Abstract
Preconditioning induces ischemic tolerance, which confers robust protection against ischemic damage. We show marked protection with polyinosinic polycytidylic acid (poly-IC) preconditioning in three models of murine ischemia-reperfusion injury. Poly-IC preconditioning induced protection against ischemia modeled in vitro in brain cortical cells and in vivo in models of brain ischemia and renal ischemia. Further, unlike other Toll-like receptor (TLR) ligands, which generally induce significant inflammatory responses, poly-IC elicits only modest systemic inflammation. Results show that poly-IC is a new powerful prophylactic treatment that offers promise as a clinical therapeutic strategy to minimize damage in patient populations at risk of ischemic injury.
Keywords: cerebrovascular disease, focal ischemia, inflammation, ischemic preconditioning and induced tolerance, neuroprotection
Introduction
Patients undergoing cardiac surgery risk ischemic injury to the brain (50% occurrence), kidneys (30% occurrence), and lungs (18% occurrence) (Barber et al, 2008; Fukada et al, 2004; Mehta et al, 2010). Despite these statistics, there remain few treatments for surgically induced ischemia or preventive measures to lessen tissue damage. A promising therapeutic approach exploits the phenomenon of ischemic tolerance whereby brief exposure to a harmful stimulus, such as ischemia, volatile anesthetics, hypothermia, hypoxia, or inflammation, when given before an ischemic challenge provides protection against lethal ischemia (Dirnagl et al, 2003). Toll-like receptors (TLRs), sentinels of the innate immune system, recognize invading pathogens and endogenous damage signals. Preconditioning with TLR ligands including lipopolysaccharide (LPS; TLR4) confers robust protection from ischemic injury (Heemann et al, 2000; Rosenzweig et al, 2007; Stevens et al, 2008). However, LPS may not be a viable option for therapeutic consideration due to its known harmful side effects, including a robust inflammatory response that can lead to serum sickness or a sepsis-like syndrome. Thus, we sought an alternate therapeutic that would have the widespread protective effects of LPS preconditioning on those organs at greatest risk, brain and kidneys, but would not induce potentially deleterious inflammation.
Polyinosinic polycytidylic acid (poly-IC) is a synthetic double-stranded RNA that activates a complex immune response via TLR3, retinoic acid-inducible gene I-like receptors, oligoadenylate synthetase, and protein kinase RNA activated. Poly-ICLC is a version of poly-IC stabilized with poly--lysine and carboxymethylcellulose moieties that have been added to improve pharmacokinetics. It has shown clinical promise in humans for various indications (e.g., vaccines, multiple sclerosis, cancer, and viral infections; Markosian and Hyde, 2005; Rosenfeld et al, 2010). Here, we show that poly-ICLC preconditioning protects against cerebral ischemic damage in an in-vitro system of modeled ischemia and in an in-vivo experimental mouse model of stroke. Additionally, we show that poly-IC preconditioning protects against damage in a model of renal ischemia. We report that unlike LPS preconditioning which produces a robust inflammatory response, poly-ICLC preconditioning induces very modest levels of pro-inflammatory cytokines. These results emphasize the promise of poly-ICLC as a novel therapeutic approach to reduce perioperative ischemic damage.
Materials and methods
Mice
Animal procedures were conducted using C57/Bl6 male mice, 8- to 12-week old (Jackson Laboratory, West Sacramento, CA, USA), according to Oregon Health and Science University (OWLAW #A3304-01) Institutional Animal Care and Use Committee and National Institute of Health Guidelines. The American Association for Laboratory Animal Care accredits the housing facility.
Reagents
Carboxymethylcellulose (Sigma-Aldrich, St Louis, MO, USA), poly-ICLC (Hiltonol; Oncovir, Washington, DC, USA), saline, poly-IC high molecular weight (Invivogen, San Diego, CA, USA), and LPS (Escherichia coli serotype 0111:B4; phenol extraction purified, protein content _3% Sigma-Aldrich) were used. Agents were delivered by either intraperitoneal or subcutaneous administration as noted in Materials and methods and figure legends. We have found both routes provide equivalent levels of protection.
