Abstract
Muscle-invasive bladder cancer is a deadly condition in dire need of effective new treatments. This unit contains a description of mouse models suitable for the evaluation of potential new therapies. Included is a genetically engineered mouse model of bladder cancer generated by the delivery of an adenovirus expressing Cre recombinase into the bladder lumen. Also described is an orthotopic mouse model created by the instillation of human bladder tumor cells into the bladder lumen of immune deficient mice. Protocols are also provided on the use of these models for the preclinical evaluation of new chemical entities, with mTOR inhibitors shown as an example.
Keywords: bladder cancer, GEM mouse models, orthotopic models, mTOR inhibition, preclinical studies
INTRODUCTION
The American Cancer Society estimates that in 2008 nearly 70,000 new cases of bladder cancer were diagnosed in the U.S., resulting in 14,000 deaths. These figures indicate that bladder cancer is now the fourth most common cancer among men and the eighth most common in women (Jemal et al., 2005). Most bladder cancers are classified as transitional cell carcinoma (TCC), originating in the bladder urothelium (Dinney et al., 2004; Eble et al., 2004; Wu, 2005; Mitra and Cote, 2009). Notably, bladder cancer arises as two distinct subtypes, a papillary form and an invasive subtype, each of which has distinct pathologies and clinical outcomes. While the former has a favorable prognosis, the survival rate for muscle-invasive bladder cancer is ~50% at 5 years. Currently, the only effective treatment for muscle-invasive bladder cancer is cystectomy, or surgical removal of the bladder, significantly affecting the quality of life. Given the need for new therapies, animal models have been developed to screen new chemical entities to determine their therapeutic potential for treating bladder cancer. Described in this unit are genetically engineered mouse models of invasive bladder cancer and complementary orthotopic models of human bladder cancer. The unit also contains examples as to how these models may be employed for drug screening.
Detailed in this unit are two distinct approaches for generating animal models of bladder cancer. One is the creation of a genetically engineered model created by the delivery of an adenovirus expressing Cre recombinase into the bladder lumen (see Basic Protocol 1). In this case, the targeted delivery of an adenovirus expressing Cre recombinase allows for precise deletion of targeted genes within the bladder epithelium (Puzio-Kuter et al., 2009). The second protocol describes an orthotopic model of human bladder cancer in immunodeficient mice that is generated by placement of cancer cells into the host bladder (see Basic Protocol 2; Chin et al., 1991). Basic Protocol 3 describes the use of these models for evaluating potentially novel treatments for this condition using, as an example, rapamycin, an mTOR inhibitor (Kinkade et al., 2008; Puzio-Kuter et al., 2009).
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) or must conform to governmental regulations regarding the care and use of laboratory animals.
STRATEGIC PLANNING
As the precise number of mice required for each study group will vary depending on the consistency of the phenotype and the efficacy of the drug treatment, the group size must be determined experimentally for each particular set of conditions and goals. However, a minimum of ten mice should be employed for each experimental condition. There are two general approaches for initiating treatment, with the selection depending on the research tools available for tumor monitoring and the experimental endpoints. For example, a study designed to study the effects of an experimental treatment on early bladder lesions may be difficult to monitor without access to imaging equipment. In general, treatment initiation should be standardized on the basis of tumor size (i.e., quantified by in vivo imaging) or the onset of initial tumor formation (i.e., a standard time after delivery of adeno-Cre virus). The dose and administration schedule of test agents should be evaluated systematically for each treatment model and may vary depending on the model being utilized. It is advisable to begin with a toxicity study followed by a target inhibition/activation study where efficacy is examined over time in the bladder (Kinkade et al., 2008; Puzio-Kuter et al., 2009).
GENETICALLY ENGINEERED MOUSE MODELS OF BLADDER CANCER
This protocol describes the use of an adenovirus to introduce Cre recombinase specifically in the bladder lumen of mice harboring relevant floxed alleles to achieve gene recombination selectively in the bladder epithelium. The Cre/loxP system is based on the bacteriophage P1 wherein Cre recombinase acts on palindromic sequences called loxP sites that have been genetically engineered into the specific sites in the mouse genome. Cre recombinase can then excise the genomic sequence between two loxP sites. Therefore, mouse alleles containing loxP sites can be used for introducing mutations, or for a temporally or spatially controlled gene knockout.
The aim of this work is to model muscle-invasive bladder cancer, the most lethal form of the disease, and most in need of new therapeutic approaches. Bladder cancer is usually diagnosed by hematuria (blood in the urine), polyuria (frequent urination), pain during urination, and/or lower back pain. Genetic alterations relevant for human muscle-invasive bladder cancer include p53, PTEN, and RB mutations (Dalbagni et al., 1993; Dinney et al., 2004; Cordon-Cardo, 2008; Knowles, 2008; Mitra and Cote, 2009). In the context of drug discovery, murine models that mimic molecular mechanisms of human disease have proven to be of value in predicting clinical utility, and thus they have been a focus of study in this area (Puzio-Kuter et al., 2009). Future studies on the molecular mechanisms of bladder cancer are likely to identify other genes of interest that could be evaluated using these approaches.
