Abstract
This study reports differential blood cells counts and their correlations with creatine kinase (CK) and C-reactive protein (CRP) levels in acute myocardial infarction (AMI) patients and normal subjects. Peripheral blood samples were obtained from all 39 AMI patients and 35 controls for blood cell counts and CK and CRP analyses. Total WBC, WBC fractions, RBC and platelets were measured with an automated hematology analyzer. The results showed a significant increase in total WBC (8.688 × 109/L versus 6.148 × 109/L), monocytes (1.271 versus 0.497 × 109/L), and neutrophils (8.367 versus 3.223 × 109/L) counts in AMI patients than controls. The RBC count was significantly less in AMI patients (4.638 × 1012/L) as compared to controls (5.105 × 1012/L). However, there was no significant difference in lymphocytes, eosinophils, basophils and platelet counts between AMI patients and controls. Both, serum CK (215.38 ± 43.15 versus 100.82 ± 8.86 U/L) and CRP (29.49 ± 7.61 versus 3.48 ± 0.60 mg/L) were significantly higher in AMI patients as compared to controls. Age of the subjects was neither correlated with blood cell counts nor CK indicating the validity of these markers irrespective of patient age. A significant correlation was observed between WBC counts and CK (R = 0.242, P = 0.041) as well as CRP (R = 0.416, P = 0.000). In conclusion, this study clearly showed significant increase in total and differential leukocyte counts indicating a pro-inflammatory cascade in AMI patients. A significant correlation between WBC counts and CK or CRP levels suggest a possible biomarker value of WBC for a quick prediction of both myocardial necrosis and inflammation in AMI patients.
Keywords: Acute myocardial infarction, blood cells count, creatine kinase, inflammation, biomarker
Introduction
Cardiovascular diseases represent one of the most important causes of death worldwide. Acute myocardial infarction (AMI) is a critical complication resulting from ischemic insult due to progressive reduction of the arterial lumen by excessive formation of atherosclerotic plaque. AMI is followed with strong systemic inflammatory response to myocardial damage. The persistence and autoamplification of immuno-inflammatory reaction contribute to the patho-genesis of atherosclerosis as well as its complications such as AMI [1]. The existence of a pro-inflammatory cascade in AMI patients has been confirmed by several biomarker studies [2-5]. Atherogenesis results from the interaction between the biology of the arterial wall and the various stress stimuli present in the circulating blood [1]. Peripheral blood mononuclear cells (PBMCs) increase after AMI and infiltrate to the infarct region. Increased PBMC count is significantly correlated with left ventricular remodeling, suggesting that PBMCs play a pivotal role for the development of left ventricular remodeling after AMI [6]. An elevated WBC count has been significantly associated with higher risk of in-hospital mortality; patients in the highest quartile of WBC count are about three times more likely to have a poor prognosis after AMI than those in the lowest quartile [7].
Basili et al [8] have shown that determination of neutrophil counts might help to improve the accuracy of AMI diagnosis in emergency patients. Hong et al [9] have suggested an important role of monocytes in the expansion of the infarct and the development of chronic ischemic heart failure after reperfusion therapy. Peripheral monocytosis is associated with left ventricular dysfunction, suggesting a possible role of monocytes in the development of left ventricular remodeling after reperfused AMI [10]. The peak monocyte count recorded during the immediate postinfarction period provides a bedside marker of the extent of myocardial damage [11]. Activated monocytes and neutrophils could be a significant source of free radicals which are involved in lipid peroxidation and cause tissue damage in early postinfarction period [12]. A higher baseline platelet count in patients with AMI is a powerful independent predictor of death and reinfarction within the first year after primary percutaneous coronary intervention [13]. Goncalves et al [14] have shown that an elevated mean platelet volume (MPV) is a strong independent predictor of long-term outcomes after percutaneous coronary intervention and possesses a prognostic value similar to that of troponin in patients with AMI.
Although the prognostic role of blood cell counts in AMI has been documented, the relationship between differential blood cell count and creatine kinase (CK), a marker of myocardial damage, is not clear. CK is a reliable marker for prediction of infarct size and left ventricular function in the acute phase as well as subsequent cardiac events after AMI [15, 16]. This study reports differential blood cells counts and their correlation with CK levels in AMI patients and normal subjects.
