Abstract
Introduction
Recently, minimally invasive parathyroidectomy (MIP) has been developed and is gaining popularity among surgeons. For this reason, preoperative localization is playing an important role to detect the precise location of the affected gland and to increase the success rate.
Material and methods
From June 2007 to June 2011, 56 consecutive patients (11 men and 45 women) with primary or secondary hyperparathyroidism in our center underwent Gray scale, color Doppler and 99m-Tc MIBI scan prior to operative management of parathyroid lesions.
Results
The sensitivity, specificity and accuracy of US and MIBI scan for pHPT was 88%, 94%, 91% and 70%, 100% and 85% respectively. In patients with sHPT, the sensitivity, specificity and accuracy of US and MIBI scan was 54%, 93%, 76% and 25%, 100% and 72.9% respectively. The overall sensitivity of combined US and MIBI scan in pHPT and sHPT was 97% and 45% respectively. The overall sensitivity, specificity and accuracy of CDUS in diagnosis of parathyroid lesions in pHPT and sHPT is 97%, 100%, 98.6% and 62%, 100% and 83% respectively.
Conclusion
The overall sensitivity and specificity of US and MIBI in preoperative localization of parathyroid adenoma in sHPT is lower than pHPT and performing CDUS can increases the overall sensitivity and specificity of imaging methods in accurate localization of parathyroid lesion.
Keywords: Preoperative localization, parathyroid lesion, diagnosis, color doppler, ultrasonography
Introduction
As the incidence of hyperparathyroidism (HPT), the third most common cause of endocrine disease, is increasing, it is no longer an uncommon problem [1]. More asymptomatic patients are being diagnosed due to yearly checkups and routine screening tests [2]. HPT is the leading cause of hypercalcemia, which is due to overproduction of parathyroid hormone (PTH) by parathyroid glands (PTGs) [3, 4].
Over 80% of patients with pHPT have solitary adenoma and the remaining have multiple adenoma, hyperplasia, and rarely carcinoma [5]. The only definite treatment for HPT is surgical resection of adenoma or hyperplastic gland. The traditional procedure is bilateral neck exploration and has a success rate of 95% if an experienced hand does it. Recently, less invasive procedures like unilateral neck exploration, minimally invasive parathyroidectomy (MIP), video assisted parathyroidectomy, etc has been developed and are gaining popularity among surgeons due to decreased operative time and decreased risk of injury to the adjacent tissue [6, 7]. For this reason, preoperative localization is playing an important role to detect the precise location of the affected gland and to increase the success rate. As for sHPT, the standard protocol is subtotal or total parathyroidectomy followed by auto-transplantation. Three and a half gland will be resected and half of the gland that is smaller in size and has low vascularity will be left in site [8]. Different modalities for preoperative diagnosis of parathyroid lesions have been proposed and each has its own advantage and limitation. Ultrasonography (US) and scintigraphy (99mTc MIBI) along with computed tomography (CT) and magnetic resonance imaging (MRI) are being used. US is preferable because of availability and being less invasive and cost-effectiveness [9, 10]. In the present study we aimed to get into challenge the utility of US and scintigraphy for pHPT and sHPT and also adding the CDUS combination wit US to improve accuracy of these modalities to direct a better surgical approach.
Material and methods
From June 2007 to June 2011, 56 consecutive patients (11men and 45women) with primary or secondary hyperparathyroidism in our center underwent Gray scale, color Doppler and 99m-Tc MIBI scan prior to operative management of parathyroid lesions.
We prospectively evaluated the imaging data of these patients and compared it with histopathological results to determine the accuracy of these imaging modalities in the diagnosis of parathyroid lesion. Patients’ sex, age, history of chronic renal failure and serum intact Parathyroid hormone (iPTH) were collected from clinical data sheet.