Materials
Dulbecco’s modified Eagle medium (D-MEM; Invitrogen, cat. no. 11995)
Hexadimethrine bromide (Sigma, cat. no. 107689)
Adenovirus expressing Cre-recombinase (University of Iowa Vector Core Facility pacAd5, high titer (4 × 1011 pfu/ml) (http://www.uiowa.edu/~gene)
Mice (with floxed alleles)
- Floxed mouse alleles (R26R reporter allele is used to monitor recombination efficacy and specificity):
- Conditional alleles for Pten (Ptenflox/flox, C57Bl6;129SVJ) (Lesche et al., 2002) and p53 (p53flox/flox, FVB;129SJv) (Jonkers et al., 2001) obtained from the NCI Mouse Models of Human Cancer Consortium repository (http://mouse.ncifcrf.gov/)
- R26R reporter allele (GT(ROSA)26Sortm1Sor) (C57Bl6;129SVJ) (Soriano, 1999) obtained from the Jackson Laboratory Induced Mutant Resource (Bar Harbor, Maine)
Isoflurane (or appropriate anesthetic)
2% Chlorhexidine solution (Sigma)
Optimal cutting temperature (OCT) compound (Tissue-Tek, cat. no. 4583)
4% paraformaldehyde/phosphate-buffered saline (PBS)
Phosphate-buffered saline (PBS; see recipe)
Staining solution (see recipe)
10% formalin
PBS-T (1 ml Tween 20 per 100 ml PBS)
Nuclear Fast Red (Vector Labs, cat. no. H3403)
50%, 70%, 95%, and 100% ethanol
Xylene
Clear Mount (American MasterTech, cat. no. MMCLEPT)
100-μl Hamilton glass syringe (Hamilton, cat. no. 81001)
30-G, ½-in. needles (BD, cat. no. 305106)
1-ml disposable tuberculin syringes (BD, cat. no. 309602)
Sterile surgical area complete with operating board
Precision vaporizer and nose cone for administering isoflurane (available at institutional animal care facility; e.g., Kent Scientific)
Animal hair clippers
- Dissecting tools (Fine Science Tools) including:
- Surgical scissors
- Serrated tissue forceps
- Dressing forceps
Dissecting microscope
5-0 coated, braided silk sutures with attached needle (Seneture, cat. no. S1173)
Wound clip applicator (BD, cat. no. 427630)
Autoclip 9-mm wound clips (BD, cat. no. 427631)
Animal warmer (such as model TR-200 from Fine Science Tools)
VWR Superfrost slides
37°C humidified chamber
NOTE: For optimal results, use high-titer virus that has been divided into aliquots at the time of its initial preparation and not subject to freeze-thawing.
NOTE: The National Cancer Institute (NCI) at Frederick Web site lists general and safety precautions for adenovirus. See http://home.ncifcrf.gov/ehs/uploadedFiles/ISM-193.pdf.
Prepare adenovirus
1. In a sterile hood, combine 40 μl DMEM, 10 μl (8 mg/ml) hexadimethrine bromide (a cationic polymer used to increase the efficiency of virus infection), and 50 μl (titer 4 × 1011 pfu/ml) adenovirus.
Maintain adenovirus on dry ice until right before use.
This will provide sufficient material for injecting five mice.
2. Draw the adenovirus combination into a 100-μl Hamilton syringe. Fit the 30-G ½-in. needle on the syringe and eliminate bubbles from the tip of the needle by holding the syringe vertically with the needle pointing up and gently pushing on the plunger.
3. Attach a second 30-G ½-in. needle onto an empty 1-ml disposable tuberculin syringe to be used for bladder evacuation.
Prepare animals
4. Prepare a sterile surgical area complete with operating board.
5. Anesthetize mice with isoflurane (4% induction, 2% maintenance). Administer isoflurane through a nose cone as an inhalant with oxygen from a precision vaporizer. Monitor the mice for depth of anesthesia by testing the rear foot reflexes before any incision is made. Continue observing respiratory pattern throughout the surgical procedure.
6. Using hair clippers, remove all hair from the lower abdomen.
7. Disinfect the surgical site with 2% chlorhexidine solution.
Instill adenovirus
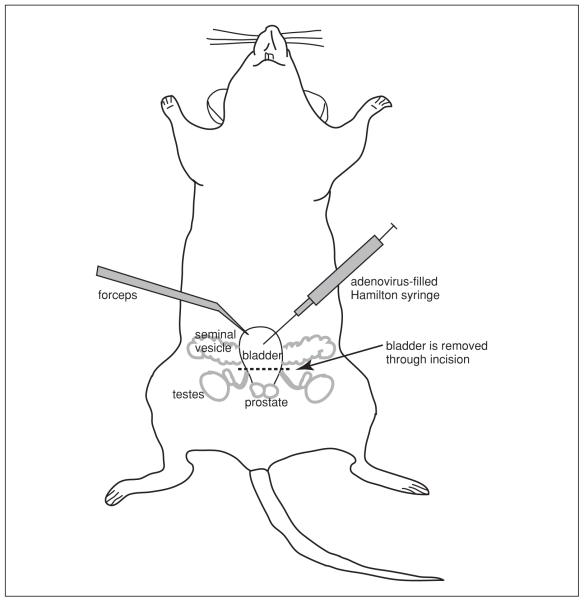
8. Grasp the skin just above the abdominal fat pad with forceps. Using sharp surgical scissors make a 1-cm incision through the skin (Fig. 14.14.1). Carefully cut the outer layers (fat and muscle) so as to avoid damaging underlying abdominal contents.
Figure 14.14.1.
Surgical procedure for instilling cells into the mouse bladder.
9. Locate the bladder, which is low and anterior in the abdomen.
10. Using serrated forceps, gently grasp with one hand the dome of the bladder, the region opposite the base of the bladder, and use the other hand to evacuate the bladder of urine using the empty syringe. Withdraw the syringe needle.
It is important to hold the bladder steady throughout this process to avoid unnecessary trauma to the organ and to ensure the hole used for bladder evacuation can be relocated.
11. Through the same hole used to evacuate urine, insert the needle from the Hamilton syringe containing the adenovirus.
12. Instill 5 to 10 μl of the adenovirus into the bladder.
The final amount will depend on the distensibility of the bladder. While the volume of a mouse bladder is more than 10 μl, there will always be some urine remaining, even after evacuation. Therefore, 5 to 10 μl is a safe and effective amount of solution for injection. The goal of this procedure is to adequately bathe the epithelium in adenovirus solution. However, overfilling of the bladder may cause reflux into the ureters or spillage into the abdominal cavity upon removal of the needle.
13. Gently allow the bladder to fall back into the abdominal cavity to its original location.
14. Close muscle and fascial layers in a single step with 5-0 silk sutures.
15. Close skin using the wound clip applicator and wound clips.
Two wound clips should be sufficient assuming a 1-cm incision. Be sure that all abdominal contents are well contained by the surgical closure.
Administer post-operative care
16. Discontinue anesthesia and allow the mouse to regain consciousness.
17. Place the mouse on the animal warmer until it returns to normal body temperature (~20 min).
18. Monitor the animal’s overall health for ~2 hr post-surgery for signs of pain or of difficult or shallow breathing. Monitor the animals until they are fully ambulatory.
If the mouse continues to exhibit the abovementioned symptoms, begin by examining the incision for signs of improper/incomplete closure (i.e., signs of bowel or testicle protrusion). Consider additional oxygen administration through the vaporizer (anesthesia at 0% with the oxygen tank on).
