Abstract
Background
Implantable Cardioverter-Defibrillators (ICDs) reduce mortality in heart failure (HF). In patients requiring ventricular assist device (VAD), the benefit from ICD therapy is not well established. The aim of the study is to define the impact of ICD on outcomes in VAD - supported patients.
Methods and Results
We reviewed data for consecutive adult HF patients receiving VAD as bridge-to-transplantation from 1996 to 2003. Primary outcome was survival to transplantation. A total of 144 VADs were implanted [85 left ventricular (LVAD), 59 biventricular (BIVAD), age 50±12 years, 77% male, LVEF 18±9%, 54% ischemic]. Mean length of support was 119 days (range 1–670); 103 (72%) patients survived to transplantation. Forty-five patients had an ICD (33 LVAD, 12 BIVAD). More LVAD patients had an appropriate ICD shock before implantation than afterwards (16 vs. 7, p=0.02). There was a trend towards higher shock frequency before LVAD implant than after (3.3±5.2 vs 1.1±3.8 shocks/year, p=0.06). Mean time to first shock after VAD implant was 129±109 days. LVAD-supported patients with an ICD were significantly more likely to survive to transplantation (LVAD: 1-year actuarial survival to transplantation 91% with ICD vs. 57% without ICD, log-rank p=0.01; BIVAD: 54% vs. 47%, log-rank p=NS). An ICD was associated with significantly increased survival in a multivariate model controlling for confounding variables (OR 2.54, 95% CI 1.04-6.21, p=0.04).
Conclusions
Shock frequency decreases after VAD implantation, likely due to ventricular unloading, but appropriate ICD shocks still occur in 21% of patients. An ICD is associated with improved survival in LVAD-supported HF patients.
Keywords: Implantable Cardioverter Defibrillators, Heart failure, Ventricular assist devices, Transplantation, Ventricular arrhythmias
Ventricular assist device (VAD) use is becoming increasingly common for patients with end-stage heart failure (HF) as bridges to transplantation (BTT), as temporary support to allow myocardial recovery and as destination therapy for transplant ineligible patients (1–6). While adverse outcomes in VAD-supported patients have decreased during the last several years, complications remain common and the optimal use of other pharmacological and non-pharmacological therapies remains uncertain (6–9). Several studies have shown significant rates of ventricular tachyarrhythmia (VA) events while on VAD support, ranging from 22–52% (9–13). The majority of events seem to occur in the early post-operative period, variably reported 1–8 weeks after implantation. At least one study has related VA events to increased mortality if occurring within the first week of VAD support (12).
Implantable cardioverter-defibrillators (ICDs) are known to improve survival in patients with both ischemic and non-ischemic HF etiologies by reducing the incidence of sudden cardiac death (14,15). Three recent reports have suggested a survival benefit with the use of ICDs in VAD-supported HF patients (16–18). Given the possible survival benefit of ICDs in VAD-supported HF patients, we sought to confirm the efficacy of ICDs in a large single center cohort of VAD-supported HF patients to improve clinical outcomes in this patient population that will surely expand in the coming years.
Methods
Study Design and Data Collection
This study was approved by the institutional review board (IRB) of the University of Pittsburgh. Data from the University of Pittsburgh Medical Center (UPMC) Cardiothoracic Transplantation Program’s Transplant Patient Management System (TPMS) were reviewed retrospectively. All adult subjects who underwent VAD support from 1996 to 2003 as a bridge to transplantation (BTT) were reviewed and included in the study; pediatric patients and those receiving devices designed for transient support were excluded. All subjects in the cohort received one of three VAD systems: Thoratec VAD (Thoratec Corp., Pleasanton, CA) implanted as either a left ventricular assist device (LVAD) or biventricular assist devices (BIVAD), Novacor LVAS (previously Baxter Healthcare Corp., now World Heart Corporation, Oakland, CA), and Heartmate LVAS XVE (previously Thermo Cardiosystems Inc., now Thoratec Corp., Pleasanton, CA). Patients with an ICD implanted prior to VAD placement were identified. All patients had the ICD turned off for VAD implantation, and only LVAD recipients had the ICD turned on post-operatively (routinely within 12 – 48 hours). BIVAD recipients had the ICD set to monitor only, based on the protocol in effect at our institution. Appropriate ICD shocks for Ventricular Tachycardia (VT) and Ventricular Fibrillation (VF) were recorded. Available ECGs were reviewed to determine which patients were paced.