One experienced radiologist in head and neck imaging performed all of the sonographic examinations. Sonographies were performed in Radiology Department of Imam training Hospital with an ultrasound device, (Toshiba Nemio 30; Toshiba CO. Ltd, Tokyo, Japan), by a high frequency (11MHZ) linear array transducer. The patients were examined in hyperextend neck in supine position from the level of mandibular angle to sterna notch.
In gray scale sonography, enlarged PTGs appear as oval or round shape homogenous hypo echoic nodules in posterior part of thyroid lobes that are clearly separated from thyroid lobes by an echogenic ring composed of capsules of adenoma and fatty tissue around the adenomas. We include this ultrasonographic feature as positive ultrasonographic result (Figure 1).
Figure 1.
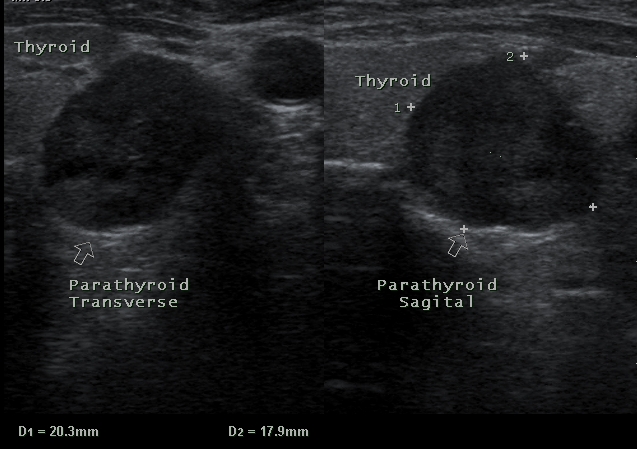
Ultrasound image shows typical hypoechoic adenoma (arrows) deep in relation to lower pole of thyroid in transverse sonogram. A. sagittal, B. transverse.
The PTG's size and Volume was measured and location of parathyroid adenoma was defined according to gray scale sonographic findings. Images were obtained in transverse and sagittal views.
Gray scale sonography results were defined as true or false positive and negative according to surgical pathology results.
Color and power Doppler sonography was performed by setting the Doppler parameter for evaluation of the vascularity of PTGs lesions. We know that parathyroid adenoma's blood supply is derived from inferior thyroid artery.
Color Doppler findings of each parathyroid lesion were defined as positive or negative on the basis of detection of the hypervascularity in PTG lesion in comparison with other adjacent structure subjectively (Figure 2).
Figure 2.
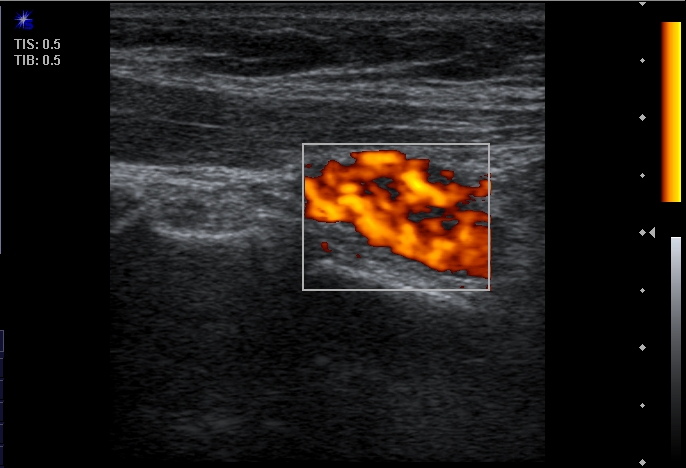
Color Doppler sonogram shows typical “spot of fire” pattern of hypervascularity of parathyroid adenoma in Power Doppler sonogram.
The thyroid scan in anterior-posterior view of the neck and upper part of thorax were acquired 20 minutes after intravenous administration of 99m-Tc pertechnetate (20mCi) with gamma camera for 5 minutes.