Materials and methods
This study was conducted on 39 AMI adult patients (29 males, 10 females) admitted to Prince Sultan Cardiac Center of the Armed Forces Hospital, Riyadh, Saudi Arabia. We also recruited and 35 normal subjects (25 males, 10 females) for comparative evaluation of various parameters. Peripheral blood samples were obtained from all patients and controls for blood cell counts (K2EDTA tubes), CK and troponin T analysis (serum separator tubes). Total WBC, WBC fractions (lymphocytes, monocytes, neutrophils, eosinophils and basophils), RBC, platelets and other hematology parameters were measured using an automated hematology analyzer, XE-2100 (Sysmex, UK). Serum CK was analyzed spectrophotometrically using COBAS Integra-800 system (Roche Diagnostics, Germany). Troponin T hs was analyzed using commercially available sandwich ELISA kit (Roche Diagnostics, Germany). Serum CRP was determined immunoturbidimetrically on COBAS system using the principle of human CRP agglutination with latex particles coated with monoclonal anti-CRP antibodies.
The data were evaluated by SPSS statistical package version 10. Independent samples Student's t-test (2-tailed) was used to compare means of different parameters between patients and controls. Pearson's correlation test was performed to examine various correlations. P values <0.05 were considered as statistically significant.
Results
All the control subjects had troponin values less than 0.003 ng/mL whereas the level of troponin in AMI patients was found to be 0.319 ± 0.091 ng/mL. There was a significant increase in WBC count in AMI patients (8.688 × 109/L) as compared to controls (6.148 × 109/L) (Table 1). The differential leucocytes counts showed significant increases in monocytes (1.271 versus 0.497 × 109/L), and neutrophils (8.367 versus 3.223 × 109/L) in AMI patients than controls. However, there was no significant difference in lymphocytes, eosinophils and basophils counts between AMI patients and controls (Table 1). RBC counts were significantly less in AMI patients (4.638 × 1012/L) than controls (5.105 × 1012/L). The platelets counts did not differ between the two groups.
Table 1.
Blood cell counts in AMI patients and normal subjects.
| Blood cells | Controls (N=35) | AMI Patients (N=39) | P value (2-tail) |
|---|---|---|---|
| WBCs | 6.148 ±0.289 | 8.688 ± 0.831 | 0.007* |
| Lymphocytes | 2.474 ±0.295 | 4.382 ± 0.904 | 0.059 |
| Monocytes | 0.497 ±0.026 | 1.271 ±0.323 | 0.027* |
| Neutrophils | 3.223 ±0.260 | 8.367 ± 2.156 | 0.028* |
| Eosinophils | 0.208 ±0.021 | 0.239 ± 0.051 | 0.593 |
| Basophils | 0.020 ± 0.006 | 0.021 ± 0.006 | 0.913 |
| RBCs | 5.105 ±0.092 | 4.638 ± 0.090 | 0.001* |
| Platelets | 268.3 ± 8.858 | 272.7 ±16.78 | 0.823 |
The values are mean ± SEM. All blood cell counts are × 109/L, except RBC (× 1012/L).
Statistically significant.
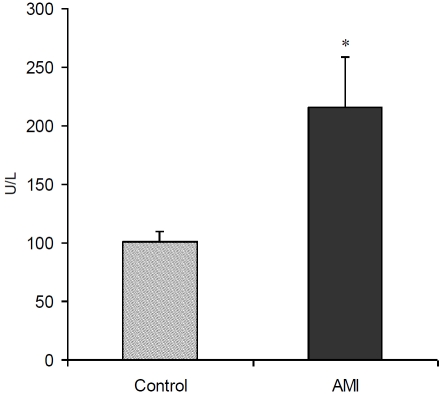
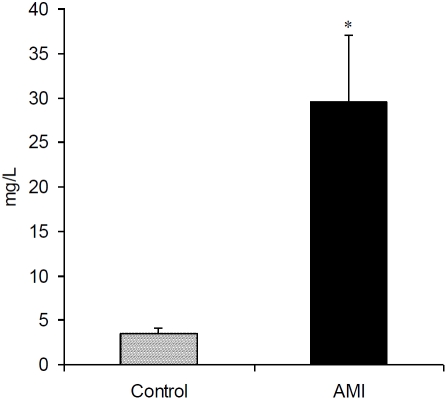
The levels of CK were significantly higher in AMI patients (215.38 ± 43.15 U/L) as compared to controls (100.82 ± 8.86 U/L) (Figure 1). There was a significant increase in serum CRP in AMI patients (29.49 ± 7.61 mg/L) than controls (3.48 ± 0.60 mg/L) (Figure 2). A significant correlation was observed between WBC count and CK (R=0.242, P = 0.041) as well as CRP (R = 0.416, P = 0.000) (Table 2). Both CK and CRP did not significantly correlate with other blood cell types except a significant correlation between CRP and platelets (R = 0.386, P = 0.001). There was no correlation between age and WBC, RBC, platelets and CK; however, CRP was significantly correlated with age (R = 0.271, P = 0.023).
