Abstract
Monocytes utilise a variety of chemokines to traffic to atherosclerotic plaques. CX3C chemokine ligand 1 (CX3CL1 & Fractalkine) and its receptor CX3CR1 and monocyte chemoattractant protein 1 (CCL2) have been identified as chemokines/receptors that have an important role in the migration and recruitment of monocytes during the pathogenesis of several inflammatory diseases including atherosclerosis. DNA vectors containing single chain variable region fragment (scFv) for DC-targeted receptor DEC205 were cloned with mouse CX3CR1 and CCL2 genes respectively, and vaccinated into C57/BL6 mice weekly for 3 weeks. Induced anti-CX3CR1 and anti-CCL2 in vaccinated mice was examined by ELISA and Western Blot analysis, while the cellular response was examined by ELISPOT. The inhibition of chemotaxis of J774 macrophages to Py-4-1 endothelial cells was examined by in vitro transwell migration assay using serum collected from vaccinated mice. All vaccinated mice generated anti-CX3CR1 and anti-CCL2 Ab and cellular response by 8 weeks after DNA vaccination. Macrophage migration towards TNF-α activated endothelial cells was significantly inhibited by serum containing both anti-CX3CR1 or anti-CCL2 Ab from vaccinated mice. These results demonstrate that DC-targeting of DNA vaccines to self-antigens generates functional immune responses which can inhibit specific key chemotactic targets. This suggests a potential therapeutic role for chemokine/receptor DNA vaccination in atherosclerosis, where chemotaxis has a pivotal role in the inflammatory process.
Keywords: DNA Vaccination, DEC205, macrophage migration, chemokine, CX3CR1, CCL2
Introduction
Monocytes have been shown to play a pivotal role in mediating the process of atherogenesis. Vascular inflammation caused by the accumulation of modified lipids in blood vessels induces the recruitment of monocytes and initiates atherosclerosis [1-4]. The actively recruited monocytes give rise to macrophages and other antigen presenting cells such as DC, when driven by macrophage colony-stimulating factor (M-CSF) and other differentiation factors [5, 6]. Monocyte-derived macrophages within the atherosclerotic lesions undergo necrosis which eventually leads to myocardial infarction, unstable angina [7], sudden cardiac death, and stroke [8].
Recruitment of blood-borne monocytes in response to inflammation-induced activation of endothelial cells is a key step of early lesion progression [9, 10]. Activated endothelial cells secrete chemokines that interact with cognate chemokine receptors on monocytes and promote their chemotaxis [11]. CX3CL1, CCL2 (MCP-1) and their receptors CX3CR1 and CCR2 are two important chemokine/receptor pairs which have been identified to contribute significantly to monocyte recruitment in atherosclerosis. Atherosclerosis is abrogated in CX3CR1-/-[12, 13] and CCL2-deficient [14] mouse models as a consequence of significant reduction of monocyte entry.
DNA vaccination delivers plasmid DNA encoding the target antigen, either alone or in combination with enhancing elements, to induce both humoral and cellular immune responses. Disadvantages of specific antibody therapy in comparison to DNA vaccination include the requirement for repeated dosing and the development of an immune response against the therapeutic antibody. However, use of DNA vaccination has been limited by its restricted ability to induce strong immune responses [7, 15]. This has been overcome by targeting vaccine antigens to dendritic cells (DC). DNA vaccine encoding a fusion protein comprised of the vaccine antigen and a single-chain Fv antibody (scFv) specific for the DC-restricted antigen-uptake receptor DEC205 was observed to substantially increase antibody levels and cellular response [16]. Previous studies from our lab and collaborators have demonstrated that DNA vaccination against T Cell Receptor (TCR) subsets in Heymann nephritis, and against the chemokine CCL2 in Adriamycin nephropathy is protective and induces specific cellular and antibody responses against the target antigen [17-19].
Our current study demonstrates that a DC-targeted DNA vaccination against CX3CR1 and CCL2 successfully induces humoral and cellular responses in mice. The generated antibodies restrict the motility of macrophages towards activated endothelial cells in an in vitro functional analysis. These findings suggest a potential therapeutic role of chemokine/receptor DNA vaccination in preventing inflammatory diseases such as atherosclerosis.