Statistical Analysis
The primary outcome was survival to transplantation. In addition to the presence of an ICD, over 250 other variables were evaluated for an effect on outcome by log-rank analysis of Kaplan-Meier survival plots. The analysis was performed for all VAD subjects and then repeated for the subgroup that received an LVAD. Variables found to have a significant effect on survival (p < 0.05) were included in a multivariate analysis by Cox regression. Data are expressed as mean ± SD. Continuous variables were analyzed using Student’s t-test. Categorical variables were analyzed using ANOVA. All tests were two-tailed. A p value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS for Windows, version 13 (SPSS, Inc., Chicago, Illinois).
Results
A total of 144 VADs were implanted for the indication of bridge to transplantation. Of these, 85 were left ventricular assist devices (LVAD) and 59 were biventricular assist devices (BIVAD). Forty-five patients had an ICD (33 LVAD, 12 BIVAD). Mean length of support was 119 days (range 1–670). Overall, 103 (72%) patients survived to transplantation. Table 1 details the devices used and survival. Baseline demographic and clinical characteristics in patients with and without an ICD prior to VAD placement were compared for all VAD patients and for the LVAD subgroup (Table 2). There were no differences in severity of heart failure as assessed by ejection fraction, cardiac index, or inotrope usage. Patients with an ICD had a longer duration of HF (68±41 vs. 33±46 months, p<0.001) with more left ventricular dilatation (7.3±1.1 vs. 6.5±1.4 cm, p=0.01). Patients with an ICD had a wider QRS complex (158±43 vs. 131±35 ms, p<0.001) and were more likely to be paced on at least one ECG (42% vs. 6%, p<0.001). More patients with an ICD were treated with digoxin (78% vs. 58%, p=0.02) and there was a trend towards higher usage of amiodarone (58% vs. 41%, p=0.07), possibly reflecting a higher incidence of known arrhythmias and/or the presence of backup pacing in the ICD group.
Table 1.
Devices used.
| Novacor® LVAS |
Heartmate® LVAS VE |
Thoratec® LVAD* |
Thoratec® BIVAD |
|
|---|---|---|---|---|
| N (% of total) | 39 (27%) | 18(13%) | 24(17%) | 59(41%) |
| Number of patients with ICD (% within device group) |
14(36%) | 12 (67%) | 7 (29%) | 12 (20%) |
| Number of patients surviving to transplant (% within device group) |
29 (74%) | 16 (89%) | 21 (88%) | 37 (63%) |
LVAD indicates left ventricular assist device; BIVAD, biventricular assist device;
includes 1 Thoratec IVAD LVAD.
Table 2.
Preoperative characteristics.
| ALL VADs No ICD (n=99) |
ALL VADs ICD (n=45) |
P | LVADs No ICD (n=52) |
LVADs ICD (n=33) |
P | |
|---|---|---|---|---|---|---|
| Age | 50 ± 12 | 51 ± 11 | NS | 51 ± 12 | 52 ± 11 | NS |
| Male gender | 71 (72%) | 40 (89%) | 0.03 | 42(81%) | 29 (89%) | NS |
| Ischemic etiology | 59 (59%) | 20 (44%) | NS | 32 (62%) | 12 (36%) | 0.02 |
| Diabetes | 31 (32%) | 10(22%) | NS | 15 (29%) | 6(18%) | NS |
| Previous sternotomy | 42 (43%) | 11 (24%) | 0.04 | 18 (36%) | 7(21%) | NS |
| Heart failure duration (months) | 33 ± 46 | 68 ± 41 | <0.001 | 36 ± 54 | 64 ± 37 | 0.01 |
