Abstract
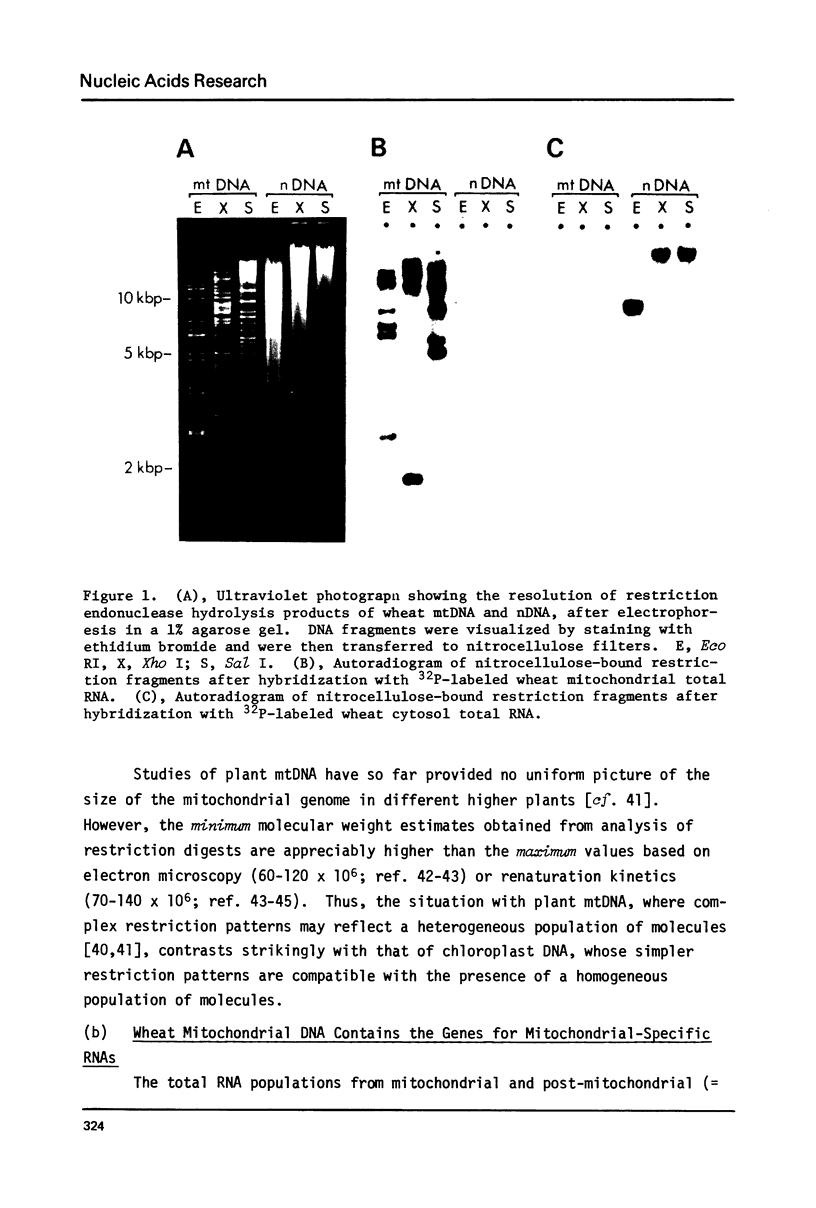
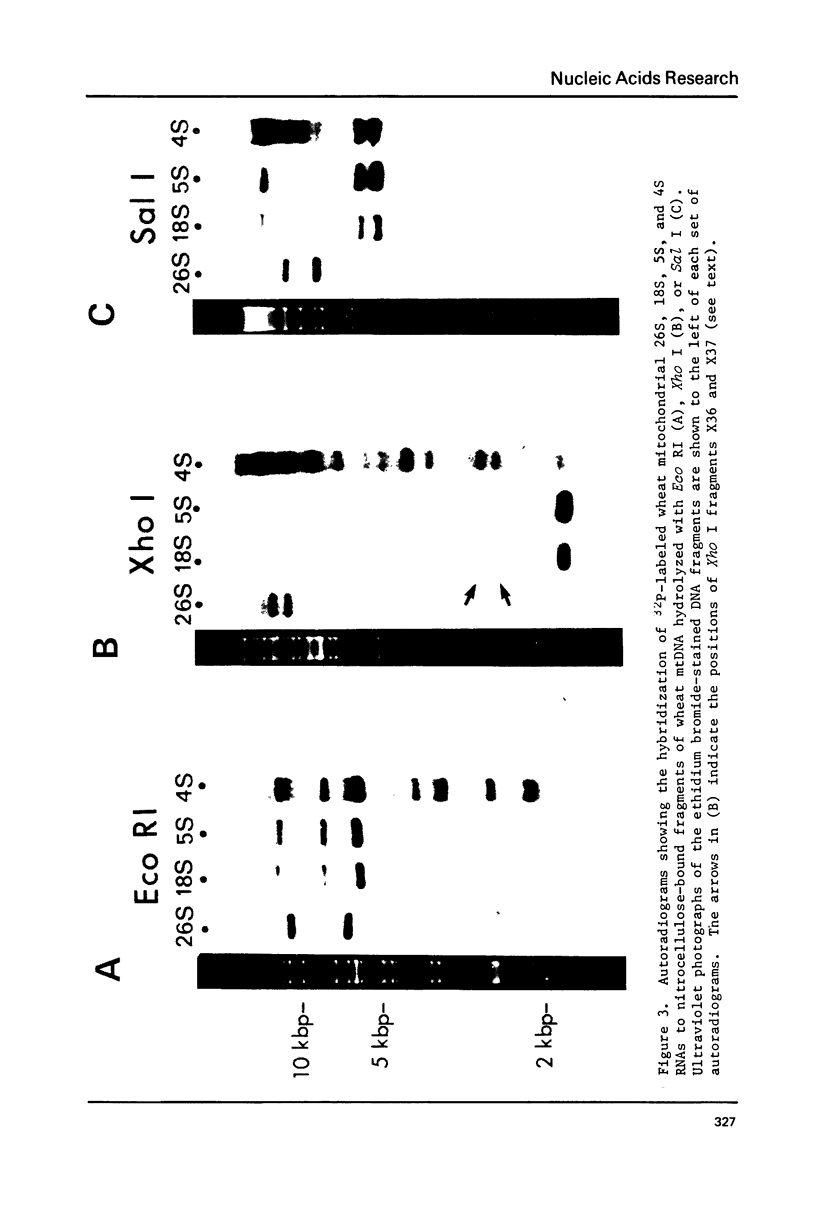
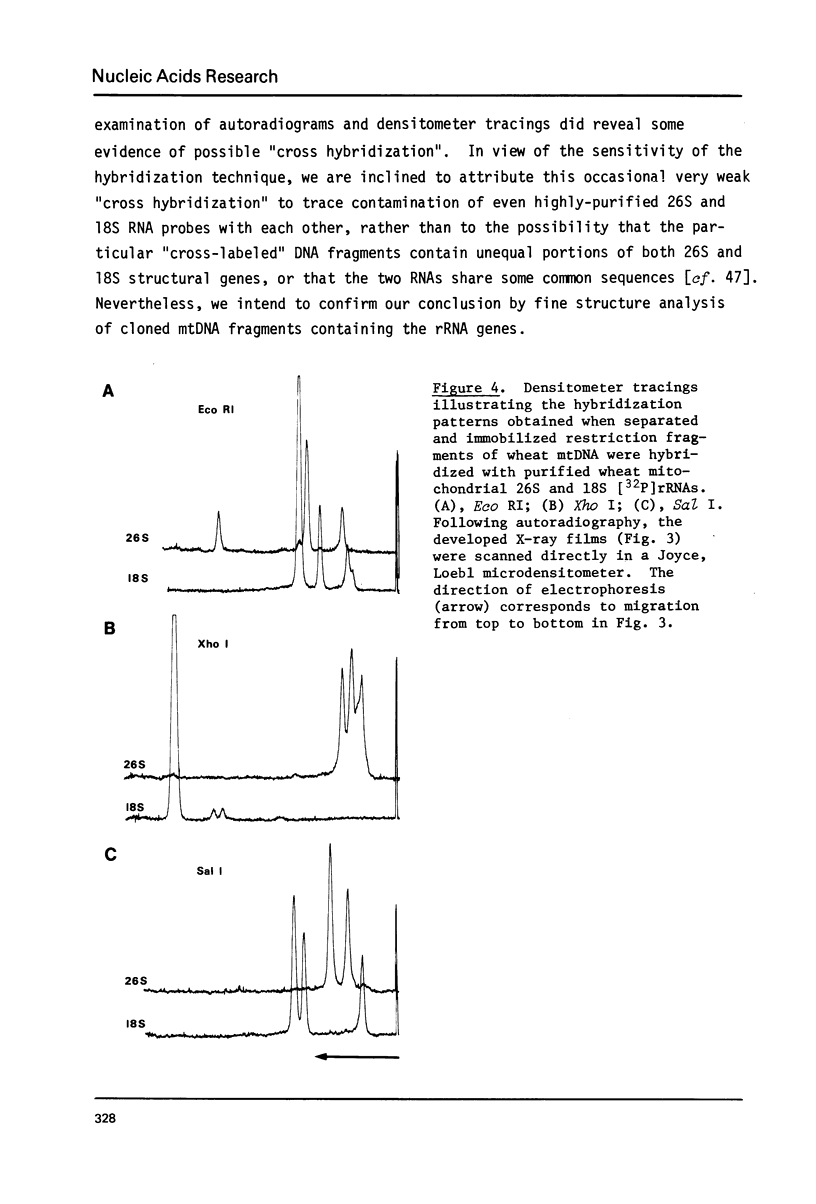
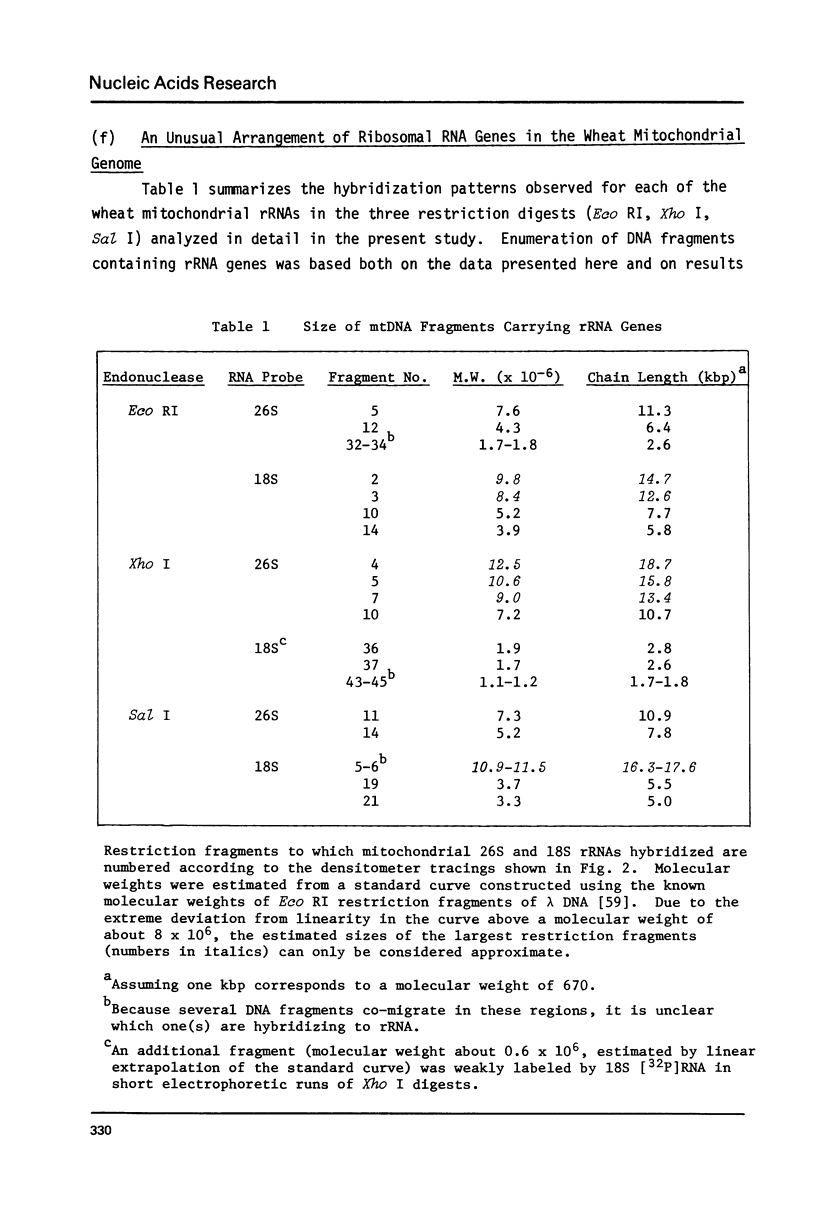
We show here that mitochondrial-specific ribosomal and transfer RNAs of wheat (Triticum vulgare Vill. [Triticum aestivum L.] var. Thatcher) are encoded by the mitochondrial DNA (mtDNA). Individual wheat mitochondrial rRNA species (26S, 18S, 5S) each hybridized with several mtDNA fragments in a particular restriction digest (Eco RI, Xho I, or Sal I). In each case, the DNA fragments to which 18S and 5S rRNAs hybridized were the same, but different from those to which 26S rRNA hybridized. From these results, we conclude that the structural genes for wheat mitochondrial 18S and 5S rRNAs are closely linked, but are physically distant from the genes for wheat mitochondrial 26S rRNA. This arrangement of rRNA genes is clearly different from that in prokaryotes and chloroplasts, where 23S, 16S and 5S rRNA genes are closely linked, even though wheat mitochondrial 18S rRNA has previously been shown to be prokaryotic in nature. The mixed population of wheat mitochondrial 4S RNAs (tRNAs) hybridized with many large restriction fragments, indicating that the tRNA genes are broadly distributed throughout the mitochondrial genome, with some apparent clustering in regions containing 18S and 5S rRNA genes.