The parathyroid scan was acquired 15 minutes (thyroid phase) and 120 minutes (parathyroid phase) after intravenous administration of 15-20 mCi of 99mTc Sesta MIBI while the patients were in supine position. The images were obtained with single head gamma camera (ADAK, Epic, Netherlands) over 10 minutes using a pinhole collimator. The Sestamibi scan was considered positive for parathyroid lesion when an increase in uptake was detected in thyroid phase.
Technetium -99m-sestamibi SPECT was performed 20-30 minutes after IV injection and reconstructed to produce 3 Dimensional projections. The SPECT was performed in full anterior (180 degree) view as 32 frames, each frame 30 seconds. Focal increased uptake in parathyroid site in considered as positive.
Statistical analysis was performed using SPSS statistical analysis software (SPSS Inc.). Continuous variables were reported as means ± SD and categorical variables as frequencies. Since the distribution of PTH and volume was not normal, the nonparametric spearman's test was used for analyzing. One-way ANOVA, chi-square test and multivariate linear model was used for evaluation of variables.
Results
Parathyroidectomy was performed in 56 patients in our hospital. Thirty-six patients had pHPT and 20 patients had sHPT. The mean age of patients for pHPT and sHPT was 52.2 ± 12.6 years respectively. Of 34 patients with PHPT, 29 were female and 7 were male and of 20 patients with sHPT, 16 were female and 4 patients were male. Our study showed that both adenoma and hyperplasia were more commonly seen in women with female to male ratio of 4 to 1.
The mean volume of involved PTGs was 2.18 ± 2.03 cc in patients with pHPT (ranging from 0.2-7.5 cc) and 1.13 ± 1.14 cc in patients with sHPT (ranging from 0.1-4.4 cc). This difference was statistically significant (p=0.001).
The mean serum iPTH level was 584 ± 510 (ranging from 80-2000) in patients with pHPT and 908.3 ± 746.9 (ranging from 76-2472) in patients with sHPT. Patients with sHPT had higher serum iPTH level (P=0.039).
Assuming that the standard surgical protocol in our department is bilateral neck dissection in sHPT and unilateral neck dissection in pHPT, total number of PTGs would be 72 for patients with pHPT and 80 for patients with sHPT. US revealed 34 Lesions in patients with pHPT and 22 lesions in patients with sHPT. MIBI scan showed 26 Parathyroid lesion in patients with pHPT and 9 lesions in patients with sHPT.
Histopathology revealed 36 solitary adenomas and 35 parathyroid hyperplasia in patients with pHPT and sHPT respectively; the remaining 36 PTGs in patients with pHPT and 45 PTGs in patients with sHPT were normal.
Histopathology revealed that three patients with pHPT had intra-thyroidal parathyroid adenoma. All of them were identified by MIBI scan but US didn't detect any of them because of concomitant multinodular goiter. In one patient with pHPT, US confirmed parathyroid lesion was lymph node and in one patient with pHPT, the lesion was exophytic thyroid nodule mimicking parathyroid adenoma and two patients with sHPT, US confirmed parathyroid lesions were lymph nodes.
Table 1 summarizes the results of MIBI, gray scale, and color Doppler US in diagnosis of parathyroid lesion in patients with pHPT and sHPT.
Table 1.
Comparative results of different imaging modalities in diagnosis of parathyroid adenoma
| pHPT | sHPT | |||||||
|---|---|---|---|---|---|---|---|---|
| HPT | ||||||||
| US | MIBI scan | combined | CDUS | US | MIBI scan | combined | CDUS | |
| Sensitivity | 88% | 70% | 87% | 97% | 53% | 25% | 45% | 62% |
| Specificity | 94% | 100% | 100% | 100% | 93% | 100% | 100% | 100% |
| Accurracy | 91% | 85% | 98.6% | 98.6% | 76% | 72.9% | 92.5% | 83% |
HPT: Hyperparathyroidism pHPT:Primary hyperparathyroidism US: Ultrasonography ; sHPT:Secondary hyperparathyroidism CDUS: Color Doppler Ultrasonography; MIBI Scan: methoxy-isobutyl-isonitrile Scan.