| IABP pre-VAD | 77 (78%) | 23 (73%) | NS | 41 (79%) | 26 (79%) | NS |
| QRS width (ms) | 131 ± 35 | 158 ± 43 | <0.001 | 130 ± 34 | 161 ± 43 | <0.001 |
| Left bundle branch block | 11 (13%) | 8(18%) | NS | 5(10%) | 7(21%) | NS |
| Paced rhythm | 5 (6%) | 19(42%) | <0.001 | 4 (8%) | 12 (36%) | 0.002 |
| Number of inotropes | 2.0 ± 1.0 | 1.8 ± 1.0 | NS | 1.9 ± 1.0 | 1.8 ± 1.0 | NS |
| ACE Inhibitors | 55 (58%) | 34 (76%) | 0.06 | 36 (72%) | 25 (76%) | NS |
| Beta-blockers | 22 (23%) | 13(31%) | NS | 16 (32%) | 10 (30%) | NS |
| Aldactone | 14(15%) | 14(33%) | 0.04 | 7(15%) | 10(31%) | 0.09 |
| Digoxin | 55 (58%) | 35 (78%) | 0.02 | 33 (66%) | 28 (85%) | 0.06 |
| Amiodarone | 39(41%) | 26 (58%) | 0.07 | 19 (38%) | 19 (58%) | 0.08 |
| Other antiarrhythmics | 18(19%) | 7 (16%) | NS | 8 (16%) | 4 (12%) | NS |
| White blood cell count (×109/L) | 10.3 ± 3.9 | 9.7 ± 4.4 | NS | 10.1 ± 4.0 | 8.2 ± 2.1 | NS |
| Hemoglobin (mg/dL) | 10.9 ± 2.0 | 11.6 ± 1.7 | 0.04 | 11.4 ± 2.1 | 11.5 ± 1.7 | NS |
| Prothrombin time (seconds) | 14.8 ± 2.9 | 14.1 ± 2.3 | NS | 14.3 ± 2.8 | 13.8 ± 2.2 | NS |
| Serum sodium (mEq/L) | 134 ± 7 | 133 ± 5 | NS | 134 ± 6 | 133 ± 4 | NS |
| Serum creatinine (mg/dL) | 1.4 ± 0.6 | 1.5 ± 0.6 | NS | 1.3 ± 0.5 | 1.4 ± 0.5 | NS |
| Creatinine clearance (mL/min) | 83 ± 37 | 79 ± 28 | NS | 89 ± 37 | 83 ± 29 | NS |
| Blood urea nitrogen (mg/dL) | 29 ± 16 | 29 ± 17 | NS | 27 ± 14 | 27 ± 16 | NS |
| PCWP (mmHg) | 26 ± 9 | 27 ± 10 | NS | 27 ± 9 | 28 ± 11 | NS |
| Mean PA pressure (mmHg) | 36 ± 11 | 39 ± 11 | NS | 36 ± 10 | 39 ± 11 | NS |
| Cardiac index (L/min/m2) | 2.0 ± 0.6 | 2.0 ± 0.5 | NS | 2.0 ± 0.6 | 2.1 ± 0.5 | NS |
| Left ventricular EF (%) | 19 ± 10 | 16 ± 5 | 0.08 | 18 ± 8 | 16 ± 4 | NS |
| LVEDD (cm) | 6.5 ± 1.4 | 7.3 ± 1.1 | 0.01 | 6.7 ± 1.3 | 7.4 ± 1.1 | 0.07 |
ICD, implantable cardioverter-defibrillator; IABP, intraaortic balloon pump; PCWP, pulmonary capillary wedge pressure; PA, pulmonary artery; EF, ejection fraction; LVEDD, left ventricle end diastolic diameter; NS, not significant.
Of the 45 VAD patients with an ICD, 13 had evidence of ventricular pacing on ECG (hereafter referred to as “paced”) but did not receive a shock, 5 received at least one ICD shock but were not paced, 3 were paced and received at least one shock, and 24 had no documented ICD therapy of any kind. There was no difference in survival to transplantation for ICD patients who were paced versus those not paced. There was no difference in survival between those paced and/or shocked and those who were neither paced nor shocked. Additionally, there was no difference in survival to transplantation after stratifying patients by occurrence of a ventricular arrhythmia.
Appropriate shocks were delivered to 7 patients after LVAD implantation (5 VT, 1 VF, 1 VT and VF). The mean time to first shock after LVAD implant was 129±109 days. In the LVAD cohort, more patients had an appropriate ICD shock for VT or VF before LVAD implant than afterwards (16 vs. 7, p=0.02). Five of the seven patients who were shocked after LVAD placement had one or more shocks pre-operatively. There was a trend to higher shock frequency before LVAD implant than after (3.3±5.2 vs. 1.1±3.8 shocks/year, p=0.06).