Materials and methods
Construction and modification of the CX3CR1 and CCL2 DNA vaccines
Vectors DEC205 (pSC-DEC-OLLA) and control (pSC-GL117-OLLA) were kindly provided by Dr Godwin Nchinda from USA [16]. Mouse CX3CR1 and CCL2 cDNA were amplified by reverse transcription-PCR from RNA extracts from kidney of C57/BL6 mouse using the specific primers, mouse CX3CR1: For 5'-CTC ACCAT GTC CAC CTC CTTtcga-gcggccgc-CTCACCATGTCCACCTCCTT-3', Rev 5'-GGA GAC CCC TTC AGA GCA Gctga-ccgcgg -GGAGACCCCTTCAGAGCAG-3', and mouse CCL2: For 5'-TCGA-gcggccgc-ACCATGCAGGTCCCTGT-3', Rev 5'-CTGA-ccgcgg-GCA TCA CAG TCCGAGTC-3'. The PCR conditions were 95°C for 5 min (1 cycle); 95°C for 45s, 60°Cfor45s, and 72°C for 1 min (35 cycles) with the final cycle at 72°Cfor 7 min. The CX3CR1 and CCL2 PCR products were cloned separately into DEC205 (DEC-CX3CR1 and DEC-CCL2) and control vector (Con -CX3CR1 and Con-CCL2) to make the two sets of DNA vaccines. The sequences of the CX3CR1 and CCL2 vaccines were confirmed by and DNA sequencing after cloning using specific primers: For 5'-GCGAATGAATTGGGACCT-3’ and Rev 5'-cttctgagatgagtttttgttcg-3'. Plasmid DNA was prepared in large-scale using Qiagen Plasmid Maxi Kit(Qiagen).
DNA vaccination
Male C57/BL6 mice at age 6 weeks (weighing 18-20g) were purchased from the Animal Resources Centre in Perth, Australia and maintained under standard sterile conditions in the Department of Animal Care at Westmead Hospital. Experiments were carried out in accordance with protocols approved by the Animal Ethics Committee of Sydney West Area Health Service. Mice were divided into four groups: CX3CR1 vaccinated (n=4), CCL2 vaccinated (n=4). CX3CR1 peptide boosts alone control (n=2), and normal control (n=2). Mice were pretreated with 0.5% bupivacaine (10 μg/g body wt; Sigma, St. Louis, MO) by intramuscular injection into tibialis anterior muscle 1 wk before plasmid DNA vaccination. Plasmid DNA (50 μg) was injected three times at the same site as bupivacaine. One week after the third DNA vaccinations, mice from the peptide boosts alone group were immunized with a prime boost of CX3CR1 peptide mixture (100 μg/mice) and Poly IC (50 μg/mice), mice from the non-vaccination control group were injected with saline only.
Serum antibody titers
Serum anti-CX3CR1 and anti-CCL2 antibody titers were evaluated by ELISA assay for all groups of mice. Briefly, 96-well Immuno ELISA microtiter plate (NUNC, in vitro Technology, Australia) was coated with CX3CR1 peptide or recombinant CCL2 at a concentration of 1 μg/well in 100 μl coating buffer. Sample mouse sera were diluted 50 and 500 times and added to the coated ELISA plate. Normal mouse serum was used as the negative control. Goat anti-mouse IgG alkaline phosphatase conjugated Ab (Sigma) and p-Nitro-phenol phosphate (Sigma) as substrate were used sequentially for the Ab titer analysis. All samples and controls were added in duplicate to the plates. Absorbance was read at 450 nm on an ELISA plate reader (Multiskan Ascent, Pathtech, Australia).
Western blot analysis
Presence of anti-CX3CR1 antibody in DEC-CX3CR1, Con-CX3CR1 and mice of CX3CR1 peptide boosted alone was further verified by Western Blot analysis. Cell lysates from NIH 3T3 Mouse embryonic fibroblast cell line were separated by SDS-PAGE (4-20% Tris-glycine gels; novex, Germany) on reducing conditions and transferred onto Immobilon-P™ membrane (Millipore, Herts, UK). Membranes were incubated in blocking buffer (TBST, 5% skim milk powder) at room temperature for 1 h and probed with diluted sera from mice mentioned above (1/100 dilution) at 4°C overnight. Washing was followed by 1 h, room temperature incubation with a horseradish-peroxidase-linked rabbit anti-mouse Ig (Amersham, Bucks, UK) (1/2000). Detection was carried out using a chemiluminescent ECL detection system (ECL; Amersham).