Oxygen Glucose Deprivation
Primary mixed cortical cultures were prepared from E15 to E17 mouse fetuses (1 litter/experiment) as previously published (Stevens et al, 2008). During oxygen glucose deprivation (OGD), medium was replaced with D-PBS (Gibco, Carlsbad, CA, USA) and cells were incubated in an anaerobic atmosphere of 85% N2, 10% CO2, 5% H2 at 37°C for 3 hours (Coy Laboratories, Grass Lake, MI, USA). After OGD, D-PBS was replaced with medium and cells returned to a normoxic incubator. Control plates remained in the normoxic incubator during OGD. Cell death was determined 24 hours after OGD using propidium iodide staining (Sigma-Aldrich) and quantified with Metmorph7 software (Molecular Devices, Downington, PA, USA). Within an experiment, each treatment was performed in triplicate and three independent experiments were performed.
Middle Cerebral Artery Occlusion
Mice were treated with poly-ICLC (0.4, 0.8 or 1.6 mg/kg), LPS (1 mg/kg), or appropriate vehicle (subcutaneous; n=9 to 10/group) 72 hours before middle cerebral artery occlusion (MCAO) (45 minutes) performed as previously described (Stevens et al, 2008). Laser Doppler (Transonic Systems Incorporated, Ithaca, NY, USA) was used to measure cerebral blood flow through the skull adjacent the middle cerebral artery to insure occlusion reduced flow to ⩽20% of baseline. In blinded manner 24 hours after MCAO, the neurologic testing was administered and indirect infarct volume determined by 2,3,5 triphenyltetrazolium chloride stain. There are two categories of neurologic scoring each based on a 28-point scale which correlates with infarct volume (Clark et al, 1997). The general score evaluates activity, posture, and grooming. The focal score evaluates body symmetry, gait, and traits characteristic of unilateral MCAO damage. The corner test evaluates body function and asymmetry by noting direction turned when mice approach a 45° corner. After MCAO, mice tend to favor their ipsilateral (right) side turning preferentially right when exiting while naive mice turn equally to either side. Each mouse was scored 10 consecutive times to calculate the percentage of right hand turns (Zhang et al, 2002). For extended recovery, mice were treated with either poly-IC (intraperitoneal; 1 mg/kg) or vehicle (n=8 to 10/group) and infarct volume was assessed 72 hours after MCAO.
Renal Ischemia Procedure
Mice were administered vehicle, LPS (1.6 mg/kg), or poly-IC (intraperitoneal; 1.6 mg/kg) 48 hours before surgery (n=6/group). Renal ischemia was induced in isoflurane (1.5% to 2%) anesthetized mice by performing a midline incision, isolating the renal vessels bilaterally, clamping the renal vessels with small bulldog clamps (45 minutes), and confirming occlusion and reperfusion by Parks Ultrasonic 811-B Doppler (Kroslak Enterprises, Riverview, FL, USA). Blood samples were taken via saphenous vein before renal ischemia surgery and 48 hours after reperfusion for creatinine level determination by the Jaffe rate method using a Beckman Coulter DXC800 (Brea, CA, USA).
Plasma Cytokine Evaluation
Blood was collected via cardiac puncture under isoflurane anesthesia 3 hours after subcutaneous administration of vehicle, LPS (1 mg/kg), or poly-ICLC (1.6 mg/kg) (n=3/group). Plasma cytokine levels were evaluated by custom multiplex ELISA for IL-1β, IL-12, IFNγ (Quansys Biosciences, Logan, UT, USA) and cytometric bead array for IL-6 and TNFα (BD Biosciences, Franklin Lakes, NJ, USA).
Data Analysis
Researchers were blinded to treatment during analyses. Significance (P<0.05) was determined using Student's t-test or one-way analysis of variance (ANOVA) with Bonferroni's post hoc test as noted using GraphPad Prism 5 software (GraphPad, La Jolla, CA, USA).
Results
Poly-ICLC Preconditioning Confers Neuroprotection in Experimental Models of Stroke In Vitro and In Vivo
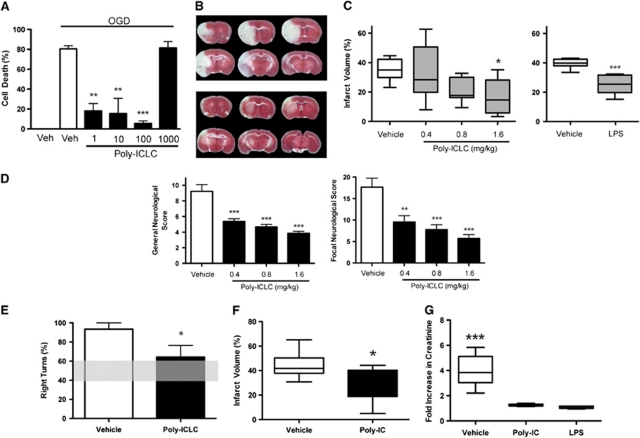
Mixed cortical cultures were used to test whether poly-IC preconditioning provides protection against modeled ischemia in vitro. Preconditioning with poly-ICLC (1 to 100 ng/ml) produced a marked attenuation of cell death after OGD compared with controls. Cells pretreated with poly-ICLC showed little cell death after OGD with the greatest protection exhibited at 100 ng/ml with 5.6±2.4% cell death compared with 80.6±3.1% cell death in vehicle-treated controls (Figure 1A). These data show that poly-ICLC preconditioning protects mixed cortical cells from OGD-induced death in vitro.
