Abstract
OBJECTIVE
To develop a system that documents the location of transrectal ultrasonography (TRUS)-guided prostate biopsies by fusing them to MRI scans obtained prior to biopsy, as the actual location of prostate biopsies is rarely known.
PATIENTS AND METHODS
Fifty patients (median age 61) with a median prostate-specific antigen (PSA) of 5.8 ng/ml underwent 3T endorectal coil MRI prior to biopsy. 3D TRUS images were obtained just prior to standard TRUS-guided 12-core sextant biopsies wherein an electromagnetic positioning device was attached to the needle guide and TRUS probe in order to track the position of each needle pass. The 3D-TRUS image documenting the location of each biopsy was fused electronically to the T2-weighted MRI. Each biopsy needle track was marked on the TRUS images and these were then transposed onto the MRI. Each biopsy site was classified pathologically as positive or negative for cancer and the Gleason score was determined.
RESULTS
The location of all (n = 605) needle biopsy tracks was successfully documented on the T2-weighted (T2W) MRI. Among 50 patients, 20 had 56 positive cores. At the sites of biopsy, T2W signal was considered ‘positive’ for cancer (i.e. low in signal intensity) in 34 of 56 sites.
CONCLUSION
It is feasible to document the location of TRUS-guided prostate biopsies on pre-procedure MRI by fusing the pre-procedure TRUS to an endorectal coil MRI using electromagnetic needle tracking. This procedure may be useful in documenting the location of prior biopsies, improving quality control and thereby avoiding under-sampling of the prostate as well as directing subsequent biopsies to regions of the prostate not previously sampled.
Keywords: prostate cancer, MRI, TRUS-guided sextant biopsy, MRI–TRUS fusion, biopsy mapping
INTRODUCTION
Prostate cancer is the most common solid organ malignancy among American men with an estimated 2009 incidence and annual death rate of 192 280 and 27 360, respectively [1]. Sextant prostate biopsy is the standard method of diagnosis. While the biopsies are theoretically obtained from all sextants, the exact location of biopsies is not routinely recorded. It is therefore possible that an area of prostate could be inadvertently under-sampled, or that subsequent biopsies may simply re-sample the original biopsy site.
Therefore, mapping of biopsy sites could be helpful in the management of patients at initial diagnosis. Moreover, the ability to document the completeness of sampling could help improve the quality of prostate biopsies. Recently, several groups have attempted to document biopsy locations by using different ultrasound methods [e.g. three-dimensional (3D) transrectal or transperineal ultrasound], however, it is difficult to interpret such images for future use because the anatomic landmarks are difficult to see on TRUS [2-6]. Magnetic resonance provides a more readily understood template on which to map biopsy tracks. Thus, a method of documenting biopsy sites on MRI is desirable. In this paper we determine the feasibility of mapping prostate biopsies by fusing the TRUS-guided needle biopsy track to the pre-procedure endorectal coil MRI.
PATIENTS AND METHODS
This prospective single institution study was approved by the local institutional review board and was compliant with Health Insurance Portability and Accountability Act (HIPAA); informed consent was obtained from each patient. Fifty patients who underwent anatomical prostate MRI at 3T and subsequent TRUS- guided prostate biopsy with electromagnetic (E-M) needle tracking were included in the study population. The median age of the patients was 61 years (mean 61.6 ± 8.4 years), and the median serum PSA level was 5.8 ng/mL (mean 8.7 ± 14.6 ng/mL).
MRI studies were performed using a combination of an endorectal coil (BPX-30, Medrad, Pittsburgh, PA, USA) and a 6-channel phased array surface coil (Philips Healthcare, Best, the Netherlands) on a 3T magnet (Achieva, Philips Healthcare) without prior bowel preparation. After digital rectal examination, the endorectal coil was inserted using a semi-anaesthetic gel (Lidocaine, Alcorn, Lake Forest, IL, USA) while the patient was in the left lateral decubitus position. The balloon surrounding the coil was distended with perfluorocarbon (Fluorinert FC-770, 3M, St. Paul, MN, USA) to a volume of approximately 50 mL. T2-weighted (T2W) images in three planes (axial, coronal and sagittal) were obtained with the parameters summarized in Table 1.
TABLE 1.
