two models, suggesting that HTN per se is not a major factor leading to the loss of barrier function. In vitro studies, using monolayers of cultured cerebral microvascular endothelial cells, have shown that angiotensin II directly increases BBB permeability in an AT-1 receptor-dependent manner (Fleegal-DeMotta et al, 2009). The absence of involvement of the renin–angiotensin system in the BBB responses to DOCA salt in this study may reflect the generation of angiotensin II concentrations by brain tissue that are sufficient to promote blood cell recruitment, but too low to elicit barrier dysfunction. Cerebral microvascular endothelial cells are likely exposed to a much higher angiotensin II level in the angiotensin II infusion model.
Our study also reveals that the recruitment of leukocytes and platelets in postischemic cerebral venules is far more intense in DOCA salt hypertensive mice, compared with control (normotensive) mice. However, a comparison of the tissue necrosis (infarct volume) manifested at 24 hours after focal cerebral ischemia and reperfusion revealed no difference between the DOCA salt and control mice. Collectively, these observations suggest that the more intense inflammatory response elicited in the brain of DOCA salt mice does not significantly influence the magnitude of the resulting injury response. The view that inflammation is an important initiator of neuronal death after ischemic stroke (Zheng et al, 2003) is more evident in other models of HTN. For example, the spontaneously hypertensive rats are known to exhibit larger cerebral infarcts after focal I/R that their normotensive counterparts (Coyle and Jokelainen, 1983; Dogan et al, 1998; Tureyen et al, 2007). Perhaps, a difference in infarct volumes between DOCA salt and control mice can be shown after longer (>24 hours) periods of reperfusion.
The plasma levels of six inflammatory cytokines (IFN-γ, tumor necrosis factor-α, MCP-1, IL-6, IL-12p70, and IL-10) were monitored in DOCA salt hypertensive mice in an effort to further define the mechanisms underlying the angiotensin II and ROS-dependent recruitment of leukocytes and platelets in cerebral venules. The cytokines levels were not altered by DOCA salt HTN, suggesting that either these factors do not contribute to the observed inflammatory response, that local tissue levels are a more important determinant of the blood cell recruitment, or that cytokine-independent factors have a more important role. Recent work by Vinh et al (2010) suggests that T-cell costimulation via B7 ligands (on antigen-presenting cells) is essential for development of both angiotensin II- and DOCA salt-induced HTN, and for the accompanying leukocyte infiltration in aorta. Whether cytokines/chemokines are responsible for the increased frequency of leukocyte/antigen-presenting cells interactions remains unclear.
Our findings show that DOCA salt HTN induces a pro-inflammatory and pro-thrombotic phenotype in the cerebral microvasculature that is further amplified after focal ischemia and reperfusion. The blood cell recruitment response to DOCA salt appears to depend on mitochondria-derived ROS and the activation of angiotensin II type-1 receptors, but independent of BP. These findings support a focus on developing strategies that target angiotensin II and mitochondrial-derived ROS to minimize the brain inflammation elicited by focal ischemic stroke in patients with salt-induced HTN.
The authors declare no conflict of interest.
Footnotes
This study was supported by a grant from the National Heart Lung and Blood Institute (HL26441).
References
- Arumugam TV, Salter JW, Chidlow JH, Ballantyne CM, Kevil CG, Granger DN. Contributions of LFA-1 and Mac-1 to brain injury and microvascular dysfunction induced by transient middle cerebral artery occlusion. Am J Physiol Heart Circ Physiol. 2004;287:H2555–H2560. doi: 10.1152/ajpheart.00588.2004. [DOI] [PubMed] [Google Scholar]
- Basso N, Ruiz P, Mangiarua E, Taquini AC.1981Renin-like activity in the rat brain during the development of DOC-salt hypertension Hypertension 3(Suppl II): II14–II7. [DOI] [PubMed] [Google Scholar]
- Bird JE, Moreland S, Waldron TL, Powell JR. Antihypertensive effects of a novel endothelin-A receptor antagonist in rats. Hypertension. 1995;25:1191–1195. doi: 10.1161/01.hyp.25.6.1191. [DOI] [PubMed] [Google Scholar]
