Abstract
Ischemic stroke affecting the adult brain causes increased progenitor proliferation in the subventricular zone (SVZ) and generation of neuroblasts, which migrate into the damaged striatum and differentiate to mature neurons. Meteorin (METRN), a newly discovered neurotrophic factor, is highly expressed in neural progenitor cells and immature neurons during development, suggesting that it may be involved in neurogenesis. Here, we show that METRN promotes migration of neuroblasts from SVZ explants of postnatal rats and stroke-subjected adult rats via a chemokinetic mechanism, and reduces N-methyl--asparate-induced apoptotic cell death in SVZ cells in vitro. Stroke induced by middle cerebral artery occlusion upregulates the expression of endogenous METRN in cells with neuronal phenotype in striatum. Recombinant METRN infused into the stroke-damaged brain stimulates cell proliferation in SVZ, promotes neuroblast migration, and increases the number of immature and mature neurons in the ischemic striatum. Our findings identify METRN as a new factor promoting neurogenesis both in vitro and in vivo by multiple mechanisms. Further work will be needed to translate METRN's actions on endogenous neurogenesis into improved recovery after stroke.
Keywords: neuronal migration, neuronal survival, neurotrophic factor, stem cells, stroke
Introduction
Following ischemic stroke in rodents, neural stem cells in the subventricular zone (SVZ) increase their proliferation and generate neuroblasts, which migrate to the damaged area in the striatum over several months (Arvidsson et al, 2002; Parent et al, 2002; Thored et al, 2006), differentiate to mature neurons, become integrated (Yamashita et al, 2006), and seem to be functional (Hou et al, 2008). In humans, there is also evidence for enhanced SVZ cell proliferation and neuroblast formation after stroke (Jin et al, 2006; Marti-Fabregas et al, 2010). To what extent endogenous neurogenesis contributes to the spontaneous functional recovery after stroke is unclear, although there is some evidence for trophic and neuroprotective actions early after the insult (Jin et al, 2010). To become therapeutically valuable, neurogenesis has to be better understood, optimized and, in particular, the number of new mature neurons surviving in the damaged area has to be increased (Arvidsson et al, 2002).
Meteorin (METRN) is a newly identified secreted protein that does not contain any motif homologous to other known neurotrophic factors. The receptor is still unknown but recent evidence indicates that METRN can act through the Jak-STAT3 pathway (Lee et al, 2010). Meteorin is highly expressed in neural stem cells and radial glial cells and in immature neurons during embryonic mouse development (Nishino et al, 2004; Lee et al, 2010). In the adult mouse brain, METRN is detected in ubiquitously distributed astrocytes, Bergmann glia, and a few discrete neuronal populations (Nishino et al, 2004; Jorgensen et al, 2009). Meteorin has been implicated in the regulation of axonal extension and glial cell differentiation (Nishino et al, 2004; Lee et al, 2010) and in angiogenesis (Park et al, 2008). Additionally, administration of METRN protects striatal neurons from excitotoxicity caused by quinolinic acid in vivo (Jorgensen et al, 2011). Taken together, these findings have raised the possibility that METRN could influence the formation of new neurons from the adult brain's neural stem cells after stroke and, potentially, contribute to functional recovery.
Our main aim was to investigate the role of METRN in SVZ neuroblast migration and cell survival in vitro, and explore the effects of infusion of recombinant METRN into the stroke-damaged rat striatum on neurogenesis in vivo. We show that METRN promotes several steps of adult neurogenesis, including SVZ cell proliferation and neuroblast migration, and leads to increased numbers of new immature and mature striatal neurons.
Materials and methods
All experimental procedures followed the guidelines established by the Malmö-Lund Ethics Committee for use and care of laboratory animals and were conducted in accordance with the European Union directive on the subject of animal rights.
Recombinant Meteorin
For in vitro studies, HIS-tagged recombinant mouse METRN was produced and purified as previously described (Jorgensen et al, 2009). Untagged recombinant mouse METRN for in vivo studies was custom-made by R&D Systems (Minneapolis, MN, USA).
Migration Assay
Rat pups were killed at postnatal days 2 to 5 (P2–P5) by rapid decapitation. The brains were placed in ice-cold Neurobasal medium (Gibco-Invitrogen, Carlsbad, CA, USA) and sliced into 1-mm coronal sections. Subventricular zone was dissected from the lateral wall of the anterior horn of the lateral ventricle and cut into small explants. These were mixed with Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA) and cultured in four-well dishes. After polymerization (25 minutes), 500 μL of Neurobasal medium supplemented with B-27, N2-supplement, glutamine, and penicillin/streptomycin (all from Gibco-Invitrogen) were added. Cultures were maintained in a humidified, 5% CO2, 37°C incubator with mouse METRN (2, 10, 20, or 50 ng/mL) or stromal cell-derived factor 1α (SDF1α; 50 ng/mL; Invitrogen). The length of migratory chains was measured from the edge of the explants at three angles using Image J software (NIH, Bethesda, MD, USA) after 24 hours. Values were divided by the average length of the vehicle group to get corresponding ratios, which were used for statistical analysis. All values were normalized to control. Subventricular zones were also isolated 4 days after 2 hours middle cerebral artery occlusion (MCAO) in adult male Wistar rats (see below), followed by the same culture and analysis as above.
Immunohistochemistry in Subventricular Zone Explants
Explants were washed with prewarmed phosphate-buffered saline (PBS) and fixed/permeabilized with 4% paraformaldehyde (PFA) and 0.05% Triton in PBS. They were then incubated with blocking buffer (2% horse serum, 1% bovine serum albumin, 0.1% gelatin, 0.1% Triton X-100, 0.05% Tween 20 in PBS, pH 7.2) for 1 hour, followed by goat antidoublecortin (DCX) antibody (1:200; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or rabbit anti-glial fibrillary acidic protein (1:200; Sigma, St Louis, MO, USA) overnight at 4°C. Explants were washed with PBS and incubated with secondary antibodies, Cy3-conjugated anti-goat or Cy2-conjugated anti-rabbit (1:200; Jackson ImmunoResearch, West Grove, PA, USA).
