Highlights
► FDA-approved drug mitoxantrone had in vitro activity against cowpox and monkeypox. ► Mitoxantrone increased survival time and survival rate in C57Bl/6 mice infected intraperitoneally with cowpox. ► Mitoxantrone showed significant in vitro synergy with cidofovir against cowpox. ► No synergy was observed in vivo between mitoxantrone and cidofovir versus cowpox in intranasally-infected BALB/c mice. ► Fewer animals survived treatment with 0.5 mg/kg MXN and 100 mg/kg CDV compared to that dose of CDV alone.
Keywords: Poxvirus, Mitoxantrone, Cowpox, Monkeypox, Synergy
Abstract
Mitoxantrone, an FDA-approved therapeutic for the treatment of cancer and multiple sclerosis, was previously reported to exhibit antiviral activity against vaccinia virus. To determine whether this activity extends to other orthopoxviruses, mitoxantrone was tested against cowpox and monkeypox. Mitoxantrone demonstrated an EC50 of 0.25 μM against cowpox and 0.8 μM against monkeypox. Intraperitoneal treatment of cowpox virus-challenged C57Bl/6 mice with 0.5 mg/kg mitoxantrone resulted in 25% survival and a significant increase in survival time. In an effort to improve its efficacy, mitoxantrone was tested for synergistic activity with cidofovir. In vitro tests demonstrated significant synergy between the two drugs against cowpox; however, no synergistic effect on animal survival or median time-to-death was seen in intranasally-infected BALB/c mice. Significantly fewer animals survived when treated with a combination of 0.5 mg/kg mitoxantrone and 100 mg/kg cidofovir than with 100 mg/kg cidofovir alone. This is, to our knowledge, the first report of limited anti-orthopoxvirus activity by mitoxantrone in an animal model.
In light of concerns regarding the use of variola virus (VARV), the etiological agent of smallpox, and monkeypox virus (MPXV) as bioterrorism weapons as well as the increase in frequency of zoonotic poxvirus infections (Essbauer et al., 2010, Rimoin et al., 2010), the development of antiviral drugs to counter the emergence of poxviruses is essential. One strategy to accelerate the development of anti-poxvirus countermeasures is to screen compounds already approved by the Food and Drug Administration for other applications for efficacy against poxviruses (Smee and Sidwell, 2003). Several licensed drugs, including cidofovir (CDV), a dCMP analog for use in the treatment of cytomegalovirus retinitis, and triflurothymidine, a nucleoside analog used to treat ocular herpes simplex virus infections, have been shown to be efficacious against orthopoxviruses both in animal models and clinically (Altmann et al., 2011, De Clercq, 2002, Vora et al., 2008), thus illustrating the utility of this strategy.
The FDA-approved anti-cancer and anti-multiple sclerosis drug mitoxantrone (MXN), an anthracycline derivative, is an intercalating agent and inhibits topoisomerase II function resulting in impaired cellular DNA replication and RNA synthesis (Fox, 2004). Recently, MXN was shown to have anti-vaccinia virus (VACV) activity in tissue culture but not in mice (Deng et al., 2007). Surprisingly, MXN blocked virion assembly rather than DNA replication or RNA synthesis (Deng et al., 2007). Although the mechanism of this inhibition is unclear, it has been suggested that MXN targets viral DNA ligase and the recruitment of host-cell topoisomerase II to the site of viral replication (Lin et al., 2008).
In vivo efficacy of a drug against one orthopoxvirus is not necessarily a reliable predictor of activity against other related viruses. For example, both cidofovir and tecovirimat (ST-246), a poxvirus egress inhibitor in phase II clinical trials, have shown a range of activity against different poxviruses (Buller et al., 2004, De Clercq, 2002, Duraffour et al., 2007, Yang et al., 2005). Thus, MXN’s lack of in vivo activity against VACV (Deng et al., 2007) does not preclude the possibility that it could be effective against other orthopoxviruses. Recently, MXN was identified as a possible inhibitor of cowpox virus (CPXV) and MPXV infection during a high-throughput screen of synthetic compound libraries (manuscript in preparation). Based on these findings, we evaluated whether MXN, either alone or in combination with CDV, was active against MPXV in vitro and against CPXV in vitro and in vivo.