T2-weighted magnetic resonance imaging parameters used in the current study
| MRI sequence | TR/TE (m/s) | FOV (mm) | Resolution (mm) | Matrix | Flip angle | Slice thickness (mm) |
|---|---|---|---|---|---|---|
| Sagittal T2W TSE | 2340/120 | 140 | 0.46 × 0.6 × 3.0 | 256 × 256 | 90 | 3 |
| Axial T2W TSE | 8852/120 | 140 | 0.46 × 0.6 × 3.0 | 256 × 256 | 90 | 3 |
| Coronal T2W TSE | 2340/120 | 140 | 0.46 × 0.6 × 3.0 | 256 × 256 | 90 | 3 |
MRI, magnetic resonance imaging; T2W, T2-weighted; TSE, turbo spin echo; TR, time of repetition; TE, time of echo; FOV, field of view.
The median interval between MRI and TRUS-guided prostate biopsy procedure was 12 days (mean 30.2 days, range 3–133 days). A 2D axial TRUS sweep was performed from the base to the apex of the prostate to reconstruct a 3D volume of the prostate before each biopsy procedure. This volume was used as a reference for MRI–TRUS registration and motion compensation (Fig. 1). TRUS-guided biopsies were performed using a navigation system that was previously developed for targeted prostate biopsy [7-9]. A disposable needle guide with two 5-degree-of-freedom electromagnetic sensors (Traxtal Inc., Waterloo, ON, Canada) was attached to an end-firing endorectal probe (C9-5 Philips Healthcare, Bothell, WA, USA), allowing the probe to be tracked throughout the procedure with 6 degrees of freedom. The real-time TRUS images were captured using a frame grabber. The tracking information and the synchronized ultrasound video stream were recorded with a dedicated workstation. A 12-core biopsy was performed for each patient and the needle track for each biopsy core was also documented. Biopsies were performed blinded to pre-procedural MRI data.
FIG. 1.
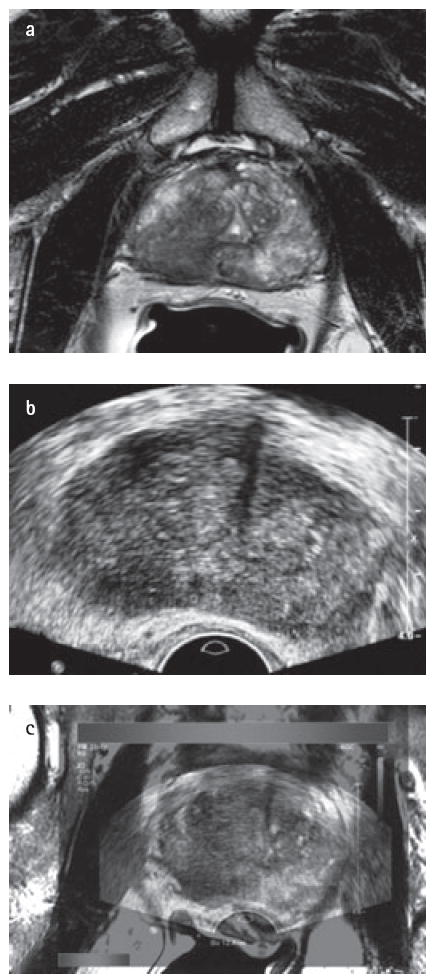
Magnetic resonance imaging-transrectal ultrasonography (MRI–TRUS) fusion in a 67-year-old male with an elevated prostate-specific antigen of 14 ng/dL. (a) Axial T2 weighted magnetic resonance image demonstrates a right-sided low signal intensity area in the right mid-peripheral zone. (b) Transrectal ultrasound image at axial plane shows a hypoechoic lesion at right mid-peripheral zone. (c) Fusion of T2-weighted MRI study with real-time transrectal ultrasound.
The position of each biopsy specimen was annotated on the MRI by translating the three coordinates of the needle track from the TRUS to the MRI. The analysis first identifies the specimen location on TRUS and then transposes the coordinates of the specimen location from TRUS to MRI using image-based registration software (Philips Research North America, Briarcliff Manor, NY, USA) that allows for image fusion between MRI and TRUS (Fig. 2). The software is customized from the software for MRI/TRUS-guided targeted biopsy [8,9] by replacing real-time ultrasound images and probe tracking with the recorded data (Fig. 3).
FIG. 2.