Enzyme-linked immunospot assay
The 96-well multiscreen plates (Millipore; Bedford, MA) were coated with capture Ab, monoclonal rat anti-mouse IFN-γ (Biosource, Camarillo, CA; 25ng/well). Lymphocytes from spleens of all groups of mice were harvested and loaded in triplicate into the pre-coated ELIspot plate at 5 × 106 cells per well in RPMI 1640 with 5% Fetal Calf Serum (GIBCO), then stimulated with either CX3CR1 peptide or recombinant CCL2 protein at concentration of 0.2 mg/ml and incubated at 37°C in 5% CO2 for 24 h. The detection Ab, biotin-conjugated rat anti-mouse IFN-γ (Biosource; 25 ng/well), was added, then kept at 4°C overnight, incubated with streptavidin alkaline phosphatase (Becton Dickinson, San Jose, CA) at room temperature for 2 h, and then developed with BCIP/NBT kit (Bio-Rad). Spots were counted under a dissecting microscope. The results are expressed as number of spot-forming cells/5 × 106 splenocytes.
Cell culture
J774 mouse macrophage cells were obtained from ATCC and were maintained as adherent culture in Dulbecco's modified Eagle's medium (GIBCO) supplemented with 10% (vol/vol) heat-inactivated Fetal Calf Serum (GIBCO) and grown in 5% CO2 at 37°C. Py-4-1 mouse endothelial cells were provided by Dr. Vicki Bautch (University of North Carolina, Chapel Hill, NC) and were grown in DMEM (GIBCO) containing 10% heat-inactivated Fetal Calf Serum (GIBCO) in 10% CO2 at 37°C as adherent culture. At confluence, cells were passaged with trypsin-EDTA (0.05%trypsin/0.53 mM Na4EDTA) for 1-5 minutes.
in vitro chemotaxis assays
Blockade of J774 macrophage migration towards activated Py-4-1 endothelial cells was measured by chemotaxis through a 5 μm-pore polycarbonate filter in Transwell chambers (Corning, Lowell, MA, USA). Py-4-1 endothelial cells used in chemotaxis assays were stimulated with recombinant mouse TNF-α (25 ng/ml; R&D Systems, Inc.) for 12 h in serum-free DMEM medium and maintained overnight in the lower chamber (7 × 104 cells/well). Macrophages were pre-incubated with sera from all groups of mice for 2 h, resuspended in serum-free DMEM and placed in the top chamber (1 × 105 cells /well). Duplicate wells were performed for each group of mice. After 2 h incubation, migrated macrophage cells were stained with DAPI (Invitrogen) and their numbers were counted by immunofluorescence microscopy. A minimum of 20 non-overlapping consecutive fields of view were taken under 200 × magnification. Results were expressed as average number of cells migrated per High Power Field (HPF).
Statistical analysis
Statistical analysis was performed using oneway analysis of variance (ANOVA) for multiple comparisons. Results are expressed as the groups mean ± SEM. Two group differences were analyzed by Student's t-test, with a p value (two-tailed) less than 0.05 considered statistically significant.
Results
Construction and expression of scDEC and scControl DNA vaccines
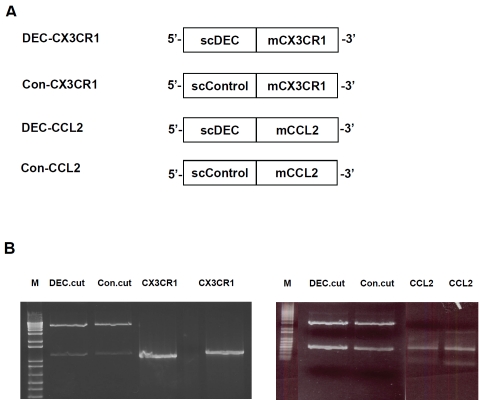
To generate DC-targeted CX3CR1 and CCL2 DNA vaccines, the open reading frame for mouse CX3CR1 and CCL2 extracellular domains were cloned separately into pSC-DEC-OLLA vector (scDEC), which is a modified pcDNA3.1 vector that contains gene encoding single-chain antibody specific for mouse DEC205 [16]. We thereby generated fusion DNA constructs with CX3CR1 or CCL2 genes fused in frame to the C-terminus of scDEC as shown in Figure 1A and 1B. For comparison, two non-DC-targeted CX3CR1 and CCL2 DNA vaccines (Con-CX3CR1 and Con-CCL2) were generated as above with the use of pSC-GL117-OLLA (scControl) vector instead of scDEC vector.