Patients with an ICD were significantly more likely to survive to transplantation, due entirely to the subset of LVAD-supported patients (LVAD: 1-year actuarial survival to transplantation 91% with ICD vs. 57% without ICD, log-rank p=0.01; BIVAD: 54% vs. 47%, log-rank p=NS; Figure 1). Improved survival in the ICD group was also found if the analysis was limited to patients with HF of greater than six months duration (to minimize the potential confounding effects of the differences in HF duration between the ICD and no-ICD groups). An ICD was associated with significantly increased survival in a multivariate model (OR 2.72, 95% CI 1.03-7.16, p=0.04) controlling for confounding variables found in Table 2 (gender, duration of heart failure, history of previous sternotomy, medications, hemoglobin) as well as variables previously reported to be associated with poor outcome in VAD-supported patients (renal function, age). There were no significant differences in VAD complications between patients with and without an ICD (Table 3). When analyzed by cause of death, patients with an ICD had lower rates of death in all categories (Table 4). There were 2 deaths directly related to arrhythmias and they both occurred in patients without an ICD. In addition, two patients without an ICD had ventricular arrhythmias requiring placement of an ICD after VAD placement.
Figure 1. Survival to cardiac transplantation.
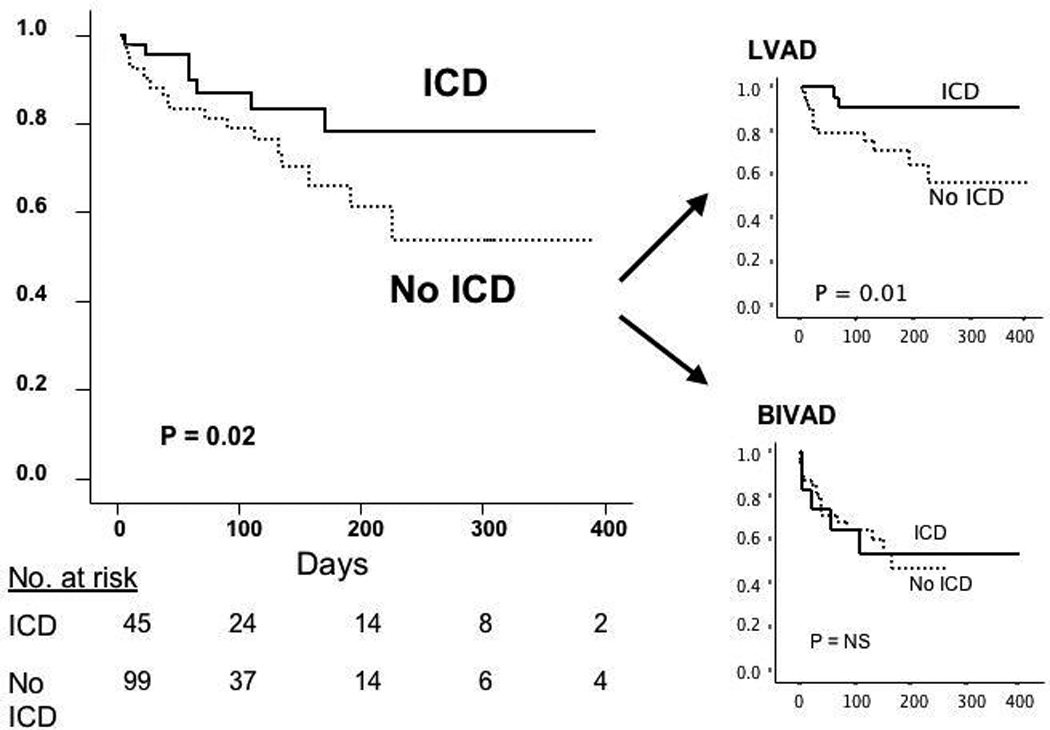
Survival to cardiac transplantation in subjects with and without an ICD. The entire cohort is divided into those implanted with an LVAD and those with a BIVAD. NS, not significant.
Table 3.