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L., Davidson N., Murphy W., Lynch D., Attardi G. An electron microscope study of the relative positions of the 4S and ribosomal RNA genes in HeLa cells mitochondrial DNA. Cell. 1976 Sep;9(1):81–90. doi: 10.1016/0092-8674(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Avadhani N. G., Lewis F. S., Rutman R. J. Mitochondrial RNA and protein metabolism. Subcell Biochem. 1976 Jun;4(2):93–145. [PubMed] [Google Scholar]
- Bedbrook J. R., Kolodner R., Bogorad L. Zea mays chloroplast ribosomal RNA genes are part of a 22,000 base pair inverted repeat. Cell. 1977 Aug;11(4):739–749. doi: 10.1016/0092-8674(77)90288-4. [DOI] [PubMed] [Google Scholar]
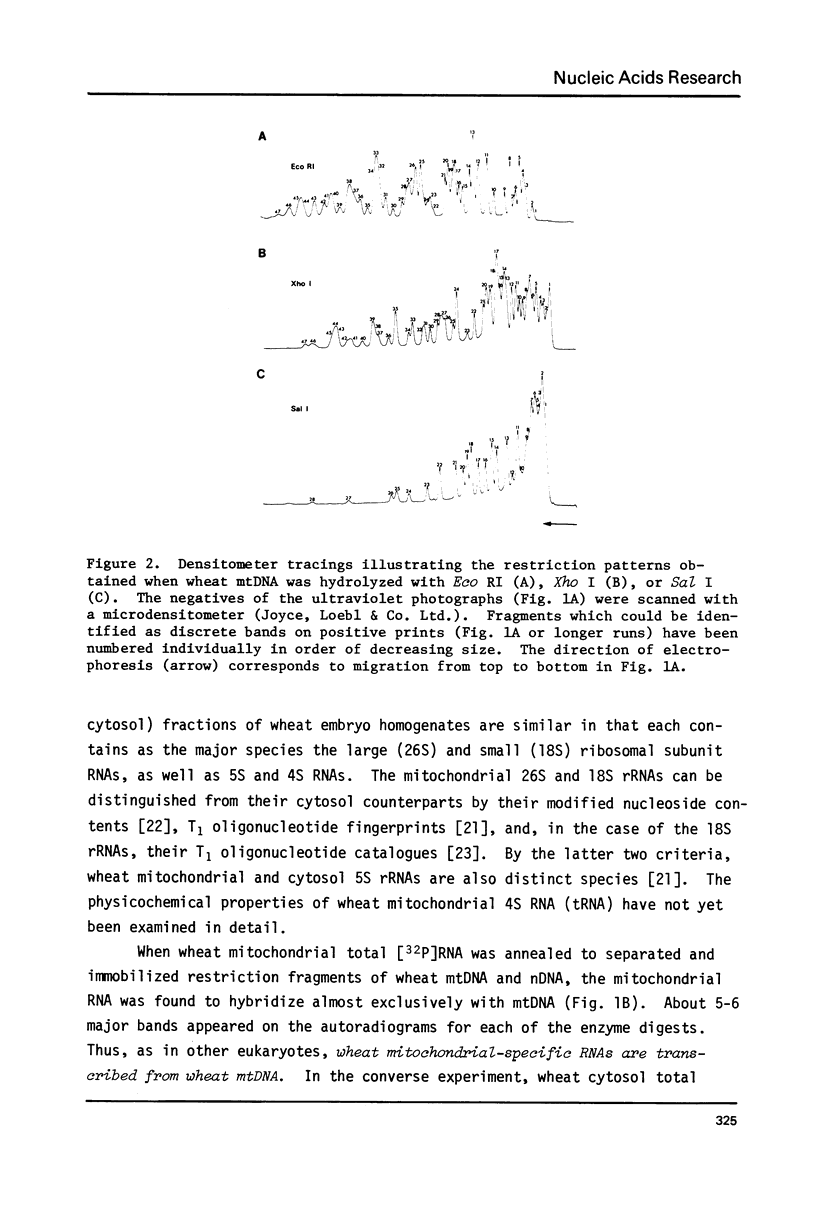
- Bonen L., Cunningham R. S., Gray M. W., Doolittle W. F. Wheat embryo mitochondrial 18S ribosomal RNA: evidence for its prokaryotic nature. Nucleic Acids Res. 1977 Mar;4(3):663–671. doi: 10.1093/nar/4.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. Mitochondrial nucleic acids. Biochimie. 1973;55(6):801–804. doi: 10.1016/s0300-9084(73)80032-x. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Heyting C., Borst P., Arnberg A. C., Van Bruggen E. F. An insert in the single gene for the large ribosomal RNA in yeast mitochondrial DNA. Nature. 1978 Sep 28;275(5678):336–338. doi: 10.1038/275336a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Cunningham R. S., Bonen L., Doolittle W. F., Gray M. W. Unique species of 5S, 18S, and 26S ribosomal RNA in wheat mitochondria. FEBS Lett. 1976 Oct 15;69(1):116–122. doi: 10.1016/0014-5793(76)80666-7. [DOI] [PubMed] [Google Scholar]
- Cunningham R. S., Gray M. W. Isolation and characterization of 32P-labeled mitochondrial and cytosol ribosomal RNA from germinating wheat embryos. Biochim Biophys Acta. 1977 Apr 4;475(3):476–491. doi: 10.1016/0005-2787(77)90063-6. [DOI] [PubMed] [Google Scholar]