Figure 1.
Construction and in vitro expression of DEC-CX3CR1, Con-CX3CR1, DEC-CCL2 and Con-CCL2 DNA vaccines. (A) Open reading frames for mouse CX3CR1 and CCL2 extracellular domain were fused in frame to the C-terminus of scDEC or scControl vector. (B) The CX3CR1 and CCL2 PCR products were ligated and cloned separately into DEC205 (DEC-CX3CR1 and DEC-CCL2) and control vector (Con-CX3CR1 and Con-CCL2), as shown in agarose gels and visualized under UV using gel-doc 1000 (BIO-RAD, Australia, two separate bands as indicated in DEC.cut and Con.cut after restriction digestion).
The fusion DNA constructs (DEC-CX3CR1, Con-CX3CR1, DEC-CCL2, and Con-CCL2) were transiently transfected into 293 cells for immunoblot analyses to verify their expressions. The sequences of each inserted genes were further confirmed by DNA sequencing.
CX3CR1 and CCL2 DNA vaccination induces anti-CX3CR1 and anti-CCL2 antibody response
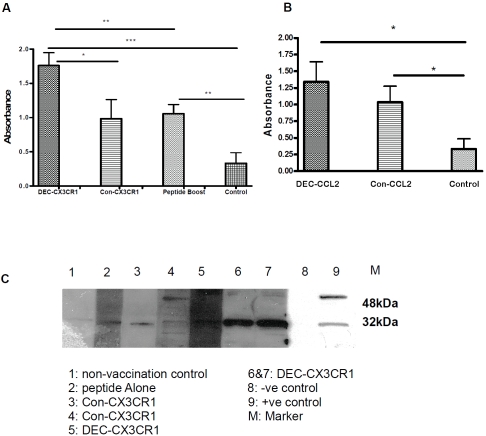
Mice were injected intramuscularly (i.m.) thrice with DEC-CX3CR1/Con-CX3CR1 or DEC-CCL2/ Con-CCL2 DNA vaccines at weekly intervals (Figure 2). One week after the third vaccination, mice from peptide boost alone group were boosted by CX3CR1 peptide. To determine whether DC-targeted vaccine could induce a stronger B cell response than control vaccine, we measured serum anti-CX3CR1 and anti-CCL2 antibody level using Enzyme-linked immunosorbent assay (ELISA). Serum collected from non-vaccinated mice was used as control. A significant increase of anti-CX3CR1 antibody level was illustrated in DEC-CX3CR1 vaccinated mice as compared to Con-CX3CR1 vaccinated mice (1.76 ± 0.19 vs 0.98 ± 0.28, p < 0.05), peptide boost alone (p < 0.01), and non-vaccinated mice (p < 0.001) as shown in Figure 3A. Interestingly, non-vaccinated mice receiving CX3CR1 peptide boost also have a minor increase of anti-CX3CR1 antibody level as compared with control (P < 0.01). We observed a three-fold increase in anti-CCL2 antibody level in DEC-CCL2 vaccinated mice as compared to non-vaccinated ones (Figure 3B, 1.34 ± 0.30 vs 0.33 ±0.15, p< 0.05).
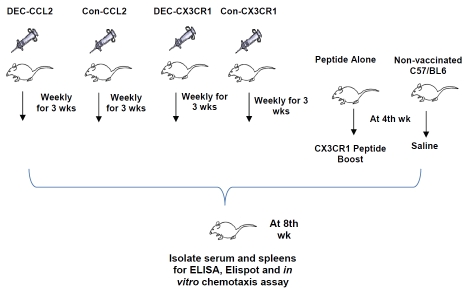
Figure 2.
Experimental protocol for DNA vaccination of C57/BL6 mice with the scDEC and scControl vectors. Mice were pretreated with bupivacaine one week before plasmid DNA vaccination. Plasmid DNA was injected three times at weekly interval. After one week, mice from peptide boosts alone group were boosted with CX3CR1 peptide mixture and mice from non-vaccination control group were injected with saline. Mice were sacrificed to harvest organs for ELISA, Western Blot, Elispot and in vitro migration assay five weeks after vaccination.
Figure 3.