- Coyle P, Jokelainen PT. Differential outcome to middle cerebral artery occlusion in spontaneously hypertensive stroke-prone rats (SHRSP) and Wistar Kyoto (WKY) rats. Stroke. 1983;14:605–611. doi: 10.1161/01.str.14.4.605. [DOI] [PubMed] [Google Scholar]
- Crofton JT, Share L, Shade RE, Lee-Kwon WJ, Manning M, Sawyer WH. The importance of vasopressin in the development and maintenance of DOC-salt hypertension in the rat. Hypertension. 1979;1:31–38. doi: 10.1161/01.hyp.1.1.31. [DOI] [PubMed] [Google Scholar]
- Dai DF, Chen T, Szeto H, Nieves-Cintrón M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Rabinovitch P. Mitochondrial oxidative stress mediates induction of autophagy and hypertrophy in angiotensin-II treated mouse hearts. Autophagy. 2011;7:917–918. doi: 10.4161/auto.7.8.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan A, Başkaya MK, Rao VL, Rao AM, Dempsey RJ. Intraluminal suture occlusion of the middle cerebral artery in Spontaneously Hypertensive rats. Neurol Res. 1998;20:265–270. doi: 10.1080/01616412.1998.11740517. [DOI] [PubMed] [Google Scholar]
- Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:640–647. doi: 10.1038/jcbfm.2008.158. [DOI] [PubMed] [Google Scholar]
- Fournie-Zaluski MC, Fassot C, Valentin B, Djordjijevic D, Reaux-Le Goazigo A, Corvol P, Roques BP, Llorens-Cortes C. Brain renin-angiotensin system blockade by systemically active aminopeptidase A inhibitors: a potential treatment of salt-dependent hypertension. Proc Natl Acad Sci USA. 2004;101:7775–7780. doi: 10.1073/pnas.0402312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd CM, Page CP. Pulmonary immune cells in health and disease: platelets. Eur Respir J. 1994;7:1145–1160. [PubMed] [Google Scholar]
- Ishikawa M, Sekizuka E, Yamaguchi N, Nakadate H, Terao S, Granger DN, Minamitani H. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007;292:H2306–H2315. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, Granger DN. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Möhring J, Möhring B, Petri M, Haack D. Is vasopressin involved in the pathogenesis of malignant deoxycorticosterone hypertension in rats. Lancet. 1976;1:170–173. doi: 10.1016/s0140-6736(76)91275-7. [DOI] [PubMed] [Google Scholar]
- Reid JL, Zivin JA, Kopin IJ. Central and peripheral adrenergic mechanisms in the development of deoxycorticosterone-saline hypertension in rats. Circ Res. 1975;37:569–579. doi: 10.1161/01.res.37.5.569. [DOI] [PubMed] [Google Scholar]
- Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sugimura K, Hasegawa K, Yoshida K, Suzuki A, Ishizuka K, Ohtsuka K, Honma T, Narisawa R, Asakura H. Activated platelets in ulcerative colitis enhance the production of reactive oxygen species by polymorphonuclear leukocytes. Scand J Gastroenterol. 2001;36:1301–1306. doi: 10.1080/003655201317097164. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Turnbull F, Blood Pressure Lowering Treatment Trialists' Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital SA, Terao S, Nagai M, Granger DN. Mechanisms underlying the cerebral microvascular responses to angiotensin II-induced hypertension. Microcirculation. 2010;17:641–649. doi: 10.1111/j.1549-8719.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Hummler E, Nussberger J, Clément S, Gabbiani G, Brunner HR, Burnier M. Blood pressure, cardiac, and renal responses to salt and deoxycorticosterone acetate in mice: role of Renin genes. J Am Soc Nephrol. 2002;13:1509–1516. doi: 10.1097/01.asn.0000017902.77985.84. [DOI] [PubMed] [Google Scholar]
- Werber AH, Fitch-Burke MC. Effect of chronic hypertension on acute hypertensive disruption of the blood-brain barrier in rats. Hypertension. 1988;12:549–555. doi: 10.1161/01.hyp.12.6.549. [DOI] [PubMed] [Google Scholar]
- WHO 2002The World Health Report 2002—Reducing Risks, Promoting Healthy Life Geneva: WHO; Available from http://www.who.int/whr/2002/en/ [DOI] [PubMed] [Google Scholar]
- Yemane H, Busauskas M, Burris SK, Knuepfer MM. Neurohumoral mechanisms in deoxycorticosterone acetate (DOCA)-salt hypertension in rats. Exp Physiol. 2010;95:51–55. doi: 10.1113/expphysiol.2008.046334. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromol Med. 2010;12:193–204. doi: 10.1007/s12017-009-8074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience. 2010;171:852–858. doi: 10.1016/j.neuroscience.2010.09.029. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Lee JE, Yenari MA. Stroke: Molecular mechanisms and potential targets for treatment. Curr Mol Med. 2003;3:361–372. doi: 10.2174/1566524033479717. [DOI] [PubMed] [Google Scholar]