VAD complications.
| NoICD (n=99) |
ICD (n=45) |
P | |
|---|---|---|---|
| Number of infections per patient | 2.0 ± 2.3 | 1.8 ± 2.2 | NS |
| Number of reoperations per patient | 1.4 ± 1.7 | 1.2 ± 1.3 | NS |
| Embolic events | 15(15%) | 5(11%) | NS |
ICD indicates implantable cardioverter-defibrillator;
NS, not significant.
Table 4.
Causes of death.
| No ICD (n = 99) | ICD (n = 45) | |
|---|---|---|
| Sepsis | 6 (6%) | 2 (4%) |
| Neurological event | 9 (9%) | 3 (7%) |
| Arrhythmia | 2 (2%) | 0 (0%) |
| Non-CNS bleed | 2 (2%) | 0 (0%) |
| Pulmonary hypertension | 1 (1%) | 0 (0%) |
| Respiratory failure* | 3 (3%) | 0 (0%) |
| Multiorgan failure | 11 (11%) | 2 (4%) |
Includes 1 pulmonary embolus. ICD indicates implantable cardioverter-defibrillator.
Discussion
We report a significant survival benefit of ICDs for VAD-supported patients. Patients with an ICD were 2.72 times more likely to survive to transplantation in a multivariable model taking into account confounding variables. Shock frequency tended to decrease after VAD implantation, but appropriate shocks for ventricular tachyarrhythmias still occurred in 21% of patients.
The early postoperative period after initiation of VAD therapy to the failing human heart is associated with a high incidence of ventricular tachyarrhythmia events with up to 35.7% at a median follow-up of 3.5 weeks as shown by Refaat et al from a single center experience at Massachusetts General Hospital (10). In this study, the transcript profiling on a subset of patients who developed ventricular tachycardias (VTs) during VAD support showed a distinct pattern of changes of specific genes that may render the heart pro-arrhythmic (10,19,20). A study from Columbia University by Ziv et al. showed that 35.2% of patients developed post-VAD ventricular arrhythmias with the majority occurring by the first week after implantation (11). In addition, studies from the University of Pittsburgh Medical Center have reported an VA incidence rate of 22% after VAD support that were associated with a 54% mortality rate if occurring within the first week of VAD support as well as an incidence of 36.4% within the first 60 days of support in a broader population (9,12). A recent study from Denmark showed sustained VT or VF in 52% of VAD treated patients with the majority of arrhythmias requiring defibrillation and occuring in the first 4 weeks after VAD implantation (13). Interestingly, we found arrhythmias to occur throughout the mechanical support period, with a mean time to clinical arrhythmia in our series of 129 days. Possible explanations of this include institutional differences in patient management of mechanical support, ICD management, medical management, or cohort variation. Regardless, our observations raise concern for arrhythmias throughout the course of mechanical support.
The mechanical unloading of the myocardium during LVAD support causes alterations in the ion channels involved in calcium handling, ion transporting genes and structural genes that may render the heart more pro-arrhythmic. Expression of Connexin 43, Na+/K+-ATPase and potassium channel Kv4.3 is down regulated while expression of the Na+/Ca2+-exchanger is upregulated (10). Such alterations can contribute to arrhythmogenesis in unloaded hearts due to dispersion of refractoriness, reduction in the repolarization reserve and delayed after depolarization.
The type of mechanical support (pulsatile versus continuous flow) may play a role in the pathogenesis of ventricular arrhythmias. For example, continuous flow support is more likely to result in septal shift. The current report describes a cohort of patients from an older era using pulsatile flow devices. In the more recent era at our institution (2004–2010), there were a total of 168 VAD implants. Of these, 95 were pulsatile devices (9 Novacor, 19 HeartMate I, 24 Thoratec PVAD LVAD, 43 Thoratec PVAD BiVAD) and 73 were continuous flow (44 HeartMate II, 22 VentrAssist, 7 HeartWare). ICDs were implanted in 44 pulsatile flow VADs and 62 continuous flow devices. Of the 44 pulsatile devices with an ICD, 15 patients had an ICD shock while on mechanical support. Shocks occurred over a range from 0 to 483 days post-VAD implant (mean 96 ± 162 days, median 39 days, 95%CI 3 – 112 days). Of the 62 continuous flow LVADs with an ICD, 8 patients had an ICD shock while on mechanical support. Shocks occurred over a range from 3 to 377 days post-VAD implant (mean 59 ± 129 days, median 6.5 days, 95%CI 2 – 89 days). These data support our findings of arrhythmias occurring in a significant portion of mechanically supported patients and throughout the support period.