Figure 1.
Serum creatine kinase (CK) levels (mean ± SEM) in controls and AMI patients. *P<0.05 versus control groups using t-test.
Figure 2.
Serum C-reactive protein (CRP) levels (mean ± SEM) in controls and AMI patients. *P<0.01 versus control groups using t-test.
Table 2.
Pearson correlation between blood cell counts and age versus CK and CRP.
| CK | CRP | |||
|---|---|---|---|---|
| R | P (2-tail) | R | P (2-tail) | |
| WBCs | 0.242 | 0.041* | 0.416 | 0.000* |
| Lymphocytes | -0.037 | 0.758 | 0.009 | 0.943 |
| RBCs | 0.071 | 0.552 | -0.151 | 0.205 |
| Platelets | -0.087 | 0.465 | 0.386 | 0.001* |
| Age | 0.091 | 0.455 | 0.271 | 0.023* |
Statistically significant.
Discussion
The results showed a significant increase in total WBC, neutrophils and monocytes counts in AMI patients as compared to normal subjects (Table 1). Age of the subjects was neither correlated with blood cell counts nor CK indicating the validity of these markers irrespective of patient age. A significant correlation between CK (representing the extent of myocardial necrosis) and WBC suggests the predictive value of WBC count for myocardial damage in AMI patients. Total WBC count has been regarded as an independent predictor of death or myocardial infarction in patients with coronary artery disease [17]. A higher WBC count may be associated with progression of myocardial damage after recanalization in patients with early recanalization of an anterior AMI [18]. Afiune Neto et al [19] have observed a higher prevalence of leukocytosis in AMI patients than the stable coronary artery disease; monocytosis being an independent variable for AMI. Dogan et al [20] have shown that basal leukocyte and neutrophil counts on admission and first day are positively correlated with peak creatine kinase-myocardial band, peak cardiac troponin and infarct size in AMI patients. Elevated leukocyte and neutrophil counts after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarctions are directly related to myocardial infarct size and the left ventricular ejection fraction and are independent predictors of cardiovascular outcomes [21]. Moreover, leukocyte thrombogenic profile is a relevant player in patients with a high risk of thromboembolic events and may represent a suitable target for molecular intervention [22].
A significant increase in CRP indicated a state of inflammation in AMI patients (Figure 2). In AMI, a loss of regulation of the inflammatory system occurs in patients with a decreased activity of regulatory T-cells, resulting in the boost of aggressive T-cells and the drop of anti-inflammatory T-cells which leads to pleiotropic proinflammatory imbalance with damaging effects in patient outcome due to this uncontrolled immune response [23]. Recently, neutro-phil/lymphocyte ratio (NLR) has been suggested as an independent predictor of short- and long-term mortalities in patients with non-ST-segment elevation myocardial infarction (NSTEMI); patients in the highest NLR tertile (NLR > 4.7) had a higher 4-year mortality rate compared to those in the lowest tertile (NLR < 3.0) [24]. Horne et al [17] have also observed greater predictive ability by high neutrophil and low lymphocyte counts while the greatest risk prediction is given by the NLR > 4.71.
Neutrophils are rapidly released into the circulation upon acute stress such as AMI [25]. Neutrophil count correlates with the risk of myocardial infarction and stroke and identify patients more susceptible to reinfarction and in-hospital death [22, 26]. Neutrophil count adds prognostic information to major adverse cardiac events in acute coronary syndrome whereas monocyte and lymphocyte counts are predictive of severity of coronary atherosclerosis [27]. The accuracy of the neutrophil count for diagnosing AMI, quantified by the area under the receiver operating characteristic curve (AUC), was significantly lower than that of cardiac troponin T [25]. Monocytes are the cells of the immune system that give rise to macrophages that participate in a maladaptive and nonresolving inflammatory response triggering acute thrombotic vascular disease including AMI [28]. Nozawa et al [29] have suggested that circulating monocytes play an important role in the progression of coronary plaque in AMI, while the peak monocyte count during might be a predictor of plaque progression. Despite significantly higher leukocytes and monocytes counts after myocardial infarction, a rapid depression of monocytic HLA-DR expression and a defective lymphocytic IFN-γ production indicate an immediate suppression of cell-mediated immune responses after myocardial infarction [30]. Although neutrophils and monocytes counts on the first days after AMI treated with primary primary coronary angioplasty are related to markers of effective myocardial reper-fusion, only monocytes are significantly and associated with contractile recovery of the in-farcted area at 6 months [31].