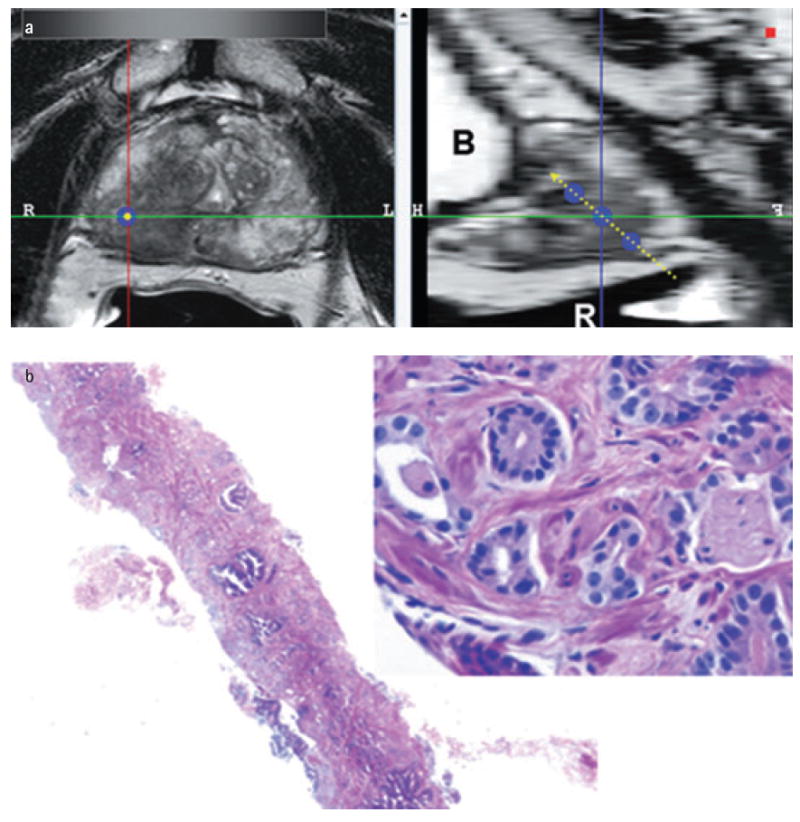
The software used for magnetic resonance imaging analysis at 12-core sextant specimen locations. (a) Left 2 column windows show multi-planar reconstructed images perpendicular to the biopsy core (single blue dot) and the sagittal view aligned with the tract of the biopsy core (triple blue dots with dashed yellow line) (B, bladder; R, rectum). (b) Right column window shows the corresponding haemotoxylin- and eosin-stained biopsy image with 2x and 40x magnification [Gleason 4+4 (70%)].
FIG. 3.
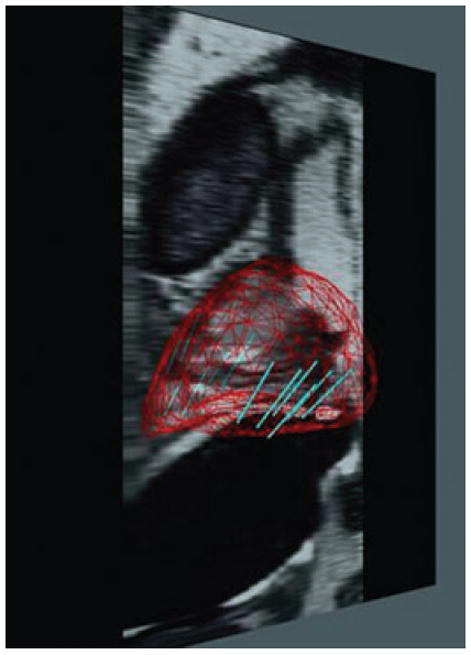
Reconstructed sagittal T2-weighted magnetic resonance image shows the map belonging 12-core sextant biopsy sites.
The site of each biopsy core was correlated with the T2W MRI findings at the anatomic location of each biopsy site. An analysis of the MRI sites was performed by two radiologists (B.T., P.L.C.) in consensus blinded to the 12-core biopsy results, using customized in-house software. The software enabled display of the multi-planar reconstructions of MR images based on the position and orientation of each specimen, allowing the radiologist to browse the T2W MRI images along the angle of each biopsy core and look for prostate cancer lesions on the MR image. Each biopsy core was modelled as a cylinder of 4 mm in diameter and 16 mm in length, which corresponded to the expected location of the biopsy core of the cutting needle. If a biopsy core intersected an MRI-visible tumour, it was classified as ‘positive’ for the sequence; otherwise it was classified as ‘negative’. On T2W MRI, the criterion for a ‘positive’ was a discrete well circumscribed, round-ellipsoid low-signal-intensity lesion within the prostate gland (Fig. 2).
MRI findings were correlated with the biopsy results. Tumour detection rate for T2W MRI was based on positive biopsy sites as well as Gleason score and this was compared with the histological biopsy result.
RESULTS
In all, 605 biopsies were obtained in 50 patients. Of the 605 cores, 56 (9.3%) were found to contain tumour tissue (n = 21 Gleason 6, n = 19 Gleason 7, n = 11 Gleason 8, n = 2 Gleason 9 and n = 3 prostatic intraepithelial neoplasia). On a per patient basis, 20 of 50 (40%) had positive biopsy cores.