More recent studies suggest a survival benefit with the use of ICDs in VAD-supported HF patients. A study of 90 VAD-treated patients with a concomitant ICD for sudden cardiac death prevention at the Cleveland Clinic showed an improved survival (16). Furthermore, in a study of 33 VAD-treated patients with a concomitant ICD at the University of Colorado, 24% of the patients received appropriate ICD shocks and appropriate ICD therapy was associated with higher mortality (17). Another study of 61 patients with LVAD and ICD followed prospectively for 1 year in Germany showed a high rate of appropriate ICD interventions of 34%, mostly for treatment of monomorphic VT in 52%, polymorphic VT in 13% and VF in 35% (18).
There may be additional benefits of combining ICD therapy with long-term mechanical circulatory assistance. It has long been appreciated that ventricular arrhythmias cause VAD flow rates to drop (11). Because the majority of VAD-supported patients only have mechanical support of the left ventricle (LVAD), the right ventricle is unprotected from the detrimental effects of ventricular tachyarrhythmias and bradyarrhythmias. Thus, ICDs likely can provide a level of protection to the RV to ensure flow to the LVAD. Indeed, the patients described in our report by and large did have VAD underfilling (low flow alarms) and/or measurable hemodynamic compromise. In addition to defining a pre and post-VAD shock frequency, we report a high incidence of ventricular pacing for bradyarrhythmias, which may be a previously unrecognized added benefit of ICDs in this population. However, it should also be noted that in rare instances, interactions between the ICD and VAD might happen (21,22).
While our overall numbers were too low to correlate with any one cause of death in this population with many reasons for morbidity and mortality, we believe that the ICD may provide a potential protective effect given the significant frequency of appropriate shocks following LVAD placement (21%) and the high rate of pacing on ECG (36%). In fact, almost 50% of the VAD subjects with ICDs were either shocked or chronically paced in our cohort. Pacing by the ICD is substantially underestimated in this study, as data on intermittent pacing by the ICD was not stored. Although arrhythmias directly accounted for only two deaths and two urgent ICD placements in the VAD-supported patients without ICDs, the true incidence of ventricular arrhythmias is unknown. In addition, the survival benefit of ICDs was not seen in BIVAD-supported patients, in whom the ICDs were left inactive according to our center’s protocol and for whom RV pacing is less likely to provide significant benefit. This group can be considered as a control arm. We therefore suggest that ventricular tachyarrhythmias and/or bradyarrhythmias may predispose to a general decline in clinical status, perhaps due to deleterious effects on right ventricular function that eventually leads to death. The significant mortality benefit in the VAD-supported patients with ICDs could also be driven by a high percentage of overdrive pacing and thus less number of appropriate shocks for ventricular tachyarrhythmia intervention. A recent study of 61 patients with LVAD and ICD followed prospectively for 1 year in Germany showed a high rate of appropriate ICD interventions of 34% for ventricular tachyarrhythmias , 71% of the ventricular tachyarrhymias were terminated by overdrive pacing while 29% of the ventricular tachyarrhythmias were terminated by appropriate ICD shocks (18).
There were several preoperative characteristics that were significantly different between the group with ICDs and the group without. Most notable was a longer duration of HF prior to VAD implantation and more dilated left ventricles. This could indicate that ICDs are merely a marker for a more slowly progressive HF in which the decision to implant a VAD was less emergent. Only 3/40 (8%) acute HF patients (duration < 6 months) had an ICD vs. 42/104 (40%) chronic HF patients (p <0.001). There was no difference in survival to transplantation in acute vs. chronic HF patients supported with VAD (when analyzed with all patients summed or stratified by the presence of an ICD). Furthermore, after eliminating the acute HF patients from analysis, there was still improved survival of VAD-supported HF pts implanted with an ICD (28/30, 93% vs. 21/32, 66%, log-rank p=0.05). Therefore, our findings appear valid for patients with chronic HF, which represents the majority of the VAD population.