We first tested the ability of MXN to inhibit CPXV and MPXV infection in tissue culture studies analogous to those by Deng et al. with VACV (Deng et al., 2007). One step growth curves of CPXV (Fig. 1 A) and MPXV (Fig. 1B) in the presence of 1 μM MXN were performed on BSC-1 cells. Cowpox growth was significantly inhibited in the presence of MXN, with virus titers remaining essentially constant over 36 h (Fig. 1A). In contrast, in the absence of the drug CPXV had a 100-fold increase in virus titer by 36 h post-infection. Mitoxantrone did not reduce MPXV replication to the same degree as it did CPXV, although the reduction was significant after 12 h post-infection (Fig. 1B). The 50% cytotoxic concentration (EC50) of MXN was at least 16 μM, indicating the antiviral effects observed were not due to drug toxicity (data not shown). To determine the 50% effective concentration of MXN, the effect of the drug on expression of green fluorescent protein (GFP) from recombinant CPXV (CPXV-GFP) and MPXV (MPXV-GFP) was determined. GFP intensity was measured in BSC-1 cells infected at a MOI of 1 with recombinant viruses in the presence of increasing concentrations of MXN at 48 h post-infection. As was seen with the one step growth curves, MXN was more effective against CPXV-GFP (EC50 = 0.25 μM) than against MPXV-GFP (EC50 = 0.8 μM) (Fig. 1C).
Fig. 1.
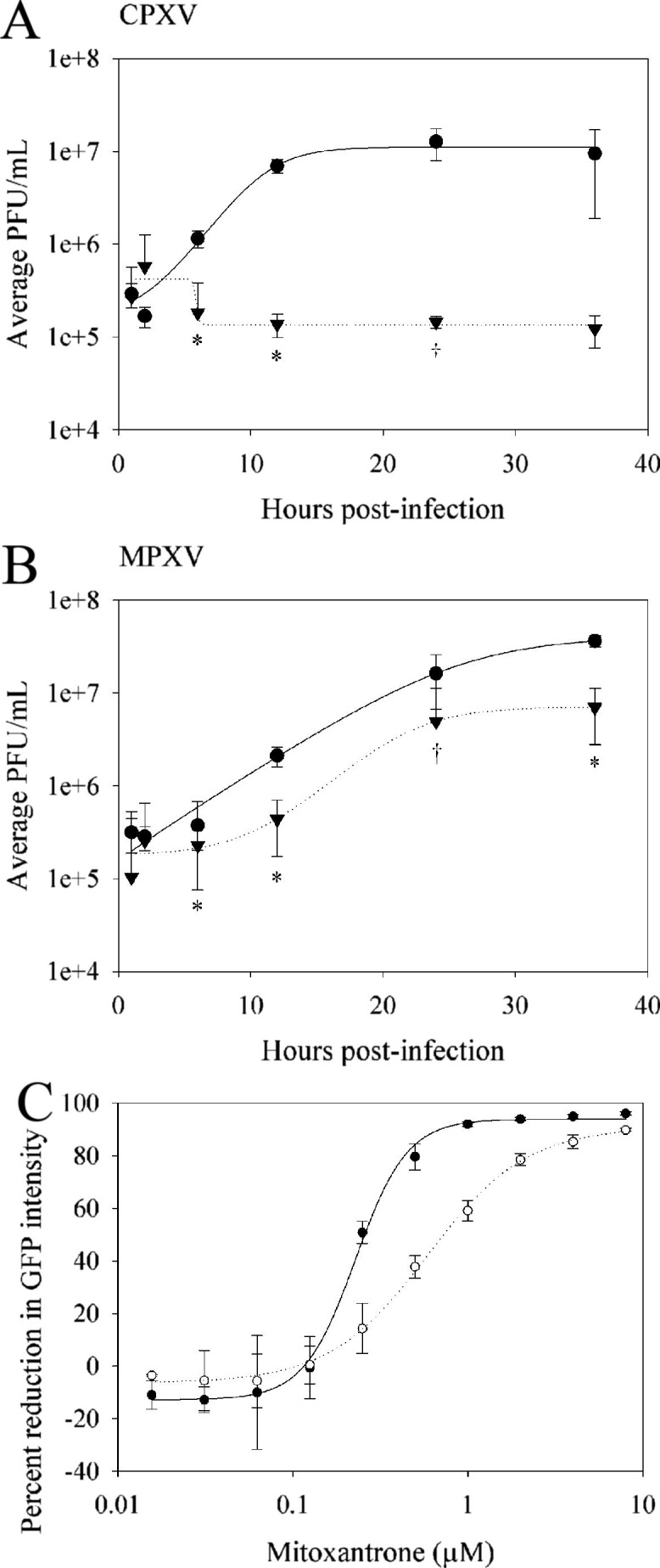
In vitro activity of mitoxantrone against cowpox and monkeypox. Mitoxantrone was tested for efficacy against CPXV (A and C) and MPXV (B and C). (A and B) One step growth curves on BSC-1 cells in the presence (dotted lines) and absence (solid lines) of 1 μM MXN. Cells were treated with MXN for 12 h prior to infection at a multiplicity of infection (MOI) of 3. Cell lysates were harvested at the indicated times post-infection and titered for virus by serial 10-fold dilution on BSC-1 cells. Virus yield in the presence of MXN was significantly reduced compared to untreated samples (†, p < 0.01; *, p < 0.001). (C) EC50 curves for CPXV-GFP (closed circles) and MPXV-GFP (open circles). Fluorescent intensity was read at 520 nm using a SpectraMax M microplate reader (Molecular Devices, Sunnyvale, CA). Data are representative of three independent experiments. Error bars represent the standard deviation of the mean for one experiment.