Figure 1.
Polyinosinic polycytidylic acid (poly-IC) preconditioning protects against both cerebral and renal ischemia-reperfusion injury. (A) Poly-ICLC preconditioning is neuroprotective in modeled ischemia in vitro. Mixed cortical cultures were preconditioned with poly-ICLC (1 to 1,000 ng/ml) 24 hours before 3-hour oxygen glucose deprivation (OGD). Cell death was determined by propidium iodide staining 24 hours after OGD. A representative example of three independent experiments is shown. Values are group means±s.e.m.; **P<0.01, ***P<0.001 versus vehicle control by analysis of variance (ANOVA) and Bonferroni's post hoc test. (B–F) Poly-ICLC preconditioning is neuroprotective in an experimental model of stroke in vivo. Mice were administered poly-ICLC (0.4, 0.8, or 1.6 mg/kg), lipopolysaccharide (LPS) (1 mg/kg) or appropriate vehicle subcutaneously 3 days before 45-minute middle cerebral artery occlusion (MCAO) (n=9 to 10/group). Extent of brain injury was assessed by measuring infarct volume 24 hours after MCAO. Neurologic outcomes were evaluated and motor deficits were quantified using the corner test. Representative images of 2,3,5 triphenyltetrazolium chloride-stained brain sections from vehicle-treated (B, top) and poly-ICLC-treated (B, bottom) animals. Collective infarct volume (C) is shown as group box and whisker (min/max) while neurologic score (D) and corner test score (E) values reflect means±s.e.m. (E) Shaded horizontal bar shows corner test score range for naive mice. For the extended recovery, experiment mice were administered poly-IC (1 mg/kg) or vehicle intraperitoneally 3 days before 45-minute MCAO (n=8 to 10/group). Damage to the brain was assessed by measuring infarct volume 72 hours after MCAO. Collective infarct volume (F) values represent group box and whisker (min/max); *P<0.05, **P<0.01, ***P<0.001 by Student's t-test and one-way ANOVA with Bonferroni's post hoc test. (G) Poly-IC preconditioning treatment improves renal function after ischemia. Mice were administered poly-IC (1.6 mg/kg), LPS (1.6 mg/kg) or vehicle intraperitoneally 48 hours before renal ischemia (n=6/group). Creatinine levels were measured in whole blood samples taken before and 48 hours after renal ischemia challenge. Values given as fold increase over baseline; ***P<0.001 by ANOVA and Bonferroni's post hoc test.
We next examined whether poly-ICLC preconditioning protects the brain from ischemic injury in an in-vivo mouse model of stroke. Poly-ICLC (0.4, 0.8, or 1.6 mg/kg) was administered 3 days before MCAO as this time point has shown consistent protection with other preconditioning stimuli including LPS. The infarct volume determined 24 hours after MCAO was significantly smaller in poly-ICLC-treated animals (1.6 mg/kg 17.64±3.89%) compared with vehicle-treated controls (35.29±2.41% P<0.05; Figures 1B and 1C). The degree of protection was similar to the known preconditioning agent, LPS (Figure 1C). As with other preconditioning stimuli, the reduction in infarct size occurred in a dose-dependent manner. Poly-IC administered at a higher dose (4 mg/kg) neither conferred neuroprotection nor exacerbated damage (vehicle 41.58±3.06%, poly-IC 43.72±2.96% P>0.05). Neurologic and motor deficits were attenuated in poly-ICLC preconditioned mice as evidenced by reduced neurologic scores and improved performance in the corner test. Preconditioned animals showed a decreased tendency to turn to the ipsilateral side compared with vehicle-treated controls (Figures 1D and 1E). Polyinosinic polycytidylic acid preconditioning has a lasting effect showing protection for 72 hours after MCAO (44.1±3.70% vehicle versus 29.1±4.48% poly-IC; Figure 1F). Thus, as hypothesized, poly-ICLC treatment before exposure to ischemia significantly reduced injury in both in-vitro and in-vivo models of cerebral ischemia.