Actin Polymerization Assay
Subventricular zone cells from P2–P5 rats were treated with accutase (PAA, Linz, Austria) to prepare monolayer cultures. They were plated on ornithine/fibronectin-coated glass cover slips and kept in culture for 48 hours in Neurobasal medium supplemented with B27, N2-supplement, glutamine, and penicillin/streptomycin. Cells were then exposed to METRN (20 ng/mL) or SDF1α (50 ng/mL) diluted in Neurobasal medium or to vehicle for 2 hours. They were washed with PBS, fixed/permeabilized with 4% PFA–0.05% Triton in PBS, and stained with fluorescein isothiocyanate-phalloidin (Invitrogen) diluted 1:50 in PBS for 20 minutes. Cells were washed, mounted, and analyzed by fluorescence microscopy. Subventricular zone explants were exposed to METRN or SDF1α for 24 hours and then stained according to the same protocol.
Chemotaxis/Chemokinesis Assay
The assay was performed according to the protocol in the Cell Migration Assay Kit (ECM510; Millipore, Billerica, MA, USA). Briefly, a Boyden assay in which cells migrated from an upper chamber to a lower chamber through a porous membrane was used to distinguish if METRN and SDF1α had chemotactic or chemokinetic properties. Subventricular zone cells were derived from P2–P5 rat pups and treated with accutase to obtain individual cells, which were placed in the upper chamber. Meteorin (20 ng/mL) or SDF1α (50 ng/mL) was added either in both upper and lower chamber or in lower chamber only. We quantified the number of cells that had crossed the membrane as relative fluorescence unit using a luminescence-assay kit.
Neurotoxicity Assay
Subventricular zone cells from P2–P5 rats were plated as monolayer cultures on ornithine/fibronectin-coated glass cover slips and kept for 8 days in vitro. Cells were exposed for 5 minutes to PBS, 100 μmol/L N-methyl--asparate (NMDA; TOCRIS, Bristol, UK), 100 μmol/L NMDA+20 ng/mL METRN, or 100 μmol/L NMDA+50 ng/mL SDF1α. The media were then replaced with PBS, METRN, and SDF1α media, respectively. Cells were fixed 24 hours thereafter, permeabilized and stained for apoptosis with TUNEL (terminal deoxynucleotidyl transferase-mediated 2'-deoxyuridine 5'-triphosphate-biotin nick end labeling) (In Situ Cell Death Detection Kit; Roche, Mannheim, Germany) and Hoechst (Invitrogen). Apoptotic cells (TUNEL+) and total number of cells (Hoechst+) were counted to calculate the ratio between them as the percentage of apoptosis.
Cannula Implantation and Design of In Vivo Experiment
Male Wistar rats (240 to 250 g at the beginning of experiment; Charles River, Wilmington, MA, USA; n=24) were implanted with infusion cannulas (Alzet Brain Infusion Kit, Durect, Cupertino, CA, USA), connected to subcutaneously placed osmotic minipumps (Alzet model 2002, Durect) loaded with PBS or METRN, into the right striatum just before induction of MCAO. Coordinates were: 1-mm rostral and 2.5-mm lateral to bregma, 5-mm ventral to dura, with toothbars at −3.3 mm. Phosphate-buffered saline or recombinant mouse METRN (0.5 μg/μL) was infused for 14 days at 0.5 μL/h. One sham-operated group (seven rats) received PBS infusion. 5′-Bromo-2′-deoxyuridine (BrdU; 50 mg/kg intraperitoneally; Sigma) was injected twice daily during the first week after stroke. Two weeks after MCAO, two rats were killed to assess the distribution of infused METRN. All rats were killed directly after the behavioral tests at 33 days after MCAO.
Induction of Stroke
Transient MCAO was performed using the intraluminal filament technique (Kokaia et al, 1995). After fasting overnight, rats were anesthetized with N2O and O2 (70%:30%) and 1.5% isofluorane. A nylon monofilament with a rounded tip was inserted into the internal carotid artery until it occluded the origin of the MCA. The filament was withdrawn 2 hours later. Only animals that showed defective limb placing/circling 24 hours after MCAO were included in the subsequent analysis. For sham surgery, the filament was advanced only a few millimeters inside the internal carotid artery.
Immunohistochemistry in the Brain Sections
All rats were deeply anesthetized and transcardially perfused with saline followed by ice-cold 4% PFA. After postfixation in PFA overnight, the brains were put in 20% sucrose, and sectioned coronally at 30 μm on dry ice. Fluorescence double staining was used for the visualization of METRN+/NeuN+, METRN+/DCX+, METRN+/S100β+, BrdU+/DCX+, BrdU+/NeuN+, and Iba1+/Ki67+ cells. In brief, for double staining with METRN, after being blocked for 1 hour in potassium PBS (KPBS) containing 0.25% Triton X-100 (t-KPBS) and 5% of the appropriate normal sera, free-floating sections were incubated with goat anti-METRN (1:200; R&D Systems), and mouse anti-NeuN (1:100; Chemicon, Billerica, MA, USA), rabbit anti-DCX (1:500; Abcam, Cambridge, UK), or rabbit anti-S100β (1:200; Millipore) in blocking solution for 36 hours at 4°C. For Iba1/Ki67 staining, sections were incubated with rabbit anti-Iba1 (1:1,000; Wako Chemicals, Osaka, Japan) and mouse anti-Ki67 antibody (1:100; Novocastra, Newcastle, UK) in blocking solution overnight. For double staining with BrdU, free-floating sections were denatured in 1 mol/L HCl at 65°C for 10 minutes, followed by a 20-minute incubation at room temperature. After 1-hour incubation in appropriate blocking solution, sections were incubated with rat anti-BrdU (1:200; Abcam), and goat anti-DCX (1:400; Santa Cruz), or mouse anti-NeuN (1:100; Chemicon) in blocking solution for 36 hours at 4°C. Thereafter, the sections were incubated for 2 hours with a combination of two of the following secondary antibodies in blocking solution at room temperature: biotinylated horse anti-goat/mouse or biotinylated goat anti-rabbit secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA), DyLight 549-conjugated donkey anti-mouse or Cy3-conjugated donkey anti-rat/rabbit/mouse secondary antibody (1:200; Jackson ImmunoResearch). Finally, sections were incubated with Alexa 488-conjugated streptavidin (1:200; Molecular Probes, Carlsbad, CA, USA) in t-KPBS for 2 hours at room temperature.