DNA vaccination induced significant humoral responses, anti-CX3CR1 and anti-CCL2 antibody levels in serum were measured by ELISA and Western Blot. Absorbance was read at 450 nm corrected against background reading at 570nm (The results represent four independent experiments). (A) DEC-CX3CR1 vaccine induces significantly higher serum anti-CX3CR1 antibody level as compared to Con-CX3CR1 vaccination, peptide alone and non-vaccinated group (*P < 0.05; **P < 0.01; ***P < 0.001). (B) Both DEC-CCL2 and Con-CCL2 vaccine induces significantly higher serum anti-CCL2 antibody as compared to non-vaccinated group (*P < 0.05; *P < 0.05). (C) Presence of anti-CX3CR1 antibody after vaccination by im-munoblot analysis. NIH/3T3 cells were probed with sera from all groups of mice. Strong bands correspond to anti-CX3CR1 of size 32kDa are shown in lane 6&7.
To further verify the presence of anti-CX3CR1 antibody in DEC-CX3CR1 vaccinated mice, an immune-blotting analysis was performed. As illustrated in Figure 3C, specific bands of 32kDa were observed in sera from DEC-CX3CR1 and Con-CX3CR1 vaccinated mice as well as mice treated with CX3CR1 peptide boost alone. An enhanced effect of CX3CR1 peptide boost on anti-CX3CR1 antibody generation was also observed .
CX3CR1 and CCL2 DNA vaccination induces anti-CX3CR1 and anti-CCL2 cellular response
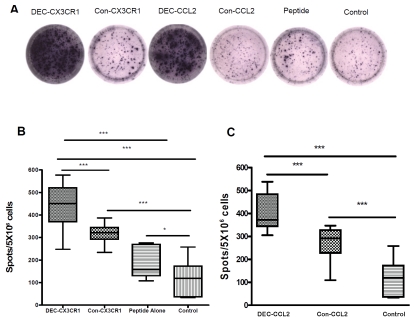
To assess the cellular response of anti-CX3CR1 and anti-CCL2 DNA vaccination in comparison with peptide boost alone and non-vaccinated control, the numbers of IFN-γ producing T cells from spleens of vaccinated mice were assessed by ELISpot assay (Figure 4). Mice vaccinated with DEC-CX3CR1 DNA had more splenic T cells that produced IFN-γ when stimulated with 0.2 mg/ml CX3CR1 peptide in vitro than mice received peptide boost alone and non-vaccinated control mice (Figure 4B, both, p < 0.001). Mice vaccinated with DEC-CCL2 DNA had more splenic T cells that produced IFN-γ when stimulated with 0.2 mg/ml recombinant CCL2 protein in vitro than non-vaccinated control mice (Figure 4C, p < 0.001). The number of IFN-γ producing T cells in non-vaccinated mice with CX3CR1 peptide boost was also greater than in non-vaccinated controls (P < 0.05). A significant increase of the number of IFN-γ producing T cells was also observed in DEC-CX3CR1 and DEC-CCL2 vaccinated mice as compared to Con-CX3CR1 and Con-CCL2 vaccinated mice respectively (both, P < 0.001)
Figure 4.
DNA vaccination induced significant cellular responses of splenic IFN-γ-producing T cells as examined by enzyme-linked immunospot assay. (A) Represented spots on each group as indicated shown a large number of spot forming cells in DEC-CX3CR1 and DEC-CCL2 vaccinated mice. (B) quantitative analysis shown that there are significantly higher number of IFN-γ-producing splenic T cells in DEC-CX3CR1 vaccinated mice than in other groups, (value = mean ± SEM, all ***p < 0.001). (C) quantitative analysis shown that there are significantly higher number of IFN-γ-producing splenic T cells in DEC-CCL2 vaccinated mice than in other groups, (value = mean ± SEM, both ***p < 0.001).