The results of this retrospective study must be interpreted with caution and considered primarily as hypothesis generating. We cannot be certain that every ICD was on during the entire period following LVAD placement; inactive devices would artificially lower the estimated shock frequency following VAD placement, but weaken the potential beneficial effect of the device. Due to the retrospective nature of this study, we did not have device electrograms to review, therefore we are unable to report on tachycardiac cycle lengths or ATP therapies which would useful to evaluate in future studies. The modest number of VAD-supported patients with ICDs limits power, particularly in subgroup analyses designed to explain the potential mechanisms of improved survival. We also cannot exclude the possibility that the ICD is associated with but not causally related to the improvement in survival. While this study cannot establish a causal link between ICD prevention of arrythmia and mortality (or morbidity) in VAD supported patients, we believe we have provided a valuable observation that should be considered for further study as well as in clinical decision making for these complex patients. Therefore, the survival benefit of an ICD for VAD-supported patients should be evaluated prospectively in a multi-center clinical trial.
Conclusions
An ICD is associated with improved survival in VAD-supported HF patients. Shock frequency decreases after VAD implantation but appropriate shocks still occur in 21% of patients. These findings should be confirmed prospectively and the mechanisms warrant further investigation.
Acknowledgements
This research was supported, in part, by the National Heart Lung and Blood Institute awards KL2 RR024154 (Dr. Simon), R01 HL75038 and K24 HL69912 (Dr. McNamara), and R01 HL062300 and R01 HL077398 (Dr. London). The authors wish to thank Pradeep Nair, MD and Michael Heffernan, MD for their efforts collecting data. The authors wish to thank Kathleen Lockard, Eileen Stanford, BSN, Lisa Carozza RN, BSN, Don Severyn, MS, Rick Schaub, PhD for their expert commentary, insight, and patient care during the preparation of this manuscript.
This research was supported, in part, by the National Heart Lung and Blood Institute awards KL2 RR024154 (Dr. Simon), R01 HL75038 and K24 HL69912 (Dr. McNamara), and R01 HL062300 and R01 HL077398 (Dr. London).
Glossary of Abbreviations
- BIVAD
biventricular assist device
- CI
confidence interval
- ECG
electrocardiogram
- HF
heart failure
- ICD
implantable cardioverter-defibrillator
- LVAD
left ventricular assist device
- OR
odds ratio
- VAD
ventricular assist device
- VA
ventricular arrhythmia
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: Dr. Kormos is on the medical advisory boards of Thoratec Corporation & World Heart Corporation. There are no other disclosures.
References
- 1.McCarthy PM, Smedira NO, Vargo RL, Goormastic M, Hobbs RE, Starling RC, Young JB. One hundred patients with the HeartMate left ventricular assist device: evolving concepts and technology. J Thorac Cardiovasc Surg. 1998;115:904–912. doi: 10.1016/S0022-5223(98)70373-3. [DOI] [PubMed] [Google Scholar]
- 2.Simon MA, Kormos RL, Murali S, Nair P, Heffernan M, Gorcsan J, Winowich S, McNamara DM. Myocardial recovery using ventricular assist devices: prevalence, clinical characteristics, and outcomes. Circulation. 2005;112:I32–I36. doi: 10.1161/CIRCULATIONAHA.104.524124. [DOI] [PubMed] [Google Scholar]
- 3.Frazier OH, Rose EA, McCarthy P, Burton NA, Tector A, Levin H, Kayne HL, Poirier VL, Dasse KA. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg. 1995;222:327–336. doi: 10.1097/00000658-199509000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL, Meier P. Long-Term Use of a Left Ventricular Assist Device for End-Stage Heart Failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009 Dec 3;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 6.Griffith BP, Kormos RL, Nastala CJ, Winowich S, Pristas JM. Results of extended bridge to transplantation: window into the future of permanent ventricular assist devices. Ann Thorac Surg. 1996;61:396–398. doi: 10.1016/0003-4975(95)01020-3. [DOI] [PubMed] [Google Scholar]