Monitor tumor growth
19. At least weekly, evaluate mice for body score index to monitor health. Body scoring is accomplished by lifting the mouse by the base of the tail and noting its body condition by passing a finger over the sacroiliac bones. Body condition is typically scored on a scale of 1 to 5:
Muscle wasting (i.e., loss of weight/muscle mass) is advanced, fat deposits are absent, and bones are very prominent.
Bones are prominent. This suggests the mouse is becoming thin and its health is declining. Further decline of condition would warrant euthanasia.
Bones are palpable but not prominent. This is an optimal condition.
The mouse is well fleshed and bones are barely felt.
The mouse is obese and bones cannot be felt.
20. Monitor tumor growth once weekly and then at least twice weekly by palpation of the mouse abdomen for detection of bladder tumors as small as 0.5 cm. Tumors may also be monitored by live animal imaging using ultrasound, bioluminescence, and/or MRI (Olive and Tuveson, 2006).
Depending on the particular genetic deletions, tumors may be present as early as one month after instillation of virus. Tumor take rate should be ~95%. Dissection of mice and collection of tissues is described in the Support Protocol.
21. To monitor adenovirus infection efficiency, reporter mice (GT(ROSA)26Sortm1Sor) can be used to verify injection efficiency by monitoring β-galactosidase activity. To accomplish this:
Use bladder tissues fixedfor 1 hr in OCT.
Dry cryosections (12 μm) for 15 min and fix with ice-cold 4% paraformaldehyde/phosphate-buffered saline (PBS).
Incubate on ice for ~10 min.
Rinse slides three times, each time with PBS (use enough PBS to completely submerge the slides) for 5 to 10 min.
Add freshly prepared staining solution to each slide, and then incubate at 37°Cin a humidified chamber.
22. Keep slides in the 37°C humidified chamber until a blue color develops (3 hr to overnight).
23. Following color development, fix slides with 10% formalin, rinse two times with PBS-T (use enough PBS-T to completely submerge the slides) for 5 to 10 min each time. Counterstain with Nuclear Fast Red for 5 sec.
24. Dehydrate the slides through an alcohol gradient (50%, 70%, 95%, 2× 100%; each time for 5 min at room temperature) into xylene and coverslip with ClearMount.
Our results showed ~10% of the bladder epithelium infected, as evidenced by B-gal staining of R26R mice.
ORTHOTOPIC MURINE MODELS OF BLADDER CANCER
Described in this protocol is the generation of primary bladder tumors by instilling cancer cells directly into the bladder of an immunodeficient host. As this technique was developed using human bladder cancer cell lines, it is a complementary strategy to the genetically engineered model described in Basic Protocol 1. Cells can be “marked” using GFP or other reporter allele to readily detect tumor cells and to distinguish them from the host bladder epithelium.
Materials
Trypsin/EDTA (Fisher Scientific, cat. no. BP2474-100)
Bladder cancer cell line (from ATCC) in appropriate medium (see Table 14.14.1)
Appropriate medium (see Table 14.14.1)
Ice
8-week-old female, athymic nude mice (Taconic)
Isoflurane
Ophthalmic ointment (Vetropolycin, Pharmaderm)
2% chlorhexidine solution (Sigma)
Surgical lubricant (Fougera)
Commercial-grade phosphate-buffered saline (PBS; see recipe for 10×), 1×
Sterile surgical area with operating board
Precision vaporizer and nose cone for administering isoflurane (available at institutional animal care facility)
5-0 coated, braided silk sutures with attached needle (Seneture, cat. no. S1173)
Forceps (Dumoxel, cat. no. 11251-35)
24-G angiocatheter (Jelco, cat. no. 4073)
Plastic syringes (BD, cat. no. 309602)
100-μl Hamilton glass syringe (Hamilton, cat. no. 81001)
Animal warmer (Gaymar T/Pump or similar)
Additional reagents and equipment for standard tissue culture procedures including trypsinization and counting cells using trypan blue (Phelan, 2006)
Table 14.14.1.
Commercially Available Bladder Cancer Cell Lines
| Malignancy characteristics | Propagation | Source | Comments | |
|---|---|---|---|---|
| UM-UC-3 | “High-grade” IFN non-responsive Hypertriploid (modal chromosome number = 80) |
McCoy’s medium (Invitrogen) + 10% heat-inactivated FBS |
Urinary bladder (epithelium), male patient |
|
| KU-7 | “High-grade” IFN non-responsive |
DMEM + 5% FBS | ||
| MGH-U3 | “Low-grade” IFN responsive |
MEM +10% FBS | ||
| RT4 | “Low-grade” Transitional cell papilloma IFN responsive |
McCoy’s medium (Invitrogen) + 10% heat-inactivated FBS |
Urinary bladder (epithelium), male patient |
|
| T24 | Transitional cell carcinoma | McCoy’s Medium + 10% FBS |
Urinary bladder (epithelium), female patient |
Contains H-ras oncogene 19 hr generation time |
| J82 | Transitional cell carcinoma | Eagle’s Minimum Essential Medium + 10% FBS |
Urinary bladder (epithelium), male patient |
Contains H-ras oncogene |
| 5637 | Grade 2 carcinoma | RPMI-1640 medium +10% FBS |
Urinary bladder (epithelium), male patient |
pRB negative p16 positive |
| HT-1376 | Grade 3 carcinoma Tumorigenic in mice |
Eagle’s Minimum Essential Medium + 10% FBS |
Urinary bladder (epithelium) female patient |
|
| TCCSUP | Grade 4 transitional cell carcinoma |
Minimum essential medium (Eagle) in Earle’s BSS with non-essential amino acids and 1 mM sodium pyruvate +10% FBS |
Bladder neck (epithelium), female patient |
|
| HT-1197 | Tumorigenic in mice | Eagle’s Minimum Essential Medium + 10% FBS |
Urinary bladder (epithelium), male patient |
|
| SW 780 | Transitional cell carcinoma | Leibovitz’s L-15 medium +10% FBS |
Urinary bladder (epithelium), female patient |
Patient had pre-op chemotherapy (Thiotepa) 41% plating efficiency |
Prepare bladder cancer cells
1. Prepare 0.2% trypsin in 0.02% EDTA
2. Remove medium from the plate and remove the cells using the trypsin/EDTA solution as per standard tissue culture procedures (Phelan, 2006).
3. Remove the cells from the plate by pipetting up and down. Transfer the cells into centrifuge tubes.
It is important to prepare a single-cell suspension to facilitate accurate counting later on.
4. Rinse the cells by resuspending them in 10 ml of medium and centrifuge 10 min at 1500 × g, 4°C, to create a cell pellet.