For DCX/TUNEL double staining, free-floating sections were first incubated with goat anti-DCX (1:400; Santa Cruz) in blocking solution overnight at 4°C, followed by 2 hours of incubation with biotinylated Cy3 donkey anti-goat secondary antibody (1:200; Jackson ImmunoResearch) in blocking solution at room temperature. Sections were mounted onto slides and dried, and then pretreated with 4% PFA for 20 minutes, followed by incubation in ice-cold permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate, freshly prepared) on ice for 2 minutes. TUNEL (Roche) enzyme solution and label solution were freshly mixed with a volume ratio of 5:45, and applied on the sections, which were then incubated for 1 hour at 37°C in a humidified chamber in the dark. Sections were counterstained with Hoechst 33342 and coverslipped.
For METRN, NeuN, Ki67, and RECA-1 single labeling, diaminobenzidine staining was used. After quenching the reaction with 3% H2O2 and 10% methanol in KPBS followed by blocking, sections were incubated with goat anti-METRN (1:500; R&D Systems), mouse anti-NeuN (1:100; Chemicon), mouse anti-Ki67 antibody (1:100; Novocastra), or mouse anti-rat RECA-1 (1:400; AbD Serotec, Oxford, UK) overnight at 4°C. Sections were then incubated with biotinylated secondary antibody (horse anti-goat, 1:200, for METRN; horse anti-mouse, 1:200, for NeuN, Ki67 and RECA-1). After incubation with avidin–biotin–peroxidase complex (Elite ABC kit, Vector Laboratories), sections were peroxidase catalyzed with diaminobenzidine and 3% H2O2. Ki67 and RECA-1 sections were then counterstained with cresyl-violet to visualize SVZ. The specificity of staining was confirmed by omitting the primary antibody.
Microscopical Analysis
Numbers of METRN+, DCX+, BrdU+/DCX+, and BrdU+/NeuN+ cells in the striatum were counted using a 0.25-mm-wide quadratic grid on an epifluorescence microscope in four sections per brain, 600 μm apart, starting +1.7 mm rostral to bregma, as previously described (Kobayashi et al, 2006). Cell counts are presented as numbers of cells per section. BrdU/DCX, BrdU/NeuN, METRN/NeuN, and METRN/DCX double labeling was validated with a confocal laser scanning microscope (Leica Microsystems GmbH, Wetzlar, Germany). When measuring the distribution of Dcx+ cells, each section of striatum was divided in segments, each stretching 0.25 mm laterally away from the lateral wall of the SVZ, thus representing increasing distances from the SVZ, and individual cell counts were recorded in each segment. The percentage of cells in each segment was then calculated by dividing the sum of cells in each corresponding segment of all sections with total striatal count.
The total number of Ki67+ cells in a rostrocaudal segment of SVZ corresponding to the area of striatal cell counting, and the length density of blood vessels (the length of blood vessels per unit volume, μm/μm3, RECA staining) in the whole striatum were estimated by Computer-Assisted Stereological Toolbox software with the optical fractionator or isotropic virtual plane methods (CAST, Visiopharm, Copenhagen, Denmark), respectively (Larsen et al, 1998; West, 1999). For each brain, stereological quantification was performed in consecutive sections in a rostrocaudal extension defined by anatomical landmarks corresponding to the region between +1.7 and −0.46 for Ki67+ cell counting and between +1.7 and −2.0 for vessel length density quantification. The crosssectional area of the intact ipsilateral hemisphere (NeuN+ region, including both striatum and cerebral cortex) and the entire contralateral hemisphere for each brain slice were measured using CAST. Stroke size was calculated by subtracting the intact volume of the ischemic hemisphere from the volume of contralateral hemisphere, and shown as the percentage of the contralateral volume.
Stepping Test
All behavioral tests were evaluated by a blinded investigator. The stepping test was used to assess forelimb function (Kirik et al, 1998). Briefly, the rat was held with its hindlimbs fixed with one hand, one forelimb with the other, and the unrestrained forepaw touching the surface of a table. The number of adjusting steps was counted as the animal was moved sideways with steady speed in both forehand and backhand direction along the table surface (90 cm in 5 seconds). Animals were tested twice daily for 5 days before MCAO. During the fifth week after MCAO, the test was repeated and mean number of steps from the last 3 consecutive days constituted the final data.
Staircase Test
This test was used to assess skilled forelimb reaching and grasping (Winkler et al, 1999). Briefly, animals were food-deprived 24 hours before the first testing day and kept on a restricted food intake (6 to 8 g/day) throughout the test period, with food administrated only after the daily test session. When testing, animals were placed in plexiglas boxes holding a removable double staircase, with 10 sugar pellets placed on each of steps 2 to 5 on both sides (total 40 pellets/side). During each session, animals were kept in the box for 15 minutes once a day, after which the number of pellets eaten on each side was calculated. Before MCAO, rats were tested for 12 consecutive days. Mean number of eaten pellets from the last 3 days was used as the preinsult performance score. During the fifth week after MCAO, rats were retested for 5 days using the same method, and the average of the last 3 days was calculated.
Statistical Analysis
Comparisons between groups were performed with Student's t-test or one-way analysis of variance with Bonferroni post hoc test. Two-way analysis of variance with Bonferroni post hoc test was used to assess differences in the distribution of DCX+ cells and behavior tests. Data are given as mean values±s.e.m. and differences are regarded as significant at P<0.05.
Results
Meteorin Promotes Subventricular Zone Neuroblast Migration In Vitro Through a Chemokinetic Mechanism
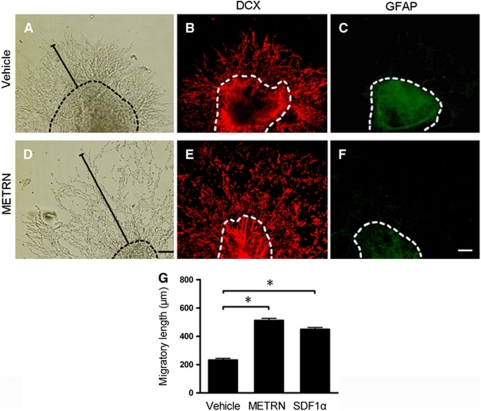
We first cultured SVZ explants from neonatal rats in the presence of METRN or SDF1α, a factor that stimulates neuronal migration (Naiyer et al, 1999; Yu et al, 2010). After 24 hours, we observed and measured migratory chains of cells extending from the explants (Figures 1A and 1D). Meteorin (20 ng/mL) increased chain length by 120%, which was similar to the increase seen with SDF1α (50 ng/mL). In a separate experiment, METRN stimulated migration in a dose-dependent manner between 2 and 10 ng/mL, a higher concentration (50 ng/mL) giving no additional effect (data not shown). The migratory cells uniformly expressed DCX, indicating they were neuroblasts (Figures 1B and 1E), whereas virtually no glial fibrillary acidic protein+ astrocytes were found in the migratory chains exposed to METRN (Figures 1C and 1F).