We observed a significant decrease in RBC counts in AMI patients however the platelets count did not differ between AMI patients and controls (Table 1). Cemin et al [32] have observed that RBC distribution width and platelets count did not differ between chest pain patients with and without AMI. However, the mean platelet volume was significantly higher in AMI patients suggesting the inclusion of these parameters along with other conventional cardiac bio-markers for evaluating patients with suspected AMI [32]. Pan et al [33] have noticed significant decrease in platelet number after AMI, especially on the second day after onset but returned to the normal on the 5-7th day after the heart attack. The decrease in platelet number was markedly present in cases with heart failure and cardiac death [33]. An increased leukocyte-platelet functional interaction in AMI at the site of plaque rupture relative to the systemic circulation may be one of the pathogenetic mechanisms responsible for myocardial dysfunction [34]. Platelet-neutrophil aggregates from ruptured plaque may be associated with impaired coronary microcirculation and resultant myocardial necrosis or dysfunction in AMI patients [35]. The interaction between circulating platelets and neutrophils influences innate immune functions, possibly contributing to regulate vascular inflammation [36].
In conclusion, this study clearly showed significant increase in total and differential leukocyte counts indicating a pro-inflammatory cascade in AMI patients. A significant correlation between WBC and CK as well as CRP suggests the relevance of WBC counts for the prediction of myocardial damage and inflammation in AMI patients.
Acknowledgments
This study was supported by National Plan for Science and Technology (NPST) Program by King Saud University Project Number 08-BIO571-02. The authors thank Adnan Ali Khan for clinical data management. The technical assistance from the nursing staff of Prince Sultan Cardiac Center and Armed Forces Hospital, Riyadh for blood collection is gratefully acknowledged.
References
- 1.Caligiuri G. Role of the immune response in atherosclerosis and acute coronary syndromes. Med Sci. 2004;20:175–181. doi: 10.1051/medsci/2004202175. [DOI] [PubMed] [Google Scholar]
- 2.Steppich BA, Demetz G, Schulz S, von Wedel J, Pogatsa-Murray G, Braun SL, Stein A, Kastrati A, Schömig A, Ott I. Effects of G-CSF on systemic inflammation, coagulation and platelet activation in patients with acute myocardial infarction. Thromb Res. 2011;127:119–121. doi: 10.1016/j.thromres.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Ferrante G, Nakano M, Prati F, Niccoli G, Mallus MT, Ramazzotti V, Montone RA, Kolodgie FD, Virmani R, Crea F. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation. 2010;122:2505–2513. doi: 10.1161/CIRCULATIONAHA.110.955302. [DOI] [PubMed] [Google Scholar]
- 4.Pasqui AL, Di Renzo M, Maffei S, Pastorelli M, Pompella G, Auteri A, Puccetti L. Pro/Anti-inflammatory cytokine imbalance in postischemic left ventricular remodeling. Mediators Inflamm. 2010. [DOI] [PMC free article] [PubMed]
- 5.Goldmann BU, Rudolph V, Rudolph TK, Holle AK, Hillebrandt M, Meinertz T, Baldus S. Neutrophil activation precedes myocardial injury in patients with acute myocardial infarction. Free Radic Biol Med. 2009;47:79–83. doi: 10.1016/j.freeradbiomed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Aoki S, Nakagomi A, Asai K, Takano H, Yasutake M, Seino Y, Mizuno K. Elevated peripheral blood mononuclear cell count is an independent predictor of left ventricular remodeling in patients with acute myocardial infarction. J Cardiol. 2011;57:202–207. doi: 10.1016/j.jjcc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Kojima S, Sakamoto T, Ishihara M, Kimura K, Miyazaki S, Tei C, Hiraoka H, Sonoda M, Tsuchihashi K, Yamagishi M, Inoue T, Asada Y, Ikeda Y, Shirai M, Ogawa H Japanese Acute Coronary Syndrome Study investigators. The white blood cell count is an independent predictor of noreflow and mortality following acute myocardial infarction in the coronary interventional era. Ann Med. 2004;36:153–160. doi: 10.1080/07853890310021553. [DOI] [PubMed] [Google Scholar]