Independent retrospective mapping of the TRUS biopsy sites on the MRI was achieved in all patients. Among the 605 total biopsy cores, 454 biopsy cores were found to be negative on T2W MRI, whereas 151 biopsy cores were found to be positive on MRI. T2W MRI thus identified 32 of 56 tumour cores (57.1%).
Among the 23 false negative MRIs, 14 (66.6%) were Gleason 6 and 9 (47.3) were Gleason 7. All 13 biopsy cores that were scored as Gleason 8 or 9 were detected with T2W MRI (Table 2).
TABLE 2.
T2-weighted magnetic resonance imaging MRI findings of detected tumour lesions according to their Gleason scores
| T2W MRI− | T2W MRI + | Total | |
|---|---|---|---|
| PIN | 1 | 2 | 3 |
| Gls 6 | 14 | 7 | 21 |
| Gls 7 | 9 | 10 | 19 |
| Gls 8 | – | 11 | 11 |
| Gls 9 | – | 2 | 2 |
| Total | 24 | 32 | 56 |
MRI, magnetic resonance imaging; T2W, T2-weighted; Gls, Gleason.
DISCUSSION
This study demonstrates that it is possible to document the location of TRUS-guided prostate biopsies using an MRI as a template. Since MRI of the prostate can be obtained with high resolution, this enables the recording of the anatomic location of each prostate biopsy on the MRI in a readily understood format. This method requires little change over the standard workflow of a 12-core biopsy, allowing it to be easily incorporated into daily practice. The MRI–TRUS fusion requires only about 5–7 min and the biopsy is otherwise exactly the same as a routine 12 core prostate biopsy. The MRI–TRUS fusion tracking prostate biopsy system was developed to prospectively guide biopsy of suspicious targets identified on MRI. However the system, as described here, can also be used to map the locations of conventionally obtained 12-core sextant biopsies back to the MRI. This enables documentation that the prostate was uniformly sampled and, if needed, additional biopsies can be obtained from regions not previously sampled. This ensures that different regions of the prostate are sampled on the subsequent biopsies. This knowledge could result in a broader geographic sampling for any patient undergoing repeat biopsies, and theoretically reduces the chance of repeatedly under-sampling specific regions of the peripheral zone. Documenting biopsy location could overcome the difficulty in sampling certain areas of the peripheral zone with specific end-fire or side-fire TRUS biopsy transducers. Additionally, accurately knowing cancer location, both on MRI and pathologically at the same location, could be useful in planning focal therapy such as radiation boost during intensity-modulated radiation therapy, placement of seeds during brachytherapy, or even more investigational types of focal therapy such as cryotherapy, laser or alcohol ablation or high-intensity focused ultrasound.
An additional advantage of this method is that it can be used to correlate imaging with pathology in patients not undergoing prostatectomy. This is especially important as the populations undergoing active surveillance or radiation therapy lack other validation techniques. Further, if repeat biopsy of an area of concern were desirable, prior suspicious biopsies could become locations for future surveillance biopsies. The radiology pathology correlation could be useful for validation of novel imaging techniques, with mapping of experimental imaging to biopsy location. Free-hand prostate biopsy is notoriously difficult to correlate with imaging because the location of the specimen is not accurately recorded. Prostatectomy-based imaging-pathology correlation may be skewed by patient selection since only surgical patients have pathology correlation. Since the population undergoing biopsy includes most patients suspected of prostate cancer, this method of validation may be more broadly representative than correlations based only on whole mount sections of prostatectomy specimens.
Results of our retrospective imaging analysis reveal that anatomic T2W MRI detected 44% (19/43) of low-intermediate risk lesions (Gleason 7 and below) [10] but 100% (13/13) of high risk lesions (Gleason 8 and above). These findings are in concordance with previous studies on the accuracy of T2W imaging for detecting prostate cancer.
Several other methods of prostate biopsy mapping have been suggested. Cool et al. developed a 3D TRUS prostate biopsy system that provided needle guidance and recorded biopsy location on TRUS. The system was validated on anatomical prostate phantoms, but not in clinical patients and it generated 3D models of the prostate with volume errors of less than 3.5% and mean boundary errors of less than 1 mm [2]. Mozer et al. used 2D TRUS guidance to register biopsy locations to a reference 3D ultrasound volume in 32 patients who underwent 12-core biopsy. They were able to improve the distribution of prostate biopsies as well as map biopsy location; however their mapping was limited to TRUS only [3]. Our system differs from the above-mentioned approach because it is readily applicable to an out-patient setting, and incorporates MRI into the biopsy mapping.