Figure 1.
Meteorin (METRN) promotes neuroblast migration in vitro. (A–F) Migratory chains from vehicle- or METRN-exposed (20 ng/mL) subventricular zone (SVZ) explants in panels A and D are DCX+ (B, E) but not glial fibrillary acidic protein+ (C, F) at 24 hours. (G) Length of migratory chains, measured from edge of explants (dashed line), exposed to vehicle, 20 ng/mL METRN or 50 ng/mL stromal cell-derived factor 1α (SDF1α). Mean values±s.e.m. n=11, 11, and 14 in vehicle, METRN, and SDF1α groups, respectively. *P<0.05 one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. Scale bar=100 μm. DCX, doublecortin.
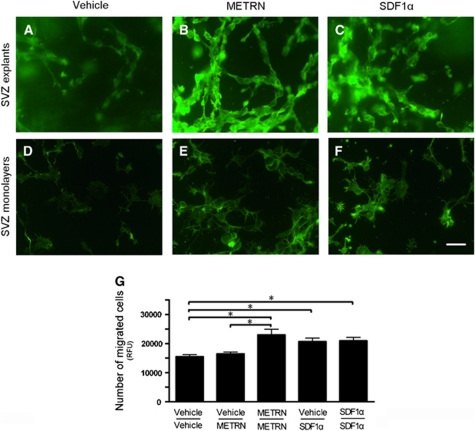
Actin polymerization is a key process in cell migration (Jones et al., 2006) and can be visualized by phalloidin-fluorescein isothiocyanate staining. We exposed SVZ explants and cell monolayers to METRN or SDF1α. Both treatments resulted in increased phalloidin-fluorescein isothiocyanate staining (Figures 2A–2F). This finding suggests that actin polymerization may be involved in the neuroblast migration stimulated by METRN and SDF1α.
Figure 2.
Meteorin (METRN) triggers actin polymerization, acts as a chemokinetic migratory factor in vitro. (A–F) Actin polymerization, visualized by fluorescein isothiocyanate-phalloidin staining, is increased in subventricular zone (SVZ) explants and monolayers after treatment with 20 ng/mL METRN or 50 ng/mL stromal cell-derived factor 1α (SDF1α). (G) Luminescence-based quantification of cells that crossed the Boyden chamber's membrane. METRN promoted migration when equally concentrated (20 ng/mL) in upper and lower chambers (METRN–METRN), whereas no effect was detected with a METRN concentration gradient (vehicle–METRN). In all, 50 ng/mL SDF1α stimulated migration both with equal concentrations and a gradient. Mean values±s.e.m. n=9 to 10 for each condition in panel G, respectively. *P<0.05 one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. Scale bar=25 μm. RFU, relative fluorescence units.
To determine if METRN has chemotactic or chemokinetic properties in migration, we used a Boyden assay. Meteorin loaded into both upper and lower chamber gave rise to increased cell migration across the membrane that separates the two chambers, while METRN loaded only into the lower chamber had no effect (Figure 2G). This finding indicated that METRN acted through a chemokinetic mechanism. In contrast, SDF1α promoted migration when present in lower as well as in both chambers (Figure 2G), indicating both chemokinetic and chemotactic properties as previously shown (Naiyer et al, 1999; Yu et al, 2010).
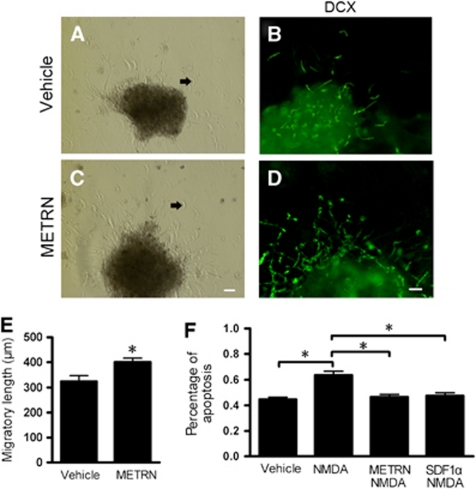
Ischemic stroke causes migration of SVZ neuroblasts into the damaged striatum (Arvidsson et al, 2002). We used explants of SVZ ipsilateral to ischemia from rats subjected to 2 hours MCAO. As shown in Figure 3, the migratory DCX+ cell chains were elongated by METRN (Figure 3E), providing evidence that the stimulatory effect of METRN on neuroblast migration also applies to stroke-subjected adult SVZ tissue.
Figure 3.
Meteorin (METRN) promotes migration of subventricular zone (SVZ) neuroblasts from stroke-damaged adult rats in vitro, and improves cell survival in vitro. (A, C, E) Explants from SVZ ipsilateral to ischemia display migratory chains (A), which are elongated by 20 ng/mL METRN (C, E). (B, D) DCX+ neuroblasts respond to METRN exposure. (F) Percentage of apoptotic cells, quantified using TUNEL (terminal deoxynucleotidyl transferase-mediated 2'-deoxyuridine 5'-triphosphate-biotin nick end labeling)/Hoechst staining, in monolayer cells after N-methyl--asparate (NMDA) exposure in the presence of 20 ng/mL METRN or 50 ng/mL stromal cell-derived factor 1α (SDF1α). Arrows indicate migrating cells in panels A and C. Mean±s.e.m. n=5 and 8 in vehicle and METRN group, respectively in panel E. n=9 for each condition in panel F. *P<0.05, Student's t-test or one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. Scale bar=50 μm. DCX, doublecortin.