The sensitivity, specificity and accuracy of US and MIBI scan for pHPT was 88%, 94%, 91% and 70%, 100% and 85% respectively.
In patients with sHPT, the sensitivity, specificity and accuracy of US and MIBI scan was 54%, 93%, 76% and 25%, 100% and 72.9% respectively. The overall sensitivity of combined US and MIBI scan in pHPT and sHPT was 97% and 45% respectively.
Assuming that parathyroid adenoma or hyperplasia has a large feeding artery derived from inferior thyroidal artery or an arc of perfusion in color Doppler ultra sonography (CDUS), we detected 35 from 36 adenoma in pHPT and 22 hyperplasia from 35 involved glands in patients with sHPT. Therefore, the overall sensitivity, specificity and accuracy of CDUS in diagnosis of parathyroid lesions in pHPT and sHPT is 97%, 100%, 98.6% and 62%, 100% and 83% respectively .
Our study showed that there is a significant positive correlation between serum intact PTH levels and positive MIBI scan (P=0.025) only in pHPT. The mean serum iPTH level in pHPT with positive MIBI scan was 666 ± 554 pg/ml and in patients with negative MIBI scan was 222 ± 146 pg/ml.
iPTH levels did not show significant differences between MIBI positive and negative patients with sHPT (p=0.45). The mean serum iPTH level in sHPT with positive MIBI scan was 1107 ± 895 pg/ml and in patients with negative MIBI scan was 822 ± 693 pg/ml.
Our study revealed that parathyroid lesions volume did not show a significant difference between MIBI positive and negative patients both in pHPT (P=0.3) and sHPT (P=0.9).
The mean volume of adenoma in patients with pHPT for positive MIBI scan was 2.42 ± 2.09 cc and for negative MIBI was 1.6 ± 1.94 cc. The mean volume of hyperplastic glands in patients with sHPT and positive MIBI scan was 1 ± 0.33 cc and in patients with negative MIBI was 1.47 ± 1.37 cc.
We found that there is a positive correlation between volume and serum iPTH levels only in pHPT (r=0.35, P=0.035). There was no correlation between volume of hyperplastic parathyroid lesions and serum iPTH levels in sHPT (r=0.49, P=0.7).
Discussion
Most of the healthy adults have four parathyroid glands, less than 5% have less than four glands and 3% healthy adults have more than four glands [11, 9]. The average dimension of parathyroid gland is 5 × 3 × 1 mm and average weight is 30-40 mg [11, 9].
The head and neck radiologists must be familiar with embryology and migration of parathyroid glands if they want to completely investigate the different patterns of hyperparathyroidism.
The parathyroid glands develop at 6 weeks of gestation and migrate caudally at 8 weeks, The upper parathyroid glands, originates from the fourth pharyngeal pouch and the lower parathyroid gland with thymus gland originates from the third pharyngeal pouch [12].
Hyperparathyroidism can be classified as primary, secondary, or tertiary. The prevalence of Primary hyperparathyroidism is 0.2% among women and 0.05% among men, most of them occur between the ages of 40-60 years [13].
Polyuria, polydipsia, and weakness accompanied with skeletal abnormality, nephrocalcinosis, nephrolithiasis, peptic ulcer disease, and psychiatric disorder are the most common clinical manifestation of hyperparathyroidism. Serum hypercalcemia and elevated serum intact PTH level are the key points in the diagnosis of hyperparathyroidism [14].
Over the past decade bilateral neck dissection and exploratory surgery was the traditional method in surgical management of hyperparathyroidism, Nowadays traditional method has been replace by focused, minimally invasive surgery in patients with single adenoma [15].