- 7.Deng MC, Edwards LB, Hertz MI, Rowe AW, Keck BM, Kormos R, Naftel DC, Kirklin JK. Mechanical Circulatory Support Device Database of the International Society for Heart and Lung Transplantation: second annual report-2004. J Heart Lung Transplant. 2004;23:1027–1034. doi: 10.1016/j.healun.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Long JW, Kfoury AG, Slaughter MS, Silver M, Milano C, Rogers J, Delgado R, Frazier OH. Long-term destination therapy with the HeartMate XVE left ventricular assist device: improved outcomes since the REMATCH study. Congest Heart Fail. 2005;11:133–138. doi: 10.1111/j.1527-5299.2005.04540.x. [DOI] [PubMed] [Google Scholar]
- 9.Genovese EA, Dew MA, Teuteberg JJ, Simon MA, Kay J, Siegenthaler MP, Bhama JK, Bermudez CA, Lockard KL, Winowich S, Kormos RL. Incidence and patterns of adverse event onset during the first 60 days after ventricular assist device implantation. Ann Thorac Surg. 2009 Oct;88(4):1162–1170. doi: 10.1016/j.athoracsur.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Refaat M, Chemaly E, Lebeche D, Gwathmey JK, Hajjar RJ. Ventricular Arrhythmias after Left Ventricular Assist Device Implantation. PACE. 2008;31:1246–1252. doi: 10.1111/j.1540-8159.2008.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziv O, Dizon J, Thosani A, Naka Y, Magnano AR, Garan H. Effects of left ventricular assist device therapy on ventricular arrhythmias. J Am Coll Cardiol. 2005;45:1428–1434. doi: 10.1016/j.jacc.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 12.Bedi M, Kormos R, Winowich S, McNamara D, Mathier M, Murali S. Ventricular Arrhythmias during left ventricular assist device support. Am J. Cardiol. 2007;99:1151–1153. doi: 10.1016/j.amjcard.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 13.Andersen M, Videbaek R, Boesgaard S, Sander K, Hansen P, Gustafsson F. Incidence of Ventricular Arrhythmias in Patients on Long-term Support with a continuous-flow Assist Device (HeartMate II) J Heart Lung Transplant. 2009;28:733–735. doi: 10.1016/j.healun.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 15.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 16.Cantillon DJ, Tarakji KG, Kumbhani DJ, Smedira NG, Starling RC, Wilkoff BL. Improved survival among ventricular assist device recipients with a concomitant implantable cardioverter-defibrillator. Heart Rhythm. 2010;7:466–471. doi: 10.1016/j.hrthm.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Ambardekar AV, Allen LA, Lindenfeld J, et al. Implantable cardioverter-defibrillator shocks in patients with a left ventricular assist device. J Heart Lung Transplant. 2010;29:771–776. doi: 10.1016/j.healun.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Oswald H, Schultz-Wildelau C, Gardiwal A, et al. Implantable defibrillator therapy for ventricular tachycardia in left ventricular assist device patients. Eur J Heart Fail. 2010:593–599. doi: 10.1093/eurjhf/hfq048. [DOI] [PubMed] [Google Scholar]
- 19.Borlak J, Thum T. Hallmarks of ion channel gene expression in end-stage heart failure. FASEB J. 2003;17:1592–1608. doi: 10.1096/fj.02-0889com. [DOI] [PubMed] [Google Scholar]
- 20.Nattel S, Frelin Y, Gaborit N, Louault C, Demolombe S. Ion-channel mRNA-expression profiling: Insights into cardiac remodeling and arrhythmic substrates. Journal of Molecular and Cellular Cardiology. 2010;48(1):96–105. doi: 10.1016/j.yjmcc.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Jacob S, Cherian PK, Ghumman WS, Das MK. “Pseudo” Faraday cage: a solution for telemetry link interaction between a left ventricular assist device and an implantable cardioverter defibrillator. J Interv Card Electrophysiol. 2009;28(3):221–225. doi: 10.1007/s10840-009-9415-6. [DOI] [PubMed] [Google Scholar]
- 22.Foo D, Walker B, Kuchar DL, et al. Left Ventricular Mechanical Assist Devices and Cardiac Device Interactions: An observational case series. PACE. 2009;32:879–887. doi: 10.1111/j.1540-8159.2009.02403.x. [DOI] [PubMed] [Google Scholar]