Meteorin Protects Subventricular Zone Cells from Apoptosis
We then exposed SVZ cells to the excitotoxin NMDA and treated them with PBS, METRN, or SDF1α. TUNEL/Hoechst staining was used to detect and quantify apoptotic cells. Apoptotic cell death was increased by 42% in the presence of NMDA (Figure 3F). The SVZ cells expressed NMDAR1 (data not shown), providing evidence that the NMDA-induced neurotoxicity was at least partly mediated through this receptor. Treatment with METRN or SDF1α was neuroprotective and reduced the number of apoptotic cells to control levels (Figure 3F).
Ischemic Stroke Upregulates Meteorin Expression in the Striatum and Subventricular Zone
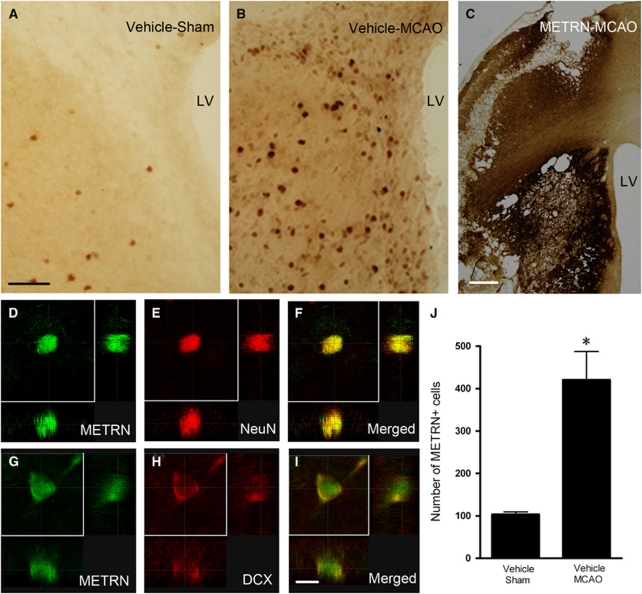
We next assessed the expression of endogenous METRN protein using immunohistochemistry in rats subjected to sham surgery or 2 hours MCAO. In sham animals, we replicated previously reported findings (Jorgensen et al, 2009), with the addition that we noted a subset of METRN-expressing cells located in the dorsomedial striatum adjacent to the SVZ (Figure 4A). At 33 days after MCAO, we found increased numbers of METRN+ cells in the striatum ipsilateral (Figures 4B and 4J) and to a lesser extent contralateral to MCAO (data not shown), most pronounced in the dorsomedial striatum close to SVZ. The vast majority of striatal METRN+ cells in both intact and ischemic rats coexpressed NeuN, indicating that they were mature neurons (Figures 4D–4F). Interestingly, a subportion of the DCX+ neuroblasts (about 10%) in the striatum after stroke also expressed METRN (Figures 4G–4I). We found only a couple of METRN+ cells in SVZ of intact rats (about half of them colabeled with DCX). After stroke, more METRN+ cells were observed in SVZ (data not shown), and about 20% of them were colabeled with DCX. We detected no METRN+ cells coexpressing the astrocytic marker S100β in striatum or SVZ. Thus, the cellular pattern of METRN expression at 33 days following MCAO differed from that observed at 4 weeks after quinolinic acid injection, when METRN was upregulated in S100β+ glial cells (Jorgensen et al, 2011). Outside the striatum and SVZ, there was a widespread distribution of METRN+ cells with neuronal morphology in deeper layers of the neocortex, and also diffuse upregulation of METRN in the borderzone of infarction in cells with astrocytic morphology (data not shown).
Figure 4.
Endogenous meteorin (METRN) is upregulated after stroke and METRN is widely distributed in the striatum following infusion. (A, B) METRN+ cells in dorsomedial striatum and subventricular zone (SVZ) in a sham-operated and a middle cerebral artery occlusion (MCAO)-treated animal. (C) Striatal distribution of METRN after 2 weeks of infusion of 0.5 μg/μL METRN at 0.5 μL/h. (D–I) Confocal photomicrographs with orthogonal reconstruction of METRN+ cells coexpressing NeuN or doublecortin (DCX). (J) Quantification of number of METRN+ cells in the striatum of sham-operated and MCAO-subjected animals (n=7 and 9, respectively). Mean values±s.e.m. *P<0.05, Student's t-test. Scale bar=50 μm (A, B), 500 μm (C), and 10 μm (D–I). The concentration of METRN antibody was 1:500 for panels A–C, and 1:200 for panels D–I. LV, lateral ventricle.
Meteorin Is Widely Distributed After Intrastriatal Infusion but Size of Ischemic Damage Is Unaffected
We wanted to explore if METRN also promotes migration and survival of SVZ-derived neurons in vivo. Meteorin or vehicle was infused into the ischemic striatum during the first 2 weeks after 2 hours MCAO. The two rats killed directly after the end of infusion showed METRN protein diffusion throughout the ipsilateral striatum, extending into the SVZ (Figure 4C) and parts of the cerebral cortex. At 33 days after MCAO, METRN could still be detected in the striatum and SVZ, although the staining was weaker than that seen at 2 weeks after MCAO (data not shown). The volume of the stroke-induced damage, at 33 days after the insult, did not differ between the groups (49.4%±5.4% in vehicle–MCAO group; 51.3%±7.1% in METRN–MCAO group; P=0.83).
Intrastriatal Meteorin Infusion Stimulates Several Steps of Stroke-Induced Striatal Neurogenesis
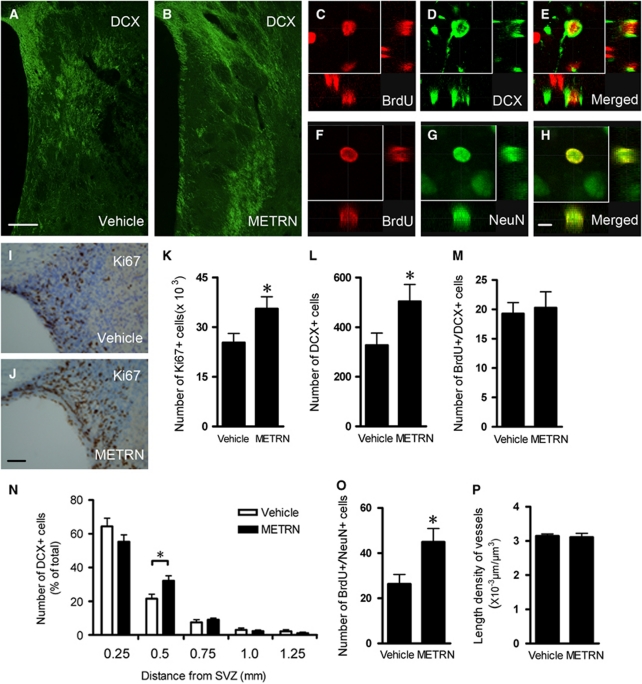
At 33 days following MCAO, we found higher number of cells expressing the mitosis marker Ki67 in the ipsilateral SVZ of METRN, compared with vehicle-treated rats (Figures 5I–5K). This finding indicated that METRN infusion had stimulated cell proliferation in the SVZ of the stroke-damaged brain.