5. Remove the medium, leaving the cell pellet at the bottom of the tube. Add another 10 ml of medium and resuspend the cells using a pipet.
6. Using a hemacytometer, count the number of living cells in 10 μl using trypan blue (Phelan, 2006).
7. Pellet the cells again as in step 4 and then resuspend them to a concentration of 1 × 105 cells per 20 μl.
8. Place cells on ice until use for up to 3 hr.
Prepare the animals
9. Prepare a sterile surgical area complete with operating board.
10. Anesthetize 8-week-old female nude mice with isoflurane (4% induction, 2% maintenance). Use an induction chamber in which mice are confined without restraint. Once adequately sedated, transfer the mice to the operating board where they are allowed to breathe only through a nose cone.
Adequate sedation is confirmed when the animals fail to react to a firm hind paw pinch.
11. Lightly apply ophthalmic ointment to each animal’s eyes to prevent them from drying and causing corneal damage.
12. Gently cleanse external urinary orifice with 2% chlorhexidine solution.
13. Place the mouse in the dorsal position.
Some investigators advocate placing the mouse in the ventral position (e.g., St. Clair et al., 1999).
14. Estimate the distance from the external urinary orifice to the bladder neck (average length is ~10 to 15 mm in a 20-g female mouse).
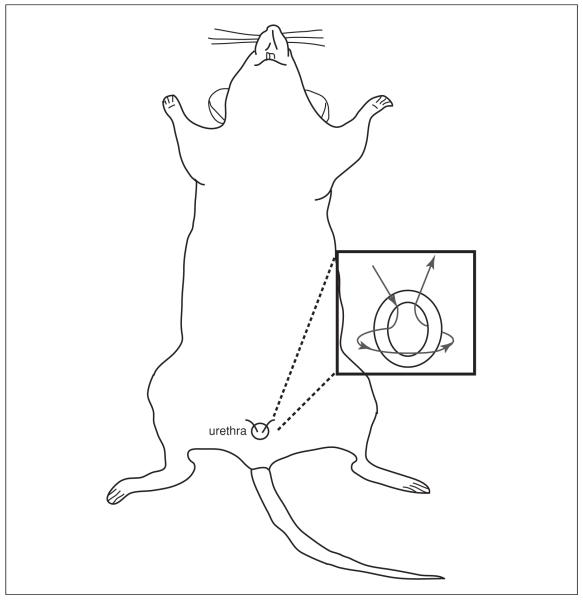
15. Place a superficial silk purse-string suture around urethral meatus (Fig. 14.14.2).
Figure 14.14.2.
Placement of a superficial silk purse-string suture around the urethral meatus.
16. Insert an empty plastic syringe into the angiocatheter.
17. Lubricate the tip of the angiocatheter with a small amount of surgical lubricant.
18. With one hand, carefully grasp the urethral meatus with the forceps, while using the other hand to advance the angiocatheter through the urethra and into the bladder.
Minimal resistance should be encountered. The tip of the catheter should be positioned so that it lies just beyond the neck of the bladder. In most mice, this means inserting the catheter to the hub. However, it is important to be aware that bladder perforation may occur if the catheter is inserted too far so great caution must be exercised if increasing resistance is encountered.
19. Draw back gently on the syringe to evacuate urine from the bladder. Remove the catheter.
This step also confirms that the catheter is in the correct position.
Pretreat the bladder
Depending on the cell line, pretreatment may not be required (Table 14.14.2). In general, those cell lines that are more aggressive can be instilled without pretreatment, but this should be determined empirically. Pretreatment with trypsin disrupts the cell membranes in the bladder epithelium, increasing the infectivity of the instilled cells.
Table 14.14.2.
Take Rates of Bladder Cancer Cells With and Without Bladder Pretreatment
| Host/cells instilled |
Bladder treatment |
Number of cells instilled |
Tumor take rate | Detection method | Reference |
|---|---|---|---|---|---|
| RT4 and EJ in athymic nude mice |
None versus electrocautery |
2 × 106 in 100 μl |
With cautery: RT4-50% EJ- 55% Without:RT4- 50% EJ- 42% |
Microscopically after sacrifice |
Ahlering, 1987 |
| KU-7 and UM-UC-2 cells in athymic nude mice |
0.2% trypsin in EDTA, PBS |
1 × 107 in 100 μl |
100% (KU-7), 80% (UM-UC-2) |
Microscopically after sacrifice |
Watanabe, 2000 |
| BIU-87 in athymic nude mice |
0.1 N HCl 0.1 N KOH PBS |
1.5 × 106 cells in 150 μl |
93% (overall), 95% (subgroup) |
MRI, gross pathology, light microscopy |
Chong, 2006 |
| KU-7 in athymic nude mice |
PBS | 2 × 106 in 50 μl |
96% | Bioluminescence imaging, microscopically after sacrifice |
Hadaschik, 2007 |
20. Instill 20 μl of 2% trypsin into the bladder using a second lubricated angiocatheter. Remove the angiocatheter from the bladder and maintain the mouse under anesthesia for a 20-min dwell time.
Dwell time refers to the amount of time the cells are in the bladder.
21. With a third lubricated angiocatheter, rinse the bladder with 50 μl 1× PBS, and then remove the catheter.
Instill cells into the bladder
22. With a fourth lubricated angiocatheter (with a 100-μl Hamilton syringe attached), instill 1 × 105 bladder cancer cells into the bladder.
While the actual final volume depends on the cell concentration, it should not exceed 60 μl to avoid reflux into the ureters.
23. To ensure adequate dwell time, tie down the purse-string suture and maintain the mouse under anesthesia for 1.5 hr. During this time, maintain the mouse under 1.75% isoflurane anesthesia and monitor the animal frequently to ensure that breathing is regular and unlabored.
If breathing pattern changes, then decrease the anesthetic.
24. Remove the sutures and isoflurane.
Administer post-operative care
25. Given the prolonged anesthesia time, make certain the mice are allowed adequate recovery time with the warmer (~20 min). After this period, mice should have returned to near-normal activity. If mice appear sluggish, consider delivering oxygen directly to them through the anesthetic machine and/or prolonging the period they are allowed to recover with the warmer.
Monitor tumor take
26. Evaluate mice at least weekly for body score index to monitor health (see Basic Protocol 1, step 19 for body scoring index procedure).