For minimally invasive surgery be successful, it dependents totally on accurate preoperative localization of parathyroid adenoma. While in secondary hyperparathyroidism imaging studies are of a limited value in surgical management of patients but have an important role when percutaneous alcohol ablation is contemplated as an adjunctive medical treatment [9].
It is uncommon to see normal parathyroid glands with ultrasonography; Diagnosis of hyperplasia is difficult by sonography because the gland volume is much less than parathyroid adenoma. Hyperparathyroidism can commonly be accompanied by thyroid disease and reactive lymph node may be particularly prominent in these patients. Therefore reactive lymph node can be mistake with parathyroid adenoma or hyperplasia [9]. In this situation CDUS can be a useful modality for accurate diagnosis of parathyroid lesion and differentiation of parathyroid adenoma from other cervical pathology.
In our study the prevalence of parathyroid adenoma was more frequent in female than male, both in primary hyperparathyroidism and secondary hyperparathyroidism, which were in concordance with previous data [4, 13].
In our study there was no correlation between serum iPTH levels and the results of MIBI scan in sHPT (P=0.45) but there was a positive correlation between the serum iPTH levels and the results of MIBI scan in PHPT (P=0.025). There was no significant difference in serum iPTH levels in patients with MIBI positive result in comparison with MIBI negative group. Previous reports showed many controversies about correlation between serum iPTH level and MIBI scan. Some investigation revealed that there is a relationship between serum iPTH levels and MIBI results [16-18] but other did not show such a correlation [19]. Sukan et al [20] reported a significant difference in intact PTH levels between MIBI positive and negative patients.
We found a relationship between serum iPTH level and ultrasonographic estimation of parathyroid adenoma's volume only in primary hyperparathyroidism. Our results are in agreement with other study that did not showed the correlation between serum iPTH and weight of the parathyroid glands [20, 21] but our results are in contrast with other reports that showed a correlation between serum iPTH and weight of the parathyroid glands. Kakuta et al showed that the volume of PTGs by US is a good indicator of their weight. Larger PTGs secrete more whole iPTH per gland [22].
We did not found relationship between ultrasonographic PTG volume determination and MIBI results both in pHPT (P=0.3) and sHPT (P=0.93). Our results were in agreement with Kasai et a l that revealed the correlation of gland weight and MIBI uptake were not significant [23] and in contrast to the results of Cechin et al [14], which demonstrated that “In MIBI positive and negative patients there was a statistically significant difference among cases in terms of adenoma volume (1.30 ± 1.51 cc vs. 0.58 ± 0.91 cc, p<0.05)".
It is obvious that sonography is operator dependent and according to previous data; the reported sensitivity of US and MIBI scan in detection of parathyroid adenoma in primary hyperparathyroidism ranging from 57-93%, 54-93%, respectively [24]. Ababa et al [25] reported that Sensitivity, specificity and diagnostic accuracy value of combined US and MIBI scan in detection of parathyroid adenoma is 94.9%, 25.0% and 91.1%.
Sikas et al [26] reported that sensitivity of US and MIBI scan in patients with secondary hyperparathyroidism is 50% and 45% respectively and the specificity of US and MIBI scan were 85% and 80% respectively.
Because of the low sensitivity of US in the detection of parathyroid hyperplasia in SHPT it is less attractive than its application in detection of parathyroid adenoma in PHPT.
We detected 35 hyperplasic PTGs in our study from 80 resected PTGs. Although hyperplasia in sHPT usually affects all glands, but not infrequently, one, two, or even three may be spared [27]. The Basis for such asymmetrical involvement is obscure.
In conclusion by performing CDUS as routinely in the evaluation of pHPT or sHPT the overall specificity and accuracy of preoperative imaging evaluation of parathyroid adenoma increase significantly and can aid to better decision making for operative management.