Inhibitory effects of antiserum neutralization on macrophage chemotaxis towards activated endothelial cells in vitro
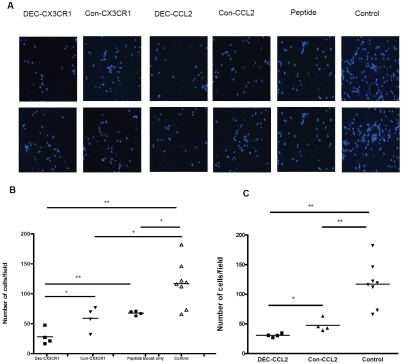
To explore the mechanism whereby DNA vaccination of CX3CR1 and CCL2 reduced monocyte infiltration, antisera from mice were tested in vitro for their blocking effects against CCL2-induced monocyte chemotaxis (Figure 5A). The total numbers of migrated macrophages were significantly lower after incubation of antisera from mice that were vaccinated with DEC-CX3CR1 and DEC-CCL2 than with antisera from non-vaccinated control mice (both, P < 0.001), whereas a minor decreased total number of migrated macrophages was also observed with antisera of mice that had undergone CX3CR1 peptide boost alone as compared to non-vaccination control (P < 0.05). The number of migrated macrophages was half as many as in DEC-CX3CR1 vaccinated mice antisera than in Con-CX3CR1 vaccinated mice (28.28 ± 6.77 vs. 59.08 ± 9.86, P < 0.05) and mice received peptide boost alone (28.28 ± 6.77 vs. 67.75 ± 1.80, P < 0.01) as shown in Figure 5B, while 50% lower in DEC-CCL2 vaccinated mice antisera as compared to Con-CCL2 vaccinated mice (30.61 ± 1.59 vs. 47.71 ± 5.35, P < 0.05), as shown in Figure 5C.
Figure 5.
Inhibition of macrophage chemotaxis by antisera of DNA vaccinated mice. (A) Number of cells migrated per High Power Field (HPF) under microscopy of each group as indicated showed significantly decreased number of migrated macrophage upon pre-incubation of antisera from DEC-CX3CR1 and DEC-CCL2 vaccinated mice (B)&(C) quantitative analysis shown that there are significantly lower number of migrated macrophages upon pre-incubation of antisera from DEC-CX3CR1 and DEC-CCL2 vaccinated mice than in other groups (Values = means ± SEM, *P < 0.05; **P < 0.01; ***P < 0.001. The results represent four independent experiments).
Discussion
Atherosclerotic disease underlies the leading cause of death in industrialized societies and is likely soon to attain this status worldwide [20]. Atherosclerosis is characterized by progressive infiltration of monocytes and formation of fibrous plaques in blood vessels leading eventually to formation and rupture of necrotic cores. Specific immunotherapeutic strategies including antibody or gene-targeted neutralization of chemokines, chemokine receptors, cytokines, or adhesion molecules and co-stimulatory pathways prevent monocyte entry to some extent in studies in mice [10, 12, 14, 25, 26]. However, these interventions lead only to partial inhibition of lesion development and fail to fully abrogate the progression of atherosclerosis. As a result, therapeutic strategies that target early stage atherogenesis could be more effective in the prevention and treatment of this disease.
DNA vaccination is a recently developed approach in which genes that encode target antigens are delivered and expressed in vivo within host cells. This technique has been proven to be very effective and promising in mouse models of infectious diseases, cancers and other autoimmune disorders including multiple sclerosis, experimental autoimmune encephalomyelitis and allergic responses [16, 21-26]. One of the major disadvantages of DNA vaccination which limits its clinical application is the restricted ability to induce strong immunity in the host [27]. This had led to the introduction and development of various strategies including adjuvant modification, electroporation delivery and self-antigen targeting to enhance the immunogenicity [16, 23, 28-30]. We therefore generated DNA vaccines encoding CX3CR1 and CCL2, which selectively targeting dendritic cells via the endocytic receptor, DEC205/CD205. The fusion vectors were delivered by electroporation to further enhance the immunogenicity. We demonstrated a significant increase of B and T cell responses against CX3CR1 and CCL2 in DC-targeted vaccinated mice in comparison with mice received non-DC targeted vaccination. These data is consistent with similar observations in recent studies [31-33].
Recently, there has been increased interest and progress in research on DNA vaccination in chronic inflammatory diseases. Immune targets include TCRs, cytokines and chemokines. Our lab and collaborators have previously established an animal model of DNA vaccination against TCR subsets in Heymann nephritis (HN) [34] and against the chemokine CCL2 in Adriamycin nephropathy (AN) [19]. In both models, DNA vaccination was shown to be protective against chronic renal injury.
Its ability to modulate inflammatory responses suggests the potential of DNA vaccination as a therapeutic approach to target early atherogenesis. Previous studies involving genetically modified mice have demonstrated that DNA vaccination against atherosclerosis disease-related immune targets including vascular endothelial growth factor receptor 2 (VEGFR2) [35, 36], CD99 [37], and TIE2 cells [38] all attenuate atherogenesis in some extent. We demonstrated that antisera from both CX3CR1 and CCL2 vaccinated mice decreased the migration of macrophages towards activated endothelial cells in an in vitro assay which mimics the situation in atherosclerosis. This suggests that, functionally blocking CX3CR1 and CCL2 by DNA vaccination may also reduce the severity of atherosclerosis.