Figure 5.
Intrastriatal meteorin (METRN) infusion (0.5 μg/μL at 0.5 μL/h) promotes neurogenesis in rats after stroke. (A, B) Doublecortin (DCX) immunostaining in the striatum of vehicle- and METRN-treated animals after stroke. (C–H) Confocal photomicrographs with orthogonal reconstruction of BrdU+ cells coexpressing DCX or NeuN. (I, J) Ki67+ cells in subventricular zone (SVZ) of vehicle- and METRN-treated animals after stroke. (K–M, O, P) Numbers of Ki67+ cells in SVZ (presented as total cell estimate) and DCX+, BrdU+/DCX+, and BrdU+/NeuN+ cells (presented as cells per section), and length density of blood vessels in striatum. (N) Distribution of DCX+ cells at different distances from the SVZ in damaged striatum. Results are presented as the percentage of the number of DCX+ cells in each segment (0 to 0.25, 0.25 to 0.5, 0.5 to 0.75, 0.75 to 1.0, and 1.0 to 1.25 mm) in relation to the total number of DCX+ cells in the striatum. Mean values±s.e.m. n=9 and 8 for vehicle- and METRN-treated group, respectively. *P<0.05, two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test for panel N, Student's t-test for the others. Scale bar=200 μm (A, B), 10 μm (C–H), and 50 μm (I, J).
We then assessed the distribution of DCX+ cells in the striatum at different distances from the SVZ, shown as a percentage of the total number of DCX+ cells in Figure 5. A higher proportion of DCX+ cells was distributed 0.25 to 0.5 mm from the SVZ in the METRN-treated group compared with control, whereas there was a trend toward a decreased proportion in the column closest to SVZ (Figures 5A, 5B, and 5N), suggesting a longer migration distance of the neuroblasts into the ischemic striatum. These data provide evidence that METRN promotes migration of newly formed cells from SVZ also in vivo.
Cell counting also revealed that at 33 days after stroke, METRN infusion gave rise to increased numbers of both DCX+ neuroblasts (by 54%) as well as of BrdU+/NeuN+ mature neurons (by 70%) in the injured striatum as compared with the vehicle-treated rats (Figures 5L and 5O). The number of BrdU+/DCX+ cells was very low and did not differ between the groups (Figure 5M).
As METRN counteracted apototic cell death in vitro and striatal neuroblasts recruited after stroke undergo apoptotic cell death (Thored et al, 2006), we stained DCX+ cells for TUNEL+ apoptotic cell death in METRN-treated and control animals at 33 days. However, we could not observe any double-labeled cells in either of the groups at this time point.
Intrastriatal Meteorin Infusion Does Not Stimulate Angiogenesis or Microglial Proliferation or Improve behavioral Recovery After Stroke
Meteorin has been reported to attenuate angiogenesis and promote vascular maturation in vitro (Park et al, 2008). We quantified the length density of blood vessels (the length of blood vessels per unit volume) in the whole striatum at 33 days after stroke. No differences in the length density were detected between the METRN- and vehicle-treated groups (Figure 5P). Thus, our findings do not support a significant role for exogenous METRN in regulating angiogenesis in the stroke-damaged striatum.
Meteorin stimulated cell proliferation in the SVZ, and stroke is associated with microglial proliferation in the SVZ (Thored et al, 2009). We analyzed double labeling for Ki67 and the microglial marker Iba1 to determine whether stimulation of microglial proliferation could account for part of the elevated SVZ cell proliferation after METRN delivery. In agreement with our previous data (Thored et al, 2009), showing that microglia proliferate early but not at 7 to 8 weeks after stroke, we did not detect any Ki67+/Iba1+ cells in the SVZ at 33 days, neither in the vehicle- nor in the METRN-infused group.
We finally evaluated whether the METRN-induced enhancement of striatal neurogenesis was accompanied by improved functional recovery. Both the number of left forehand steps in the stepping test (Figure 6A) and the number of pellets eaten by the left paw (contralateral to MCAO) in the staircase test (Figure 6B) were markedly reduced after stroke. However, the METRN treatment did not influence the performance in either of the tests during the fifth week after the ischemic insult (Figures 6A and 6B).
Figure 6.
Intrastriatal meteorin (METRN) infusion has not improved functional recovery in rats at 5 weeks after stroke. Both number of left forehand steps in stepping test (A) and number of eaten pellets by left paw in staircase test (B) are markedly reduced during the fifth week after stroke, demonstrating a clear functional deficit. No improvement is detected in the METRN- compared with the vehicle-treated group. Mean values±s.e.m. n=8 to 9. Mean values±s.e.m. *P<0.05, two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test.
Discussion
This study provides the first evidence that METRN promotes neurogenesis and cell migration in vitro and in vivo. Infusion of METRN after stroke gave rise to increased SVZ cell proliferation and neuroblast migration, and higher numbers of new neurons in the striatum.
At the end of infusion, 2 weeks after the insult, pumps were virtually empty and METRN had diffused extensively in the stroke-damaged hemisphere and reached the SVZ. We observed METRN immunoreactivity in the striatum also at 33 days, which suggests that METRN could exert biological effects also after the cessation of protein infusion. In support of this idea, we found increased cell proliferation in the SVZ and higher number of DCX+ cells in the striatum at 33 days in the METRN-treated group. The marker DCX is transiently expressed in neuroblasts during the first 2 weeks after their generation (Brown et al, 2003). Therefore, the DCX+cells in the striatum at 33 days most likely represented neuroblasts formed during the preceding 2 weeks. Accordingly, at 33 days only few DCX+ cells were colabeled with BrdU, which was administered during the first week after stroke.