References
- 1.Wermers RA, Khosla S, Atkinson EJ, Achenbach SJ, Oberg AL, Grant CS, Melton LJ., 3rd Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease. J Bone Miner Res. 2006;21:171–177. doi: 10.1359/JBMR.050910. [DOI] [PubMed] [Google Scholar]
- 2.Mihai R, Wass JA, Sadler GP. Asymptomatic hyperparathyroidism-need for multicentre studies. Clinical Endocrinology. 2008;68:155–164. doi: 10.1111/j.1365-2265.2007.02970.x. [DOI] [PubMed] [Google Scholar]
- 3.MacKenzie-Feder J, Sirrs S, Anderson D, Khan A. Primary Hyperparathyroidism: An Overview. Int J Endocrinol. 2011;2011:251410. doi: 10.1155/2011/251410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taniegra ED. Hyperparathyroidism. Am Fam Physician. 2004;69:333–339. [PubMed] [Google Scholar]
- 5.Marx SJ. Hyperparathyroid and hyperparathyroid disorders. N Engl J Med. 2000;343:1863–1875. doi: 10.1056/NEJM200012213432508. [DOI] [PubMed] [Google Scholar]
- 6.Carling T, Udelsman R. Focused approach to parathyroidectomy. World Journal of Surgery. 2008;32:1512–1517. doi: 10.1007/s00268-008-9567-z. [DOI] [PubMed] [Google Scholar]
- 7.Starker L, Fonseca A, Carling T, Udelsman R. Minimally Invasive Parathyroidectomy. Int J Endocrinol. 2011;2011:206502. doi: 10.1155/2011/206502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tominaga Y. Surgical treatment of secondary hyperparathyroidism due to chronic kidney disease. Uppsala J Med Sci. 2006;3:277–292. doi: 10.3109/2000-1967-047. [DOI] [PubMed] [Google Scholar]
- 9.Johnson NA, Tublin ME, Ogilvie JB. Parathyroid Imaging: Technique and Role in the Preoperative Evaluation of Primary Hyperparathyroidism. AJR Am J Roentgenol. 2007;188:1706–1715. doi: 10.2214/AJR.06.0938. [DOI] [PubMed] [Google Scholar]
- 10.De Feo ML, Colagrande S, Biagini C, Tonarelli A, Bisi G, Vaggelli L, Borrelli D, Cicchi P, Tonelli F, Amorosi A, Serio M, Brandi ML. Parathyroid glands: combination of (99m) Tc MIBI scintigraphy and US for demonstration of parathyroid glands and nodules. Radiology. 2000;214:393–402. doi: 10.1148/radiology.214.2.r00fe04393. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen BD. Parathyroid Imaging with Tc-99m Sestamibi Planar and SPECT Scintigraphy. Radiographics. 1999;19:601–614. doi: 10.1148/radiographics.19.3.g99ma10601. [DOI] [PubMed] [Google Scholar]
- 12.Simeone DM, Sandelin K, Thompson NW. Undescended superior parathyroid gland: a potential cause of failed cervical exploration for hyperparathyroidism. Surgery. 1995;118:949–956. doi: 10.1016/s0039-6060(05)80099-6. [DOI] [PubMed] [Google Scholar]
- 13.Suh JM, Cronan JJ, Monchik JM. Primary hyperparathyroidism: is there an increased prevalence of renal stone disease? AJR Am J Roentgenol. 2008;191:908–911. doi: 10.2214/AJR.07.3160. [DOI] [PubMed] [Google Scholar]
- 14.Suter-Widmer I, Kraenzlin ME, Meier C. Primary hyperparathyroidism. Ther Umsch. 2011;68:321–326. doi: 10.1024/0040-5930/a000172. [DOI] [PubMed] [Google Scholar]
- 15.Patel CN, Scarsbrook AF. Multimodality imaging in hyperparathyroidism. Postgrad Med J. 2009;85:597–605. doi: 10.1136/pgmj.2008.077842. [DOI] [PubMed] [Google Scholar]