- Gillemaut P., Weil J. H. Aminoacylation of Phaseolus vulgaris cytoplasmic, chloroplastic and mitochondrial tRNAsMet and of Escherichia coli tRNAsMet by homologous and heterologous enzymes. Biochim Biophys Acta. 1975 Oct 1;407(2):240–248. doi: 10.1016/0005-2787(75)90288-9. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Borst P., Bollen-de Boer J. E., van Bruggen E. F. The organization of ribosomal RNA genes in the mitochondrial DNA of Tetrahymena pyriformis strain ST. Biochim Biophys Acta. 1978 Nov 21;521(1):169–186. doi: 10.1016/0005-2787(78)90260-5. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Hallick R. B. Physical mapping of the Euglena gracilis chloroplast DNA and ribosomal RNA gene region. Biochemistry. 1978 Jan 24;17(2):284–289. doi: 10.1021/bi00595a015. [DOI] [PubMed] [Google Scholar]
- Guderian R. H., Pulliam R. L., Gordon M. P. Characterization and fractionation of tobacco leaf transfer RNA. Biochim Biophys Acta. 1972 Feb 23;262(1):50–65. doi: 10.1016/0005-2787(72)90218-3. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Steinmetz A., Burkard G., Weil J. H. Aminoacylation of tRNA-Leu species from Escherichia coli and from the cytoplasm, chloroplasts and mitochondria of Phaseolus vulgaris by homologous and heterologous enzymes. Biochim Biophys Acta. 1975 Jan 6;378(1):64–72. doi: 10.1016/0005-2787(75)90137-9. [DOI] [PubMed] [Google Scholar]