Meteorin did not mitigate ischemic damage, which is in contrast to previous findings that METRN protects against excitotoxic injury caused by quinolinic acid (Jorgensen et al, 2011). This may suggest that METRN's neuroprotective action is insult specific and not effective in counteracting the mechanisms underlying ischemic neuronal death. However, the discrepancy could also be due to differences in mode and timing of METRN administration. We used a clinically feasible approach and started METRN infusion after the occlusion and continued for 2 weeks. In contrast, in the study of Jorgensen et al (2011), METRN was delivered using a lentiviral vector 2 weeks before the quinolinic acid injection. Thus, METRN expression was already high in striatum at the time of the excitotoxic insult. It is conceivable that with the slow release rate of the osmotic pump at 0.5 μL/h, sufficiently high, effective levels of METRN, covering the whole striatum, were not reached in time to salvage injured striatal neurons, their damage being complete at 48 hours after stroke (Kokaia et al, 1998). Conversely, the lack of effect of METRN treatment on functional recovery could be explained by the timing of delivery, with METRN level between week 3 and 5 being too low to stimulate an effect on neurogenesis and other plastic changes of such a magnitude as to induce behavioral improvement.
The most well-established mechanism regulating migration of stroke-induced new neurons is SDF1α and its receptor CXCR4. Stromal cell-derived factor 1α levels are increased in the injured hemisphere (Robin et al, 2006) and CXCR4 is expressed on neural progenitors and stroke-generated neuroblasts (Robin et al, 2006). Neuroblast migration is markedly attenuated by blocking CXCR4 (Robin et al, 2006; Thored et al, 2006). Other factors promoting neuroblast migration after stroke are monocyte chemoattractant protein-1 (Yan et al, 2007), matrix metalloproteinase-9 (Lee et al, 2006), and osteopontin (Yan et al, 2009). We found that METRN stimulates neuroblast migration in vitro to the same extent as SDF1α. The distribution of DCX+ cells after stroke in the METRN-treated animals was shifted laterally from SVZ, indicating a facilitation of neuroblast migration also in vivo. Hypothetically, accumulation of DCX+ cells in a segment of striatum could be due to local neuroprotection or delayed maturation. However, this interpretation is unlikely since immunohistochemistry showed an even distribution of METRN in the striatum.
Results from the Boyden chamber assay indicate that METRN promotes neuroblast migration through a chemokinetic mechanism (Parameswaran et al, 2002). Meteorin and SDF1α increased the polymerization of actin, a cytoskeleton protein that actively remodels during cell migration and is important for forming cell protrusions (Insall and Machesky, 2009). Chemoattractants such as chemokines are believed to act through localized and specific alterations of the actin cytoskeleton (Yamaguchi and Condeelis, 2007). Exposure of migratory cancer cells to chemokines is associated with actin polymerization (Muller et al, 2001). Our data indicate that the METRN-evoked stimulation of actin cytoskeleton reorganization may mediate its effect on neuroblast migration.
Two other actions of METRN could contribute to the elevated numbers of new neuroblasts and mature neurons in striatum. The increased numbers of Ki67+ cells in the SVZ following METRN infusion suggested a stimulatory effect on neural progenitor proliferation. At 33 days, the increased SVZ cell proliferation triggered by stroke during the first weeks (Jin et al, 2001; Arvidsson et al, 2002) has tapered off. Delivery of METRN seems to be able to stimulate progenitor proliferation in SVZ at this late time point, which may increase the pool of newly formed neuroblasts entering the striatum from SVZ. Our in vitro data indicated that METRN can improve survival of SVZ cells exposed to NMDA. Thus, METRN may promote striatal neurogenesis also by preventing the apoptosis of the newly formed neuroblasts. However, no TUNEL+/DCX+ cells were observed in the SVZ and striatum in either group at 33 days. These data do not exclude an in vivo effect on apoptosis, as it is likely that the methods at hand do not detect limited apoptosis in a subset of cells at sufficient levels to compare group effects. In our previous work, we found only few scattered DCX+/caspase-cleaved poly polymerase+ (cPARP; a marker of apoptosis) cell in the striatum after stroke (Thored et al, 2006).
Since the METRN receptor has not been identified, it is unclear whether METRN exerts its effects on striatal neurogenesis directly on SVZ progenitors, neuroblasts, or mature neurons, or indirectly through other cells. Meteorin-induced promotion of axonal extension in neurons is mediated through glia (Nishino et al, 2004). Glia are present in our SVZ explants, and high numbers of activated glia are found in close proximity to the chains of migrating cells after stroke (Thored et al, 2006; Yamashita et al, 2006). A similar indirect effect of METRN via glia is possible also here. Another potential mediator could be blood vessels. Neurogenesis and angiogenesis are closely linked in SVZ and peri-infact regions after stroke (Leventhal et al, 1999; Ohab et al, 2006). Neuroblasts migrate along blood vessels toward the poststroke striatum (Kojima et al, 2010). Meteorin has been reported to attenuate angiogenesis and promote vascular maturation in vitro (Park et al, 2008). However, we obtained no evidence that the METRN infusion had altered angiogenesis in striatum after stroke. Thus, it seems unlikely that METRN promotes stroke-induced neurogenesis through an action on blood vessels.
Despite the increased number of new striatal neurons induced by METRN infusion, we observed no improvement of behavioral outcome. Circumstantial evidence suggests that endogenous neurogenesis can contribute to functional recovery after stroke. The administration of molecules that promote neural proliferation in the SVZ and striatal neurogenesis after stroke is associated with improved functional outcome (Lindvall and Kokaia, 2008). Genetic ablation of newly formed neuroblasts caused worsening of behavior and more extensive ischemic lesion as early as 24 hours after stroke (Jin et al, 2010), which suggests a trophic action but does not exclude functional improvement through neuronal replacement later after stroke. It is possible that the enhancement of neurogenesis found here, leading to 70% higher number of new mature neurons in the striatum at 33 days after the insult, is still too low to be translated into a behavioral improvement. Another possibility is that the behavioral assessments at 33 days after stroke were performed too early, since functional development and integration of the new neurons are not completed until 6 to 8 weeks, even several months, post-ischemia (Hou et al, 2008; Lai et al, 2008; Kadam et al, 2010).
Our data indicate that exogenous METRN, delivered by intracerebral infusion, can promote intrinsic neuroregenerative responses in the adult brain after stroke. The upregulation of METRN protein in the stroke-damaged striatum, close to SVZ, suggests that also endogenous METRN may have a similar role. Future studies should aim at optimizing the stimulatory effect of METRN and other factors on neurogenesis to significantly improve functional restoration after stroke.