- 8.Basili S, Di Francoi M, Rosa A, Ferroni P, Diurni V, Scarpellini MG, Bertazzoni G. Absolute neutrophil counts and fibrinogen levels as an aid in the early diagnosis of acute myocardial infarction. Acta Cardiol. 2004;59:135–140. doi: 10.2143/AC.59.2.2005167. [DOI] [PubMed] [Google Scholar]
- 9.Hong YJ, Jeong MH, Ahn Y, Yoon NS, Lee SR, Hong SN, Moon JY, Kim KH, Park HW, Kim JH, Cho JG, Park JC, Kang JC. Relationship between peripheral monocytosis and nonrecovery of left ventricular function in patients with left ventricular dysfunction complicated with acute myocardial infarction. Circ J. 2007;71:1219–1224. doi: 10.1253/circj.71.1219. [DOI] [PubMed] [Google Scholar]
- 10.Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, Mitamura H, Ogawa S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol. 2002;39:241–246. doi: 10.1016/s0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 11.Meisel SR, Pauzner H, Shechter M, Zeidan Z, David D. Peripheral monocytosis following acute myocardial infarction: incidence and its possible role as a bedside marker of the extent of cardiac injury. Cardiology. 1998;90:52–57. doi: 10.1159/000006817. [DOI] [PubMed] [Google Scholar]
- 12.Durdević PM, Baskić DD, Dukić ALj, Popović S, Jakovljević VLj, Arsenijevic NN. Phagocytic activity of peripheral blood leukocytes during acute myocardial infarction. Med Pregl. 2003;56:97–102. [PubMed] [Google Scholar]
- 13.Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, Mehran R, Lansky AJ, Na Y, Stone GW. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am J Cardiol. 2007;99:1055–1061. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 14.Goncalves SC, Labinaz M, Le May M, Glover C, Froeschl M, Marquis JF, O'Brien E, Shukla D, Ruchin P, Sookur D, Ha A, So D. Usefulness of mean platelet volume as a biomarker for long-term outcomes after percutaneous coronary intervention. Am J Cardiol. 2011;107:204–209. doi: 10.1016/j.amjcard.2010.08.068. [DOI] [PubMed] [Google Scholar]
- 15.Mayr A, Mair J, Klug G, Schocke M, Pedarnig K, Trieb T, Pachinger O, Jaschke W, Metzler B. Cardiac troponin T and creatine kinase predict mid-term infarct size and left ventricular function after acute myocardial infarction: a cardiac MR study. J Magn Reson Imaging. 2011;33:847–854. doi: 10.1002/jmri.22491. [DOI] [PubMed] [Google Scholar]
- 16.Kazmi KA, Iqbal SP, Bakr A, Iqbal MP. Admission creatine kinase as a prognostic marker in acute myocardial infarction. J Pak Med Assoc. 2009;59:819–822. [PubMed] [Google Scholar]
- 17.Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, Renlund DG, Muhlestein JB; Intermountain Heart Collaborative Study Group. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 18.Kosuge M, Kimura K, Ishikawa T, Shimizu T, Takamura T, Tsukahara K, Tahara Y, Nozawa N, Furukawa E, Tochikubo O, Sugiyama M, Umemura S. Relation between white blood cell counts and myocardial reperfusion in patients with recanalized anterior acute myocardial infarction. Circ J. 2004;68:526–531. doi: 10.1253/circj.68.526. [DOI] [PubMed] [Google Scholar]
- 19.Afiune Neto A, Mansur Ade P, Avakian SD, Gomes EP, Ramires JA. Monocytosis is an independent risk marker for coronary artery disease. Arq Bras Cardiol. 2006;86:240–244. doi: 10.1590/s0066-782x2006000300013. [DOI] [PubMed] [Google Scholar]
- 20.Dogan I, Karaman K, Sonmez B, Celik S, Turker O. Relationship between serum neutrophil count and infarct size in patients with acute myocardial infarction. Nucl Med Commun. 2009;30:797–801. doi: 10.1097/MNM.0b013e32832e3a16. [DOI] [PubMed] [Google Scholar]
- 21.Chia S, Nagurney JT, Brown DF, Raffel OC, Bamberg F, Senatore F, Wackers FJ, Jang IK. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol. 2009;103:333–337. doi: 10.1016/j.amjcard.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 22.Maugeri N, Manfredi AA, Maseri A. Clinical and experimental evidences on the prothrombotic properties of neutrophils. Srp Arh Celok Lek. 2010;138:50–52. doi: 10.2298/sarh10s1050m. [DOI] [PubMed] [Google Scholar]