- 16.Hung GU, Wang SJ, Lin WY. Tc-99m MIBI parathyroid scintigraphy and intact parathyroid hormone levels in hyperparathyroidism. Clin Nucl Med. 2003;28:180–185. doi: 10.1097/01.RLU.0000053529.71776.37. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosoni P, Heuguerot C, Olaizola I, Acuña G, Fajardo L, Petraglia A, Caorsi H, López J, Kurdian M, Jorgetti V, Aznárez A. Can we use 99mTc-MIBI in functional studies of the parathyroid gland? Nephrol Dial Transplant. 1998;13:33–36. doi: 10.1093/ndt/13.suppl_3.33. [DOI] [PubMed] [Google Scholar]
- 18.Stawicki SP, El Chaar M, Baillie DR, Jaik NP, Estrada FP. Correlations between biochemical testing, pathology findings and preoperative sestamibi scans: a retrospective study of the minimally invasive radioguided parathyroidectomy (MIRP) approach. Nucl Med Rev Cent East Eur. 2007;10:82–86. [PubMed] [Google Scholar]
- 19.Carpentier A, Jeannotte S, Verreault J, Lefebvre B, Bisson G, Mongeau CJ, Maheux P. Preoperative localization of parathyroid lesions in hyperparathyroidism: relationship between technetium-99m-MIBI uptake and oxyphil cell content. J Nucl Med. 1998;39:1441–1444. [PubMed] [Google Scholar]
- 20.Sukan A, Reyhan M, Aydin M, Yapar AF, Sert Y, Canpolat T, Aktas A. Preoperative evaluation of hyperparathyroidism: the role of dual-phase parathyroid scintigraphy and ultrasound imaging. Ann Nucl Med. 2008;22:123–131. doi: 10.1007/s12149-007-0086-z. [DOI] [PubMed] [Google Scholar]
- 21.Saxe AW, Lincenberg S, Hamburger SW. Can the volume of abnormal parathyroid tissue be predicted by preoperative biochemical measurement? Surgery. 1987;102:840–845. [PubMed] [Google Scholar]
- 22.Kakuta T, Tanaka R, Kanai G, Miyamoto Y, Inagaki M, Suzuki H, Fukagawa M, Saito A. Relationship between the weight of parathyroid glands and their secretion of parathyroid hormone in hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial. 2008;12:385–390. doi: 10.1111/j.1744-9987.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 23.Kasai ET, da Silva JW, Mandarim de Lacerda CA, Boasquevisque E. Parathyroid glands: combination of sestamibi-(99m)Tc scintigraphy and ultrasonography for demonstration of hyperplasic parathyroid glands. Rev Esp Med Nucl. 2008;27:8–12. doi: 10.1157/13114364. [DOI] [PubMed] [Google Scholar]
- 24.Cecchin D, Motta R, Zucchetta P, Bui F, Basso SM, Lumachi F. Imaging Studies in Hypercalcemia. Curr Med Chem. 2011;18:3485–3493. doi: 10.2174/092986711796642607. [DOI] [PubMed] [Google Scholar]
- 25.Akbaba G, Berker D, Isik S, Aydin Y, Ciliz D, Peksoy I, Ozuguz U, Tutuncu YA, Guler S. A co-parative study of preoperative imaging methods in patients with primary hyperparathyroidism: US, MIBI, SPECT and MRI. J Endocrinol Invest. 2011 doi: 10.3275/7764. [DOI] [PubMed] [Google Scholar]
- 26.Sikas N, Gakis D, Takoudas D, Vergoulas G, Fouzas I, Karatzas N, Antoniadis A. Preoperative localization of parathyroid glands in secondary hyperparathyroidism and concomitant thyroid disease. Hippokratia. 2000;4:19–25. [Google Scholar]
- 27.Kumar v, Abbas AK, Fausto N, Aster J. Robbins & Cotran Pathologic Basis of Disease. 8 edition. W.B. Saunders Co; 2009. pp. 1097–1164. [Google Scholar]