Poly-IC Preconditioning Protects from Renal Ischemic Damage
We hypothesized that poly-IC preconditioning would also shield the kidneys from ischemic damage. To test this, mice were preconditioned with LPS, poly-IC, or vehicle 2 days before bilateral renal ischemia. Serum creatinine levels were assessed as a measure of kidney function 2 days after reperfusion and compared with baseline samples taken before ischemia. As with LPS, creatinine levels were significantly attenuated (74% reduction) in poly-IC preconditioned mice (1.26±0.036 fold increase) compared with vehicle (3.98±0.523 fold increase; P<0.001; Figure 1G). Thus, poly-IC preconditioning protects from functional deficits incurred in the setting of renal ischemia.
Poly-ICLC Treatment Elicits Reduced Systemic Plasma Cytokine Levels Compared with Lipopolysaccharide
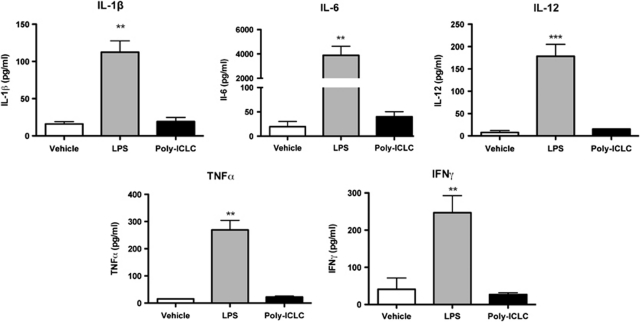
The known pro-inflammatory properties of LPS limit its use in a therapeutic setting, despite the fact that LPS preconditioning induces ischemic tolerance in multiple organs. To test whether preconditioning doses of poly-ICLC elicits systemic cytokines, we compared plasma cytokine levels 3 hours after administration of protective doses of poly-ICLC or LPS. Mice treated with poly-ICLC had significantly decreased plasma levels of IL-1β, IL-6, IL-12, TNFα and IFNγ compared with mice treated with LPS (P<0.01; Figure 2). Thus, poly-ICLC provides protection without the robust and considerably detrimental inflammatory response observed after LPS treatment.
Figure 2.
Poly-ICLC treatment resulted in reduced systemic plasma cytokine levels at 3 hours after administration compared with lipopolysaccharide (LPS) treatment. Plasma cytokine levels were measured from samples taken at 3 hours after subcutaneous injection of vehicle, LPS (1 mg/kg), or poly-ICLC (1.6 mg/kg) (n=3/treatment). Plasma cytokine levels were evaluated by custom multiplex ELISA for IL-1β, IL-12, and IFNγ and cytometric bead array for IL-6 and TNFα. Cytokine levels are significantly lower in poly-ICLC-treated animals compared with LPS treatment: **P<0.01, ***P<0.001 by analysis of variance (ANOVA) and Bonferroni's post hoc test.
Discussion
We have discovered that preconditioning with poly-IC induces tolerance to both cerebral and renal ischemic injury. We show decreased tissue damage after cerebral ischemia and improved function based on neurologic testing and measurement of creatinine levels, respectively. Numerous patients at risk of ischemic damage due to surgical procedures would benefit from antecedent treatment that induces protection against ischemia-reperfusion injury. In addition to the high risk of ischemic injury after cardiac surgery, cognitive dysfunction after major non cardiac surgery affects ∼40% of patients (Monk et al, 2008). Importantly, poly-IC preconditioning may provide a benefit to patients who undergo major surgery by reducing ischemic damage and improving functional outcomes.
Additionally, prophylactic ischemic tolerance may protect organs from transplant ischemia-reperfusion damage that is inherent in this procedure. Despite advances in transplant technologies there remains high demand for transplanted organs and the ability to use organs under suboptimal conditions, such as prolonged ischemia, could increase the available organ pool. The duration of ischemia before transplant predicts long-term kidney failure in humans (Salahudeen et al, 2004). Prophylactic ischemic preconditioning was reported to improve viability of transplanted organs such as liver and kidney (Ambros et al, 2007). We believe that pharmacological preconditioning using poly-IC administration offers a more practical therapeutic approach to protect transplant organs from ischemia-reperfusion injury although further studies are needed to investigate this potential.