27. Monitor tumor growth twice weekly by palpation of the mouse abdomen for detection of bladder tumors as small as 0.5 cm.
Tumors may be also monitored by live-animal imaging with ultrasound, bioluminescence, and/or MRI (Olive and Tuveson, 2006).
Dissection of mice and collection of bladder tissues is described in the Support Protocol.
Primary bladder tumors resulting from orthotopic implantation can develop as early as 2 weeks after instillation of cells, depending on the cell line. The time course of tumor development and take rate varies as a function of the cell line, as well.
USING MOUSE MODELS TO EVALUATE TREATMENTS FOR BLADDER CANCER
Genetically engineered mouse models and orthotopic tumor models provide complementary approaches for evaluating novel therapies for the treatment of invasive bladder cancer. As an example, this protocol describes the evaluation of the mTOR inhibitor, rapamycin, for the treatment of bladder tumors in genetically engineered p53flox/flox;Ptenflox/flox mice following instillation of adenovirus expressing Cre-recombinase (see Basic Protocol 1). Rapamycin is used because of the observation that mTOR signaling is frequently deregulated in invasive human and mouse bladder tumors (Puzio-Kuter et al., 2009). Furthermore, in this study it is reported that rapamycin treatment of mice with muscle-invasive bladder tumors completely suppresses tumorigenesis.
Materials
Rapamycin (see recipe)
Animal balance
1-ml plastic syringes, (BD, cat. no. 309602)
26-G, 3/8-inch needles (BD, cat. no. 305110)
Additional reagents and equipment for in vivo imaging using bioluminescence, ultrasound, or MRI imaging (Olive and Tuveson, 2006) and euthanizing the mice (Donovan and Brown, 2006)
1. Perform initial toxicity assays to determine optimal dosing schedules and dosage.
The toxicity assays should consist of a dose escalation study in which the maximum tolerated dose is the highest dose at which there is acceptable compromise to animal health after administration of multiple doses.
For additional discussion of dose and dosing schedule generation, see Kinkade et al. (2008).
2. Choose relevant endpoints and outcomes and pre-evaluate them in pilot studies.
Define the experimental endpoints based on the goals of the study. Typical outcomes are based on objective measures of tumor size (volume, weight), as well as biologic measures including semi-quantitative assessment of histology, proliferation index (the percentage of cells that stain positive when treated with Ki67), and measures of target and/or pathway activation (immunostaining for target protein expression/activation).
3. At the initiation of treatment (using either Basic Protocol 1 or 2), randomize the mice into treatment and control groups.
Randomization is essential as it controls for variation in materials and technique, genetic variation among litters, and other inherent variables.
4. Weigh mice daily or prior to each treatment.
Daily weights are used primarily for determining dose, although they can also serve as a measure of animal health.
5. Using 1-ml syringes equipped with a 26-G needle, administer rapamycin at 10 mg/kg (i.p.), an optimal dose in most instances (see Kinkade et al., 2008; Puzio-Kuter et al., 2009). Treatment can continue for 5 consecutive days, followed by 2 “rest” days (Kinkade et al., 2008; Puzio-Kuter et al., 2009).
6. If available, monitor mice by in vivo imaging during the treatment phase using bioluminescence, ultrasound, or MRI imaging (Olive and Tuveson, 2006).
7. At the conclusion of treatment, euthanize mice by CO2 asphyxiation (Donovan and Brown, 2006). See the Support Protocol for details about tissue/data collection.
ANALYSES AND TISSUE COLLECTION OF MICE FOLLOWING BLADDER CANCER TREATMENT EVALUATION
At the end of the in vivo portion of the study, the mice are sacrificed and tissues collected for analyses. Tissue collection following sacrifice should be conducted expeditiously to prevent protein degradation that could compromise study results. To achieve this it is important to organize laboratory supplies prior to beginning the dissection procedure. In general, certain methods of tissue collection may lend themselves to analysis of the effects of certain compounds better than others. For example, when evaluating compounds that target a specific molecule, care must be taken to ensure that inhibition of the target can be demonstrated (e.g., formalin-fixed tissue for analysis by immunohistochemistry or snap-frozen tissue for analysis by immunoblotting).
Materials
Phosphate-buffered saline (PBS; see recipe for 10× stock), 1×
10% buffered formalin (Fisher, cat. no. SF93-4)
4% paraformaldehyde (PFA; Sigma, cat. no. 158127)
Optimal cutting temperature (OCT) compound (Tissue-Tek, cat. no. 4583)
Liquid nitrogen
Processing cassettes (Fisher, cat. no. 15-182-705)
Cryogenic vials (VWR, cat. no. 479-081)
EDTA precoated tubes (Greiner Bio-One, cat. no. 450403)
22-G 1-in. needle (BD, cat. no. 305768)
3-ml disposable syringe (BD, cat. no. 309585)
1.5-ml tubes
- Dissecting tools including: (Fine Science Tools)
- Fine forceps
- Surgical scissors
- Disposable scalpel (Bard-Parker, cat. no. 371611)
Petri dishes
Dissecting microscope
Additional reagents and equipment for euthanizing the mice (Donovan and Brown, 2006)
Prepare materials and animal prior to dissection
1. Label all tubes, cassettes, and vials with the animal number and tissue to be collected.
A typical list may include tail, bladder tumor, lymph nodes, lung, liver, and plasma. However, the organs collected should be dictated by the tissues known to be affected by the disease and treatment.
2. Prior to sacrifice, record the body condition of the mouse (see Basic Protocol 1, step 19) and the body weight.
3. Sacrifice the mouse by CO2 asphyxiation (Donovan and Brown, 2006) or other institutionally approved procedure.
Collect tissues
Collect plasma
4. Collect the plasma as follows:
Using a 3-ml syringe equipped with a 22-G 1-in. needle, insert needle just to the left of the sternum approximately one fingerbreadth below the clavicle.
Withdraw ~5 ml blood. Remove the needle from the syringe and transfer the blood to an EDTA precoated tube.
Invert the tube several times to mix and place it on ice.
Centrifuge the tube 15 min at 1500 × g, 4°C.
Transfer the plasma supernatant layer to a 1.5-ml tube. Store the sample indefinitely at −80°C.
Plasma can be stored on ice until the end of the dissection period, but not longer than 1 hr.
Collect tissue for verification of genotype
5. Collect a 0.5-cm length of tail for confirmatory genotyping.
This is particularly important when using genetically engineered models. Store tail segments at −80°C.