- 23.Bodi V, Sanchis J, Nunez J, Mainar L, Minana G, Benet I, Solano C, Chorro FJ, Llacer A. Uncontrolled immune response in acute myocardial infarction: unraveling the thread. Am Heart J. 2008;156:1065–1073. doi: 10.1016/j.ahj.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, Gobunsuy R, Jadonath S, Baldari D, McCord D, Lafferty J. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 25.Meissner J, Irfan A, Twerenbold R, Mueller S, Reiter M, Haaf P, Reichlin T, Schaub N, Winkler K, Pfister O, Heinisch C, Mueller C. Use of neutrophil count in early diagnosis and risk stratification of AMI. Am J Med. 2011;124:534–542. doi: 10.1016/j.amjmed.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Karabinos I, Koulouris S, Kranidis A, Pastromas S, Exadaktylos N, Kalofoutis A. Neutrophil count on admission predicts major in-hospital events in patients with a non-ST-segment elevation acute coronary syndrome. Clin Cardiol. 2009;32:561–568. doi: 10.1002/clc.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang G, Zhong XN, Zhong B, Chen YQ, Liu ZZ, Su L, Ling ZY, Cao H, Yin YH. Significance of white blood cell count and its subtypes in patients with acute coronary syndrome. Eur J Clin Invest. 2009;39:348–358. doi: 10.1111/j.1365-2362.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 28.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nozawa N, Hibi K, Endo M, Sugano T, Ebina T, Kosuge M, Tsukahara K, Okuda J, Umemura S, Kimura K. Association between circulating monocytes and coronary plaque progression in patients with acute myocardial infarction. Circ J. 2010;74:1384–1391. doi: 10.1253/circj.cj-09-0779. [DOI] [PubMed] [Google Scholar]
- 30.Haeusler KG, Schmidt WU, Foehring F, Meisel C, Guenther C, Brunecker P, Kunze C, Helms T, Dirnagl U, Volk HD, Villringer A. Immune responses after acute ischemic stroke or myocardial infarction. Int J Cardiol. 2010. [Epub ahead of print] [DOI] [PubMed]
- 31.Mariani M, Fetiveau R, Rossetti E, Poli A, Poletti F, Vandoni P, D'Urbano M, Cafiero F, Mariani G, Klersy C, De Servi S. Significance of total and differential leucocyte count in patients with acute myocardial infarction treated with primary coronary angioplasty. Eur Heart J. 2006;27:2511–2515. doi: 10.1093/eurheartj/ehl191. [DOI] [PubMed] [Google Scholar]
- 32.Cemin R, Donazzan L, Lippi G, Clari F, Daves M. Blood cells characteristics as determinants of acute myocardial infarction. Clin Chem Lab Med. 2011;49:1231–1236. doi: 10.1515/CCLM.2011.183. [DOI] [PubMed] [Google Scholar]
- 33.Pan Y, Wu B, Hong X. The change of platelet number in patient with acute myocardial infarction and its clinical significance. Zhonghua Nei Ke Za Zhi. 1995;34:19–21. [PubMed] [Google Scholar]
- 34.Botto N, Sbrana S, Trianni G, Andreassi MG, Ravani M, Rizza A, Al-Jabri A, Palmieri C, Berti S. An increased platelet-leukocytes interaction at the culprit site of coronary artery occlusion in acute myocardial infarction: a pathogenic role for “no-reflow” phenomenon? Int J Cardiol. 2007;117:123–130. doi: 10.1016/j.ijcard.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 35.Arakawa K, Yasuda S, Hao H, Kataoka Y, Morii I, Kasahara Y, Kawamura A, Ishibashi-Ueda H, Miyazaki S. Significant association between neutrophil aggregation in aspirated thrombus and myocardial damage in patients with ST-segment elevation acute myocardial infarction. Circ J. 2009;73:139–144. doi: 10.1253/circj.cj-08-0609. [DOI] [PubMed] [Google Scholar]
- 36.Maugeri N, Rovere-Querini P, Evangelista V, Covino C, Capobianco A, Bertilaccio MT, Piccoli A, Totani L, Cianflone D, Maseri A, Manfredi AA. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and ﹛beta﹜2 integrin-dependent cell clearance program. Blood. 2009;113:5254–5265. doi: 10.1182/blood-2008-09-180794. [DOI] [PubMed] [Google Scholar]