This study has several limitations. For example, it was assumed that the MRI/TRUS fusion used for retrospective analysis was accurate. However this system may introduce some errors secondary to deformation of the prostate gland caused by the endorectal coil thereby making it difficult to exactly fuse with the TRUS images. Phantom and cadaver studies with this device have demonstrated that the accuracy of the TRUS/MRI fusion system is approximately 2.4 ± 1.2 mm [9]. This would limit the reasonable detectable lesion size to approximately 5 × 5 mm lesions which is probably the minimal lesion size that can be identified with confidence on prostate MRI. Additionally, the number of patients enrolled in the current study is relatively small and most of the patients were low–intermediate risk patients. However this group is representative of the majority of patients with prostate cancer in the United States who have undergone screening with annual serum PSA. The MRI–TRUS fusion tracking prostate biopsy system was designed for prospective targeting of lesions that are suspicious on MRI. However, this tool may also be used for mapping locations of conventional, systematic sextant biopsies.
CONCLUSION
In conclusion, MRI–TRUS-fused tracked biopsy mapping is a feasible method of documenting the anatomic locations of prostate biopsies and validating multi-parametric MRI results with histology correlation. This method can be applied to those patients who have prostate cancer who are not undergoing surgery but rather surveillance or radiation therapy. Moreover, it can be useful in patients on active surveillance who undergo subsequent repetitive biopsy for rising PSA values because samples can be obtained from regions of the prostate not previously evaluated. MRI–TRUS-fused tracked biopsy mapping may be useful for improving the diagnosis of prostate cancer and potentially for directing focal nerve-sparing therapies in the future.
What’s known on the subject? and What does the study add?
Currently, systematic prostate biopsies are obtained with minimal information about their actual location.
This study demonstrates that a electromagnetically tracked ultrasound probe can be used to guide biopsies into specific areas of the prostate. By registering the ultrasound to an MRI scan of the prostate, obtained prior to biopsy, it is possible to accurately map the location of biopsies. Thus, if a patient requires a repeat biopsy, or there is a question about whether a specific area of the prostate was sampled, this system can be used to more accurately guide biopsies in the future.
Acknowledgments
NIH and Philips have intellectual property in the field. This study was supported in part by the Intramural Research Program of the NIH. NCI contract number HHSN261200800001E.
Abbreviation
- T2W
T2-weighted
Footnotes
CONFLICT OF INTEREST
Sheng Xu and Jochen Kruecker are employees of Philips Research North America. Yuxi Pang is an employee of Philips Healthcare.
References
- 1.American Cancer Society. Cancer Facts & Figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 2.Cool D, Sherebrin S, Izawa J, Chin J, Fenster A. Design and evaluation of a 3D transrectal ultrasound prostate biopsy system. Med Phys. 2008;35:4695–707. doi: 10.1118/1.2977542. [DOI] [PubMed] [Google Scholar]
- 3.Mozer P, Baumann M, Chevreau G, et al. Mapping of transrectal ultrasonographic prostate biopsies: quality control and learning curve assessment by image processing. J Ultrasound Med. 2009;28:455–60. doi: 10.7863/jum.2009.28.4.455. [DOI] [PubMed] [Google Scholar]
- 4.Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol. 2009;27:4321–6. doi: 10.1200/JCO.2008.20.3497. [DOI] [PubMed] [Google Scholar]
- 5.Onik G, Barzell W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol Oncol. 2008;26:506–10. doi: 10.1016/j.urolonc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Merrick GS, Taubenslag W, Andreini H, et al. The morbidity of transperineal template-guided prostate mapping biopsy. BJU Int. 2008;101:1524–9. doi: 10.1111/j.1464-410X.2008.07542.x. [DOI] [PubMed] [Google Scholar]
- 7.Wood BJ, Zhang H, Durrani A, et al. Navigation with electromagnetic tracking for interventional radiology procedures: a feasibility study. J Vasc Interv Radiol. 2005;16:493–505. doi: 10.1097/01.RVI.0000148827.62296.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh AK, Kruecker J, Xu S, et al. Initial clinical experience with real-time transrectal ultrasonography-magnetic resonance imaging fusion-guided prostate biopsy. BJU Int. 2008;101:841–5. doi: 10.1111/j.1464-410X.2007.07348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI–TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13:255–64. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooperberg MR, Lubeck DP, Mehta SS, Carroll PR CaPSURE. Time trends in clinical risk stratification for prostate cancer: implications for outcomes (data from CaPSURE) J Urol. 2003;170:S21–5. doi: 10.1097/01.ju.0000095025.03331.c6. [DOI] [PubMed] [Google Scholar]