Acknowledgments
The authors thank Koichi Oki for help with design of behavioral assessments, and Camilla Ekenstierna and Birgitte Romme Lisdorf for technical support. The authors are grateful to Dr Vassilios Kalabokis and Dr Jason Patzlaff for the production of in vivo grade recombinant METRN.
MT and JRJ are employed by NsGene, holding patent on METRN.
Footnotes
This study was supported by the Swedish Research Council, EU 7th work program through the European Stroke Network (Grant no. 201024) and Swedish Government Initiative for Strategic Research Areas (Stem Therapy).
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Hou SW, Wang YQ, Xu M, Shen DH, Wang JJ, Huang F, Yu Z, Sun FY. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008;39:2837–2844. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell. 2009;17:310–322. doi: 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci USA. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- Jorgensen JR, Emerich DF, Thanos C, Thompson LH, Torp M, Bintz B, Fjord-Larsen L, Johansen TE, Wahlberg LU. Lentiviral delivery of Meteorin protects striatal neurons against excitotoxicity and reverses motor deficits in the quinolinic acid rat model. Neurobiol Dis. 2011;41:160–168. doi: 10.1016/j.nbd.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Jorgensen JR, Thompson L, Fjord-Larsen L, Krabbe C, Torp M, Kalkkinen N, Hansen C, Wahlberg L. Characterization of Meteorin--an evolutionary conserved neurotrophic factor. J Mol Neurosci. 2009;39:104–116. doi: 10.1007/s12031-009-9189-4. [DOI] [PubMed] [Google Scholar]
- Kadam SD, Smith-Hicks CL, Smith DR, Worley PF, Comi AM. Functional integration of new neurons into hippocampal networks and poststroke comorbidities following neonatal stroke in mice. Epilepsy Behav. 2010;18:344–357. doi: 10.1016/j.yebeh.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke. 2006;37:2361–2367. doi: 10.1161/01.STR.0000236025.44089.e1. [DOI] [PubMed] [Google Scholar]
- Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Andsberg G, Martinez-Serrano A, Lindvall O. Focal cerebral ischemia in rats induces expression of P75 neurotrophin receptor in resistant striatal cholinergic neurons. Neuroscience. 1998;84:1113–1125. doi: 10.1016/s0306-4522(97)00579-4. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Zhao Q, Kokaia M, Elmer E, Metsis M, Smith ML, Siesjo BK, Lindvall O. Regulation of brain-derived neurotrophic factor gene expression after transient middle cerebral artery occlusion with and without brain damage. Exp Neurol. 1995;136:73–88. doi: 10.1006/exnr.1995.1085. [DOI] [PubMed] [Google Scholar]
- Lai B, Mao XO, Xie L, Jin K, Greenberg DA. Electrophysiological neurodifferentiation of subventricular zone-derived precursor cells following stroke. Neurosci Lett. 2008;442:305–308. doi: 10.1016/j.neulet.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JO, Gundersen HJ, Nielsen J. Global spatial sampling with isotropic virtual planes: estimators of length density and total length in thick, arbitrarily orientated sections. J Microsc. 1998;191:238–248. doi: 10.1046/j.1365-2818.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- Lee HS, Han J, Lee SH, Park JA, Kim KW. Meteorin promotes the formation of GFAP-positive glia via activation of the Jak-STAT3 pathway. J Cell Sci. 2010;123:1959–1968. doi: 10.1242/jcs.063784. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Rogowska J, Zhao BQ, Bhide P, Parent JM, Lo EH. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–3495. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z.2008Neurogenesis following stroke affecting the adult brain Adult Neurogenesis(Gage FH, Kempermann G, Song H, eds),Cold Spring Harbor Laboratory Press: Woodbury, NY; 549–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Fabregas J, Romaguera-Ros M, Gomez-Pinedo U, Martinez-Ramirez S, Jimenez-Xarrie E, Marin R, Marti-Vilalta JL, Garcia-Verdugo JM. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357–365. doi: 10.1212/WNL.0b013e3181cbccec. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Naiyer AJ, Jo DY, Ahn J, Mohle R, Peichev M, Lam G, Silverstein RL, Moore MA, Rafii S. Stromal derived factor-1-induced chemokinesis of cord blood CD34(+) cells (long-term culture-initiating cells) through endothelial cells is mediated by E-selectin. Blood. 1999;94:4011–4019. [PubMed] [Google Scholar]
- Nishino J, Yamashita K, Hashiguchi H, Fujii H, Shimazaki T, Hamada H. Meteorin: a secreted protein that regulates glial cell differentiation and promotes axonal extension. EMBO J. 2004;23:1998–2008. doi: 10.1038/sj.emboj.7600202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran K, Cox G, Radford K, Janssen LJ, Sehmi R, O'Byrne PM. Cysteinyl leukotrienes promote human airway smooth muscle migration. Am J Respir Crit Care Med. 2002;166:738–742. doi: 10.1164/rccm.200204-291OC. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Park JA, Lee HS, Ko KJ, Park SY, Kim JH, Choe G, Kweon HS, Song HS, Ahn JC, Yu YS, Kim KW. Meteorin regulates angiogenesis at the gliovascular interface. Glia. 2008;56:247–258. doi: 10.1002/glia.20600. [DOI] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, Lindvall O. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Winkler C, Bentlage C, Nikkhah G, Samii M, Bjorklund A. Intranigral transplants of GABA-rich striatal tissue induce behavioral recovery in the rat Parkinson model and promote the effects obtained by intrastriatal dopaminergic transplants. Exp Neurol. 1999;155:165–186. doi: 10.1006/exnr.1998.6916. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, Lang BT, Vemuganti R, Dempsey RJ. Osteopontin is a mediator of the lateral migration of neuroblasts from the subventricular zone after focal cerebral ischemia. Neurochem Int. 2009;55:826–832. doi: 10.1016/j.neuint.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Yu T, Huang H, Li HF. Stromal cell-derived factor-1 promotes migration of cells from the upper rhombic lip in cerebellar development. J Neurosci Res. 2010;88:2775–2786. doi: 10.1002/jnr.22454. [DOI] [PubMed] [Google Scholar]