In comparison to various current treatments of atherosclerosis, DNA vaccination can provide a relatively cheap and fast alternative way since atherosclerosis initiates at young ages and such patients will benefit from the vaccination strategy early in their lives that prevents the formation and development of atherosclerotic lesions. Although the results from our current study are consistent with some of others, it should be mentioned that our study was only performed in a non-disease model and with limited number of experimental objects. In order to extrapolate these data, further studies are required in animal models with experimental atherosclerosis induced. More importantly, all data collected from animal models must be further verified by performing studies in the human situations, while the risks of side effects as a consequence of blocking bioactive molecules also needed to be carefully assessed.
Conclusively, in this study, we vaccinated mice by specifically targeting the chemokine receptor CX3CR1 and chemokine CCL2, both of which are important to monocyte recruitment in atherosclerosis. Upon vaccination, mice generated significant cellular and humoral responses, and the inhibitory effects of the antibodies on macrophage transmigration were demonstrated in vitro. In addition, on average a doubling increase of antibody levels and T-cell responses was induced by DC-targeting of DNA vaccination as compare to non-DC-targeting DNA vaccination. This pilot study suggests that DNA vaccination using self-targets against key chemokines and their receptors involved in monocyte recruitment maybe an attractive therapeutic strategy to prevent and treat atherosclerosis.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia (NHMRC grant 571343 and 632519). We thank the animal house staff in the Westmead Hospital Animal House Facility for care of the animals.
References
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 3.Rollins BJ. Chemokines and atherosclerosis: what Adam Smith has to say about vascular disease. J Clin Invest. 2001;108:1269–1271. doi: 10.1172/JCI14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol. 2009;20:370–378. doi: 10.1097/MOL.0b013e3283309848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky Ml. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 7.Ada G. Vaccines and vaccination. N Engl J Med. 2001;345:1042–1053. doi: 10.1056/NEJMra011223. [DOI] [PubMed] [Google Scholar]
- 8.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 9.Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 10.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 13.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1-/- mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 15.Ada G, Ramshaw I. DNA vaccination. Expert Opin Emerg Drugs. 2003;8:27–35. doi: 10.1517/14728214.8.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Nchinda G, Kuroiwa J, Oks M, Trumpfheller C, Park CG, Huang Y, Hannaman D, Schlesinger SJ, Mizenina O, Nussenzweig MC, Uberia K, Steinman RM. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest. 2008;118:1427–1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H, Wang Y, Tay YC, Zheng G, Zhang C, Alexander SI, Harris DC. DNA vaccination with naked DNA encoding MCP-1 and RANTES protects against renal injury in adriamycin nephropathy. Kidney Int. 2005;67:2178–2186. doi: 10.1111/j.1523-1755.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Walters G, Knight JF, Alexander SI. DNA vaccination against specific pathogenic TCRs reduces proteinuria in active Heymann nephritis by inducing specific autoantibodies. J Immunol. 2003;171:4824–4829. doi: 10.4049/jimmunol.171.9.4824. [DOI] [PubMed] [Google Scholar]
- 19.Zheng G, Wang Y, Xiang SH, Tay YC, Wu H, Watson D, Coombes J, Rangan GK, Alexander SI, Harris DC. DNA vaccination with CCL2 DNA modified by the addition of an adjuvant epitope protects against “nonimmune” toxic renal injury. J Am Soc Nephrol. 2006;17:465–474. doi: 10.1681/ASN.2005020164. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: heart disease and stroke statistics-2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 21.Fissolo N, Montalban X, Comabella M. DNA-based vaccines for multiple sclerosis: Current status and future directions. Clin Immunol. 2010 doi: 10.1016/j.clim.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Opriessnig T, Gomes-Neto JC, Hemann M, Shen HG, Beach NM, Huang Y, Halbur PG, Meng XJ. An experimental live chimeric porcine circovirus 1-2a vaccine is efficacious at decreasing porcine circovirus 2b viremia when administered intramuscularly or orally in a porcine circovirus 2b and porcine reproductive and respiratory syndrome virus dual-challenge model. Microbiol Immunol. 2011;55:863–873. doi: 10.1111/j.1348-0421.2011.00385.x. [DOI] [PubMed] [Google Scholar]