Perform bladder dissection
6. Using one hand, grasp the body wall with forceps. With the other hand, make a horizontal incision in the abdominal wall ~2-cm in length using surgical scissors. Using the scissors, incise from the center of the original opening to the sternum taking care not to damage underlying organs. Note the general appearance of the abdominal organs.
7. Locate the bladder and record its condition. Remove the bladder at the base using the surgical scissors and transfer it to a 100-mm petri dish containing 1× PBS (enough PBS to cover the bottom of the petri dish). Clean the specimen under the dissecting microscope. Photograph the bladder and record its size/volume and weight. Measure volume either by water displacement or by measurement of the tumor dimensions (length × width × height = volume).
Other tissues of interest may also be collected and fixed in 10% formalin.
Fix the tissues
8. Bladder (without tumor): Fix 2 hr to overnight in 10% formalin.
Tissues may alternatively be fixed in 4% PFA. In general, formalin is best for histological preservation while PFA may be preferred for immunofluorescence analyses.
9. Collect bladder tumors: Divide the bladder into three parts. Section the lower-most portion closest to the urethra (which should contain normal or pre-neoplastic tissue) and place in 10% formalin overnight for histological and immunohistochemical analyses. Place the middle portion in OCT compound and store at −80°C for future immunohistochemical analysis. Snap-freeze the distal portion of the bladder in liquid nitrogen for later preparation of protein, genomic DNA, or RNA for analyses.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see SUPPLIERS APPENDIX.
Phosphate-buffered saline (PBS)
10× stock solution, 1 liter:
80 g NaCl (J. T. Baker, cat. no. 3624-05)
2.0 g KCl (J. T. Baker, cat. no. 3040-05)
14.4 g Na2HPO4 (EM Science, cat. no. SX0720-5)
2.4 g KH2PO4 (J. T. Baker, cat. no. 3246-05)
Rapamycin stock solution
Dissolve rapamycin (LC Labs, cat. no. R-5000) in 100% ethanol at 25 mg/ml. To make 4 ml of working stock, add 200 μl of 25 mg/ml rapamycin solution to 3.8 ml of vehicle (see recipe).
25 mg/ml rapamycin can be stored up to 1 year at −80°C.
Staining solution (final concentrations)
1M MgCl2 (1.3 mM)
50 mM Na2HPO4/50 mM NaH2PO4 (5 mM)
25 mg/ml X-gal (1 mg/ml)
20% IGEPAL (Sigma-Aldrich, cat. no. I8896; 0.02%)
10% sodium deoxycholate (Sigma-Aldrich, cat. no. D6750; 0.01%)
1× phosphate-buffered saline (PBS; see recipe), pH 7.4
Prepare fresh
Vehicle for dilution of rapamycin (5.2% Tween 80, 5.2% PEG400 in sterile water)
35.84 ml of sterile water
2.08 ml Peg400 (Fluka, cat. no. 81170)
2.08 ml Tween 80 (Sigma, cat. no. P8074)
Mix using an end-over-end or rotating shaker
Store up to 1 week at 4°C
Discard if there are signs of contamination (e.g., cloudiness, particulates, etc.).
COMMENTARY
Background Information
Although the spontaneous occurrence of carcinomas in mice is rare, they are an attractive species for studying human cancer because of the ease of manipulating their genome. Indeed, as a consequence of advances in gene targeting techniques in recent years (Branda and Dymecki, 2004), mouse models based on targeted alterations of the genome have become increasingly more common and sophisticated. Conditional, as well as inducible deletion of tumor suppressor genes, or activation of oncogenes in tissue-specific compartments, have become routine procedures. This has led to the emergence of a new generation of mouse models appropriate for drug development and translational studies. Generally speaking, these genetically modified mouse models enable the investigation of cancer phenotypes in the context of the tumor microenvironment and an intact immune system, distinguishing them from orthotopic or xenograft models in which exogenous tumor cells or tissues are typically grown in immune-deficient mice.
Despite these technological advances, there remain significant differences between cancer development in humans and in genetically engineered mice (Rangarajan and Weinberg, 2003). The question, however, is whether such differences limit the utility of these animal models in studying human cancer. If the intent is to study the evolution of the disease as it occurs in humans, then the differences can be significant. However, if the aim is to investigate the molecular mechanisms underlying a cancer phenotype based on a particular genetic alteration, or to facilitate biomarker analyses, then genetically engineered mouse models can serve as surrogates for the human condition. Indeed, analyses of gene expression changes in relatively homogeneous mouse models can help to identify biomarkers that may not be evident in more heterogeneous human cancer specimens (Ouyang et al., 2008).
The relative homogeneity of cancer development in mouse models, relative to humans, is of considerable benefit for compound testing. Mouse models based on the perturbation of specific molecular pathways can enable a detailed interrogation of the involvement of such pathways in tumorigenesis, as well as investigations of potential therapeutics that target them specifically. Generally, cancer develops more uniformly and more rapidly in mouse models which, from a pharmacological standpoint, can be highly advantageous for compound testing (Singh and Johnson, 2006). Moreover, mouse models provide ready access to cancer tissue at all disease stages, greatly facilitating pharmacokinetic and pharmacodynamic studies (Olive and Tuveson, 2006; Singh and Johnson, 2006). Such studies provide valuable information about whether a given agent is effective in the target tissue. Finally, the ability to monitor the consequences of drug delivery by in vivo imaging of mouse models (Fomchenko and Holland, 2006; Olive and Tuveson, 2006) makes possible a quantitative assessment of the consequences of drug action for tumor development.
Critical Parameters
While the procedures described in this unit require some expertise, they become routine with practice. Aseptic technique is absolutely critical to ensure that mice survive the surgical procedures. As such, surfaces must be properly disinfected prior to initiation of any study. Sterile gloves should always be worn by the investigator and hands cleaned with 70% ethanol between animals. Tools and solutions should be sterilized, with segregation of tools used for surgeries and dissection protocols.
Given the variability in the time course to tumor development, daily monitoring of mice is necessary. Signs of animal distress include lethargy, hunching, decreased grooming, weight loss, ascites (can be detected as weight gain in conjunction with a distended abdomen), bleeding (including blood in the urine), infection of wound or tumor, impaired ability to consume food or water, and vocalization as a result of pain. Such signs are indicative of poor health and should be used to decide whether a mouse should be sacrificed prior to the study conclusion.