- Hahn U., Lazarus C. M., Lünsdorf H., Küntzel H. Split gene for mitochondrial 24S ribosomal RNA of Neurospora crassa. Cell. 1979 May;17(1):191–200. doi: 10.1016/0092-8674(79)90307-6. [DOI] [PubMed] [Google Scholar]
- Hartley M. R., Ellis R. J. Ribonucleic acid synthesis in chloroplasts. Biochem J. 1973 May;134(1):249–262. doi: 10.1042/bj1340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassur S. M., Whitlock H. W., Jr UV shadowing--a new and convenient method for the location of ultraviolet-absorbing species in polyacrylamide gels. Anal Biochem. 1974 May;59(1):162–164. doi: 10.1016/0003-2697(74)90020-7. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., RajBhandary U. L. Organization of tRNA and rRNA genes in N. crassa mitochondria: intervening sequence in the large rRNA gene and strand distribution of the RNA genes. Cell. 1979 Jul;17(3):583–595. doi: 10.1016/0092-8674(79)90266-6. [DOI] [PubMed] [Google Scholar]
- JOHNSTON F. B., STERN H. Mass isolation of viable wheat embryos. Nature. 1957 Jan 19;179(4551):160–161. doi: 10.1038/179160b0. [DOI] [PubMed] [Google Scholar]
- Jeannin G., Burkard G., Weil J. H. Aminoacylation of Phaseolus vulgaris cytoplasmic, chloroplastic and mitochondrial tRNAsPro and tRNAsLys by homologous and heterologous enzymes. Biochim Biophys Acta. 1976 Aug 2;442(1):24–31. doi: 10.1016/0005-2787(76)90171-4. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Physicochemical characterization of mitochondrial DNA from pea leaves. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1830–1834. doi: 10.1073/pnas.69.7.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. The molecular size and conformation of the chloroplast DNA from higher plants. Biochim Biophys Acta. 1975 Sep 1;402(3):372–390. doi: 10.1016/0005-2787(75)90273-7. [DOI] [PubMed] [Google Scholar]