- 23.Potter H, Heller R. Transfection by electroporation. Curr Protoc Neurosci. 2011 doi: 10.1002/0471142301.nsa01es57. Appendix 1: 1E. [DOI] [PubMed] [Google Scholar]
- 24.Zhou B, Ensell M, Zhou Y, Nair U, Glickstein J, Kermany MH, Cai Q, Cai C, Liu W, Deng YP, Kakigi A, Barbieri M, Mora M, Kanangat S, Yoo TJ. Prevention and treatment of DNA vaccine encoding cockroach allergen Bla g 1 in a mouse model of allergic airway inflammation. Allergy. 2011 doi: 10.1111/j.1398-9995.2011.02727.x. [DOI] [PubMed] [Google Scholar]
- 25.Cho HI, Celis E. Design of immunogenic and effective multi-epitope DNA vaccines for melanoma. Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libbey JE, Fujinami RS. Experimental autoimmune encephalomyelitis as a testing paradigm for adjuvants and vaccines. Vaccine. 2011;29:3356–3362. doi: 10.1016/j.vaccine.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youssef S, Wildbaum G, Maor G, Lanir N, Gour-Lavie A, Grabie N, Karin N. Long-lasting protective immunity to experimental autoimmune encephalomyelitis following vaccination with naked DNA encoding C-C chemokines. J Immunol. 1998;161:3870–3879. [PubMed] [Google Scholar]
- 28.Diehl L, Den Boer AT, van der Voort EI, Melief CJ, Offringa R, Toes RE. The role of CD40 in peripheral T cell tolerance and immunity. J Mol Med (Berl) 2000;78:363–371. doi: 10.1007/s001090000126. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds J, Khan SB, Allen AR, Benjamin CD, Pusey CD. Blockade of the CD154-CD40 costimulatory pathway prevents the development of experimental autoimmune glomerulonephritis. Kidney Int. 2004;66:1444–1452. doi: 10.1111/j.1523-1755.2004.00907.x. [DOI] [PubMed] [Google Scholar]
- 30.Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, Leung L, Otten GR, Thudium K, Selby MJ, Ulmer JB. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164:4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 31.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste Dl, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demangel C, Zhou J, Choo AB, Shoebridge G, Halliday GM, Britton WJ. Single chain antibody fragments for the selective targeting of antigens to dendritic cells. Mol Immunol. 2005;42:979–985. doi: 10.1016/j.molimm.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 33.Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, Huang Y, Schlesinger SJ, Park CG, Nussenzweig MC, Granelli-Piperno A, Steinman RM. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H, Walters G, Knight JF, Alexander SI. DNA vaccination against specific pathogenic TCRs reduces proteinuria in active Heymann nephritis by inducing specific autoantibodies. J Immunol. 2003;171:4824–4829. doi: 10.4049/jimmunol.171.9.4824. [DOI] [PubMed] [Google Scholar]
- 35.Hauer AD, van Puijvelde GH, Peterse N, de Vos P, van Weel V, van Wanrooij EJ, Biessen EA, Quax PH, Niethammer AG, Reisfeld RA, van Berkel TJ, Kuiper J. Vaccination against VEGFR2 attenuates initiation and progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2050–2057. doi: 10.1161/ATVBAHA.107.143743. [DOI] [PubMed] [Google Scholar]
- 36.Petrovan RJ, Kaplan CD, Reisfeld RA, Curtiss LK. DNA vaccination against VEGF receptor 2 reduces atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vase Biol. 2007;27:1095–1100. doi: 10.1161/ATVBAHA.106.139246. [DOI] [PubMed] [Google Scholar]
- 37.van Wanrooij EJ, de Vos P, Bixel MG, Vestweber D, van Berkel TJ, Kuiper J. Vaccination against CD99 inhibits atherogenesis in low-density lipoprotein receptor-deficient mice. Cardiovasc Res. 2008;78:590–596. doi: 10.1093/cvr/cvn025. [DOI] [PubMed] [Google Scholar]
- 38.Hauer AD, Habets KL, van Wanrooij EJ, de Vos P, Krueger J, Reisfeld RA, van Berkel TJ, Kuiper J. Vaccination against TIE2 reduces atherosclerosis. Atherosclerosis. 2009;204:365–371. doi: 10.1016/j.atherosclerosis.2008.09.039. [DOI] [PubMed] [Google Scholar]