Troubleshooting
The production of consistent mouse models of bladder cancer may be complicated by poor animal viability peri-operatively, development of infection post-operatively, and lack of tumor development. See Table 14.14.3 for suggested solutions in case anticipated results are not obtained.
Table 14.14.3.
Troubleshooting Genetically Engineered and Orthotopic Mouse Models of Bladder Cancer
| Genetically engineered model | Orthotopic model | |
|---|---|---|
| Mice die during surgery |
Most likely a result of improper anesthesia control. Monitor breathing of mouse throughout any procedure. If breathing becomes irregular or excessively labored, remove mouse from nose cone until breathing becomes more normal. |
|
| Mice develop infection |
Practice aseptic technique when carrying out any surgical procedure. Make sure to properly disinfect mouse and surgical tools. It is recommended that mice be placed in a clean cage following surgical procedures. |
|
| Tumor does not develop |
Virus infectivity compromised due to lower than reported titer or to prolonged time at room temperature. Confirm titer and use virus promptly once it is thawed. Virus leaked from the bladder following delivery. |
Cell line is not adequately aggressive to ensure consistent take. If not carried out previously, consider pretreatment with trypsin (as outlined in Basic Protocol 2). Inadequate dwell time within the bladder. Inadequate number of cells instilled. |
| Tumor is not of epithelial origin |
Virus not delivered properly to bladder lumen. Ensure correct technique when delivering adenovirus to the bladder in order to minimize infection of alternate tissues and/or organs. |
N/A |
Anticipated Results
Adenovirus-injected mouse model
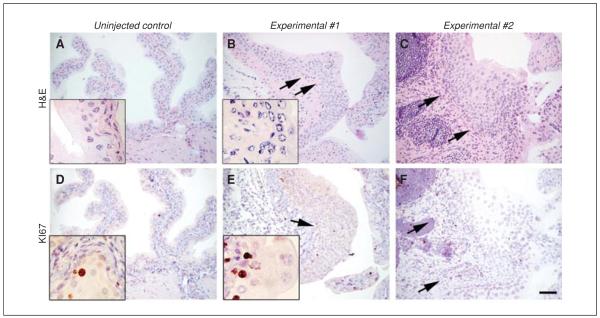
The time course of tumor development in this model will vary depending on the mouse alleles used and the experimental parameters. In the case of p53floxP/floxP ; PtenfloxP/floxP mice, tumors generally develop by 12 weeks, with more than 90% of experimental animals harboring deletions of both alleles developing tumors by 6 months of age (Puzio-Kuter et al., 2009). Figure 14.14.3 shows stained sections from bladders of control and experimental mice (Seager et al., 2009).
Figure 14.14.3.
Modeling early-stage bladder cancer in mutant mice. (A to C) Representative H&E-stained sections from bladders of a control mouse (uninjected p53flox/flox; Ptenflox/flox) or two representative experimental mice (Adeno-Cre–injected p53flox/flox; Ptenflox/flox) 6 weeks subsequent to delivery of Adeno-Cre (or mock injection for the control). Shown are representative histologic sections from a total of 10 mice in each of the experimental and control groups. Note that the experimental, but not control, mice have a dysplastic and expanded epithelium (arrows) as well as abnormal stroma. Inset, high power view. (D to F) KI67-immunostained adjacent sections show elevated proliferation in the bladder epithelium of the experimental mice relative to the control mice. Inset, high power view. Scale bar, 100 μm. Reprinted with permission from Seager et al. (2009).
Orthotopic model
Primary bladder tumors resulting from orthotopic implantation develop as early as 2 weeks after instillation of cells, depending on the cell line. The time course of tumor development and take rate will vary based on the cell line employed.
Preclinical studies
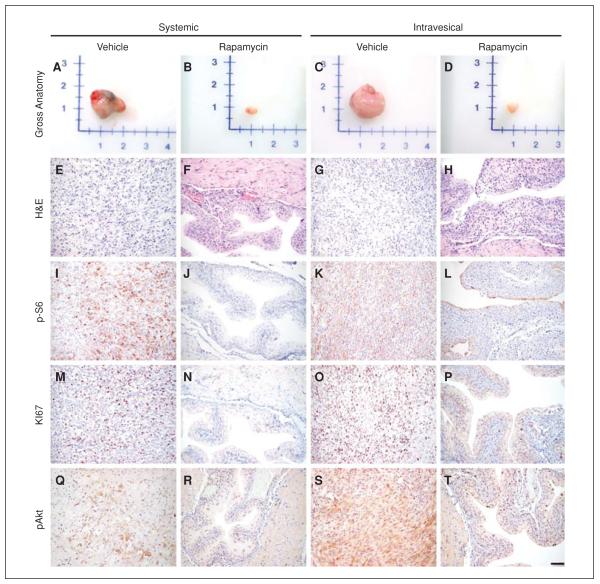
The example provided in this unit, namely preclinical evaluation of rapamycin in the adenovirus-injected model, completely suppresses bladder tumors (Puzio-Kuter et al., 2009). Figure 14.14.4 shows the effect of rapamycin on bladder tumor progression.
Figure 14.14.4.
Rapamycin inhibits bladder tumor progression. Representative examples from the vehicle- or rapamycin-treated mutant mice show the following: (A to D) gross anatomy of bladder tissues/tumors; (E to H) H&E-stained sections; or (I to T) immunostained sections using the indicated antibodies. In all groups, gross analyses of bladder tissues and H&E analyses were done on all control and experimental mice in each group; immunohistochemistry was done on a minimum of four animals from each group; representative data are shown. Scale bars, 100 μm. Reprinted with permission from Seager et al. (2009).
Time Considerations
Adenovirus-injected mouse model
Initially, delivery of adenovirus requires 15 to 20 min per mouse after induction of anesthesia. With experience, the amount of time needed to complete this protocol will decrease significantly. It is not unreasonable, once well practiced, to infect 15 to 20 mice in a half day.
Orthotopic model
Instillation of cells into the bladder is not time consuming once the catheterizing procedure has been perfected. With practice, the entire catheterizing process, including washings, should not take more than 5 min for each animal. However, pretreatment requires a dwell time of 20 min and cells should be retained in the bladder for at least 1 hr while mice are under anesthesia. This takes up to 1.5 hr per mouse. Preparation of cells requires significant advance planning.