- Leaver C. J., Harmey M. A. Higher-plant mitochondrial ribosomes contain a 5S ribosomal ribonucleic acid component. Biochem J. 1976 Jul 1;157(1):275–277. doi: 10.1042/bj1570275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Harmey M. A. Plant mitochondrial nucleic acids. Biochem Soc Symp. 1973;(38):175–193. [PubMed] [Google Scholar]
- Leaver C. J. Molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1973 Sep;135(1):237–240. doi: 10.1042/bj1350237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane A. W., Nagley P. Structural mapping of mitochondrial DNA. Arch Biochem Biophys. 1978 Apr 30;187(2):277–289. doi: 10.1016/0003-9861(78)90036-x. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E., Lindahl L., Jaskunas S. R., Dennis P. P., Nomura M. Transfer RNA genes between 16S and 23S rRNA genes in rRNA transcription units of E. coli. Cell. 1976 Feb;7(2):165–177. doi: 10.1016/0092-8674(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Maizels N. Dictyostelium 17S, 25S, and 5S rDNAs lie within a 38,000 base pair repeated unit. Cell. 1976 Nov;9(3):431–438. doi: 10.1016/0092-8674(76)90088-x. [DOI] [PubMed] [Google Scholar]
- Marcus A., Efron D., Weeks D. P. The wheat embryo cell-free system. Methods Enzymol. 1974;30:749–754. doi: 10.1016/0076-6879(74)30073-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng R. L., Vanderhoef L. N. Mitochondrial tyrosyl transfer ribonucleic Acid in soybean seedlings. Plant Physiol. 