We show, compared with preconditioning doses of LPS, poly-ICLC produces a very modest pro-inflammatory plasma cytokine response, which lowers the risk of toxicity and thereby offers a more favorable approach to generate ischemic tolerance. Lipopolysaccharide preconditioning requires TNFα—an inflammatory cytokine not strongly induced by poly-ICLC (Rosenzweig et al, 2007). This suggests that poly-ICLC may work through an entirely novel mechanism of protection that is TNFα independent. One candidate mediator of ischemic protection may be type-1 interferon, which is induced by poly-ICLC (Markosian and Hyde, 2005) and has previously been shown to be neuroprotective (Marsh et al, 2009).
Poly-ICLC as a clinical-stage therapeutic being evaluated for multiple indications, looks promising as a safe and effective preconditioning agent for ischemic injury. More than two-dozen phase I/II clinical trials have been initiated with poly-ICLC for a wide range of indications including cancer, AIDS, malaria, hepatitis, multiple sclerosis, and viral infection (Markosian and Hyde, 2005; Rosenfeld et al, 2010). Hundreds of patients have been safely treated with poly-ICLC 2 to 3 times per week for years; therefore, the use of poly-ICLC as a prophylactic treatment for patients at risk of ischemic damage holds true clinical potential.
Acknowledgments
The authors would like to thank Valerie Conrad and Tao Yang for their excellent technical support.
Dr Andres M Salazar is CEO of and owns stock in Oncovir, which is developing poly-ICLC (Hiltonol).
Footnotes
This work was supported by funding from the National Institutes of Health–National Institute of Allergy and Infectious Diseases T32A107472 and National Institute of Neurological Disorders and Stroke NS050567 and NS062381.
References
- Ambros JT, Herrero-Fresneda I, Borau OG, Boira JM. Ischemic preconditioning in solid organ transplantation: from experimental to clinics. Transpl Int. 2007;20:219–229. doi: 10.1111/j.1432-2277.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–1433. doi: 10.1161/STROKEAHA.107.502989. [DOI] [PubMed] [Google Scholar]
- Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–648. doi: 10.1080/01616412.1997.11740874. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Fukada J, Morishita K, Ingu A, Kawaharada N, Fujisawa Y, Hasegawa T, Abe T. Comparative study of the effect on clinical outcome of the use of an open circuit and the use of a closed circuit in cardiopulmonary bypass for a graft replacement of the descending thoracic or thoracoabdominal aorta. Surgery Today. 2004;34:11–15. doi: 10.1007/s00595-003-2639-7. [DOI] [PubMed] [Google Scholar]
- Heemann U, Szabo A, Hamar P, Muller V, Witzke O, Lutz J, Philipp T. Lipopolysaccharide pretreatment protects from renal ischemia/reperfusion injury: possible connection to an interleukin-6-dependent pathway. Am J Pathol. 2000;156:287–293. doi: 10.1016/S0002-9440(10)64729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markosian M, Hyde RM. Oligonucleotides and polyribonucleotides: a review of antiviral activity. Antivir Chem Chemother. 2005;16:91–102. doi: 10.1177/095632020501600202. [DOI] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RH, Honeycutt E, Patel UD, Lopes RD, Shaw LK, Glower DD, Harrington RA, Califf RM, Sketch MH., Jr Impact of recovery of renal function on long-term mortality after coronary artery bypass grafting. Am J Cardiol. 2010;106:1728–1734. doi: 10.1016/j.amjcard.2010.07.045. [DOI] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MR, Chamberlain MC, Grossman SA, Peereboom DM, Lesser GJ, Batchelor TT, Desideri S, Salazar AM, Ye X. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro Oncol. 2010;12:1071–1077. doi: 10.1093/neuonc/noq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig HL, Minami M, Lessov NS, Coste SC, Stevens SL, Henshall DC, Meller R, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning protects against the cytotoxic effects of TNFa after stroke: a novel role for TNFa in LPS-ischemic tolerance. J Cereb Blood Flow Metab. 2007;27:1663–1674. doi: 10.1038/sj.jcbfm.9600464. [DOI] [PubMed] [Google Scholar]
- Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004;65:713–718. doi: 10.1111/j.1523-1755.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]