Preclinical analyses
Developing the parameters for the preclinical study (dose analyses, etc.) can proceed rapidly or may take up to 2 months as pilot studies may have to be repeated until optimal conditions are determined. The actual preclinical studies can take up to several months, during which time mice need to be monitored and drug administration performed each week. Animal monitoring and drug administration can take several hours per week.
Acknowledgements
Funding for this project was provided by a grant from the Alexander and Margaret Stewart Trust provided to the Institute of Cancer Genetics at Columbia University Medical Center. This work was further supported by grants UO1 CA084294 (C.A.-S.) and NCI P50 CA91846 (C.C.-C.), the TJ Martell Foundation (C.A.-S. and C.C.-C.), and a Ruth L. Kirschstein National Research Service Award (CA1106625) to A.P.-K. C.A.-S. and C.C.-C. are members of the NCI Mouse Models of Human Cancer Consortium.
Literature Cited
- Ahlering TE, Dubeau L, Jones PA. A new in vivo model to study invasion and metastasis of human bladder carcinoma. Cancer Res. 1987;15:6660–6665. [PubMed] [Google Scholar]
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev. Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Chin J, Kadhim S, Garcia B, Kim YS, Karlik S. Magnetic resonance imaging for detecting and treatment monitoring of orthotopic murine bladder tumor implants. J. Urol. 1991;145:1297–1301. doi: 10.1016/s0022-5347(17)38618-4. [DOI] [PubMed] [Google Scholar]
- Chong L, Ruping Y, Jiancheng B, Guohong Y, Yougang F, Jiansong W, Xiang G, Jie H, Shusheng X. Characterization of a novel transplantable orthotopic murine xenograft model of a human bladder transitional cell tumor (BIU-87) Cancer Biol. Ther. 2006;4:394–398. doi: 10.4161/cbt.5.4.2509. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C. Molecular alterations associated with bladder cancer initiation and progression. Scand. J. Urol. Nephrol. Suppl. 2008;42:154–165. doi: 10.1080/03008880802291915. [DOI] [PubMed] [Google Scholar]
- Dalbagni G, Presti J, Reuter V, Fair WR, Cordon-Cardo C. Genetic alterations in bladder cancer. Lancet. 1993;342:469–471. doi: 10.1016/0140-6736(93)91595-d. [DOI] [PubMed] [Google Scholar]
- Dinney CP, McConkey DJ, Millikan RE, Wu X, Bar-Eli M, Adam L, Kamat AM, Siefker-Radtke AO, Tuziak T, Sabichi AL, Grossman HB, Benedict WF, Czerniak B. Focus on bladder cancer. Cancer Cell. 2004;6:111–116. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Donovan J, Brown P. Euthanasia. Curr. Protoc. Immunol. 2006;73:1.8.1–1.8.4. doi: 10.1002/0471142735.im0108s73. [DOI] [PubMed] [Google Scholar]
- Eble J, Sauter G, Epstein J, Sesterhenn I. Tumors of the Urinary System—Pathology and Genetics. IARC Press; Lyon, France: 2004. [Google Scholar]
- Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin. Cancer Res. 2006;12:5288–5297. doi: 10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- Hadaschik BA, Black PC, Sea JC, Metwalli AR, Fazli L, Dinney CP, Gleave ME, So AI. A validated mouse model for orthotopic bladder cancer using transurethral tumour inoculation and bioluminescence imaging. BJU Int. 2007;100:1377–1384. doi: 10.1111/j.1464-410X.2007.07165.x. [DOI] [PubMed] [Google Scholar]
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J. Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J. Clin. Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MA. Bladder cancer subtypes defined by genomic alterations. Scand. J. Urol. Nephrol. Suppl. 2008 Sep;(218):116–130. doi: 10.1080/03008880802284605. 2008. [DOI] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- Mitra AP, Cote RJ. Molecular Pathogenesis and Diagnostics of Bladder Cancer. Annu. Rev. Pathol. 2009;4:251–285. doi: 10.1146/annurev.pathol.4.110807.092230. [DOI] [PubMed] [Google Scholar]
- Olive KP, Tuveson DA. A “mouse hospital” for preclinical testing of novel cancer therapeutics. Clin. Cancer Res. 2006;12:5277–5287. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- Ouyang X, Jessen WJ, Al-Ahmadie H, Serio AM, Lin Y, Shih WJ, Reuter VE, Scardino PT, Shen MM, Aronow BJ, Vickers AJ, Gerald WL, Abate-Shen C. Activator protein-1 transcription factors are associated with progression and recurrence of prostate cancer. Cancer Res. 2008;68:2132–2144. doi: 10.1158/0008-5472.CAN-07-6055. [DOI] [PubMed] [Google Scholar]
- Phelan MC. Techniques for mammalian cell tissue culture. Curr. Protoc. Mol. Biol. 2006;74:A.3F.1–A.3F.18. doi: 10.1002/0471142727.mba03fs74. [DOI] [PubMed] [Google Scholar]
- Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, Wang X, Shen TH, Matos T, Shen MM, Cordon-Cardo C, Abate-Shen C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–680. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Weinberg RA. Opinion: Comparative biology of mouse versus human cells: Modelling human cancer in mice. Nat. Rev. Cancer. 2003;3:952–959. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- Seager CM, Puzio-Kuter AM, Trushar Patel T, Jain S, Carlos Cordon-Cardo C, McKiernan J, Abate-Shen C. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev. Res. 2009;2:1008–1014. doi: 10.1158/1940-6207.CAPR-09-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Johnson L. Using genetically engineered mouse models of cancer to aid drug development: An industry perspective. Clin. Cancer Res. 2006;12:5312–5328. doi: 10.1158/1078-0432.CCR-06-0437. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- St. Clair MB, Sowers AL, Davis JA, Rhodes LL. Urinary bladder catheterization of female mice and rats. Contemp. Top. Lab. Anim. Sci. 1999;38:78–79. [PubMed] [Google Scholar]
- Watanabe T, Shinohara N, Sazawa A, Harabayashi T, Ogiso Y, Koyanagi T, Takiguchi M, Hashimoto A, Kuzumaki N, Yamashita M, Tanaka M, Grossman HB, Benedict WF. An improved intravesical model using human bladder cancer cell lines to optimize gene and other therapies. Cancer Gene Ther. 2000;7:1575–1580. doi: 10.1038/sj.cgt.7700261. [DOI] [PubMed] [Google Scholar]
- Wu XR. Urothelial tumorigenesis: A tale of divergent pathways. Nat. Rev. Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]