1972 Aug;50(2):298–302. doi: 10.1104/pp.50.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. R. Structure and synthesis of the ribosomal ribonucleic acid of prokaryotes. Bacteriol Rev. 1973 Dec;37(4):562–603. doi: 10.1128/br.37.4.562-603.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish J. H., Kirby K. S. Reagents which reduce interactions between ribosomal RNA and rapidly labelled RNA from rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- Payne P. I., Loening U. E. RNA breakdown accompanying the isolation of pea root microsomes. An analysis by polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1970 Nov 12;224(1):128–135. doi: 10.1016/0005-2787(70)90626-x. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring D. R. Maize mitochondria: purification and characterization of ribosomes and ribosomal ribonucleic Acid. Plant Physiol. 1974 May;53(5):677–683. doi: 10.1104/pp.53.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring D. R., Thornbury D. W. Molecular weights of maize mitochondrial and cytoplasmic ribosomal RNAs under denaturing conditions. Biochim Biophys Acta. 1975 Mar 10;383(2):140–146. doi: 10.1016/0005-2787(75)90255-5. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Malnoe P. Anatomy of the chloroplast ribosomal DNA of Chlamydomonas reinhardii. Cell. 1978 Oct;15(2):661–670. doi: 10.1016/0092-8674(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Scott N. S., Ingle J. The genes for cytoplasmic ribosomal ribonucleic Acid in higher plants. Plant Physiol. 1973 Apr;51(4):677–684. doi: 10.1104/pp.51.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Bonner W. D. DNA from plant mitochondria. Plant Physiol. 1966 Mar;41(3):383–388. doi: 10.1104/pp.41.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Vedel F., Quetier F. Physico-chemical characterization of mitochondrial DNA from potato tubers. Biochim Biophys Acta. 1974 Apr 10;340(4):374–387. doi: 10.1016/0005-2787(74)90059-8. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Wells R., Birnstiel M. Kinetic complexity of chloroplastal deoxyribonucleic acid and mitochondrial deoxyribonucleic acid from higher plants. Biochem J. 1969 May;112(5):777–786. doi: 10.1042/bj1120777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R., Ingle J. The constancy of the buoyant density of chloroplast and mitochondrial deoxyribonucleic acids in a range of higher plants. Plant Physiol. 1970 Jul;46(1):178–179. doi: 10.1104/pp.46.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfeld P. R., Herrmann R. G., Bottomley W. Mapping of the ribosomal RNA genes on spinach chloroplast DNA. Nucleic Acids Res. 1978 Jun;5(6):1741–1751. doi: 10.1093/nar/5.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Gross N. J. The form and size of mitochondrial DNA of the red bean, Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1968 Sep;61(1):245–252. doi: 10.1073/pnas.61.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries H., de Jonge J. C., Bakker H., Meurs H., Kroon A. Laboratory of Physiological Chemistry, State University Groningen, Netherlands. Nucleic Acids Res. 1979;6(5):1791–1803. doi: 10.1093/nar/6.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]