To date, few immunocompetent small-animal models of MPXV infection have been described (Americo et al., 2010, Hutson et al., 2009, Tesh et al., 2004) and only one of these, prairie dogs, has been used to evaluate anti-monkeypox drug efficacy (Smith et al., 2011). As none of these models has been fully characterized, the in vivo efficacy of MXN was tested only against CPXV in well-established mouse models of infection. To determine whether MXN was effective against CPXV in vivo, 0.25 or 0.5 mg/kg of drug was administered intraperitoneally to 4–6 week old female C57Bl/6 mice 1 day post-intraperitoneal inoculation. Log-rank tests were performed comparing survival times between the two MXN treatment groups relative the mock-treated group. A p-value of 0.025 was considered statistically significant, after a Bonferroni correction for the comparisons of the two MXN dose groups to the mock-treated. The lower dose of MXN significantly improved the survival time of infected animals, with the median day-of-death (MDD) in infected animals increasing from day 7 to day 9 (p = 0.0015). The 0.5 mg/kg dose of MXN improved the MDD to 12 days (p = 0.0005). Twenty-five percent of the animals treated with 0.5 mg/kg MXN survived compared to 5% of mock-treated animals (Fig. 2 ). Administration of higher levels of MXN or additional dosages were not beneficial (data not shown).
Fig. 2.

Mitoxantrone prolongs survival in CPXV-infected C57Bl/6 mice. Female C57Bl/6 mice were inoculated intraperitoneally with 1 × 106 PFU CPXV. One day post-inoculation, animals (n = 20) received 0.5 mL of MXN at the indicated dosage or saline solution intraperitoneally. Data are a compilation of two independent experiments. Kaplan–Meier survival curves plot the survival times by dose. The lower dose of MXN significantly improved the survival time of infected animals, with the median day-of-death (MDD) in infected animals increasing from day 7 to day 9 (p = 0.0015; log-rank test), while 0.5 mg/kg of MXN improved the MDD to 12.5 (p = 0.0005; log-rank test). All animal procedures were approved by the NIAID Animal Care and Use Committee and adhered to National Institutes of Health policies.
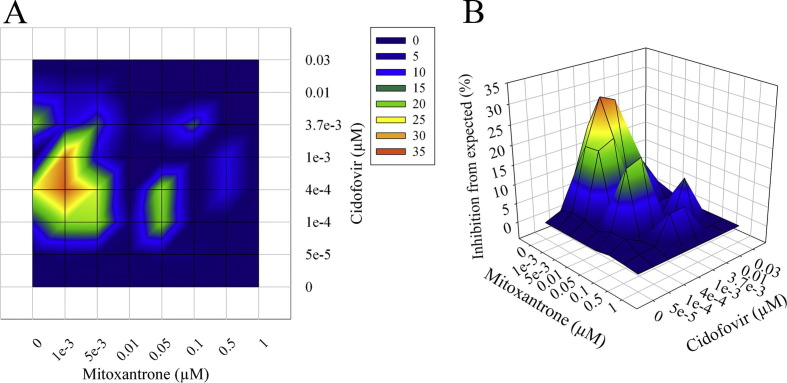
Concentrations of MXN higher than those used here can be toxic in mice ((Deng et al., 2007) and data not shown). One strategy that can be used to mitigate toxicity issues is to use a combination of drugs for treatment that display synergistic activity, thereby reducing the concentration of the individual drugs needed to see an antiviral effect. To evaluate the possibility that MXN could act synergistically in combination with other drugs, MXN was screened for in vitro synergy with CDV against CPXV-GFP. Infected BSC-1 cells were incubated for 24 h with increasing concentrations of MXN and CDV either separately or in combination. Synergy (percent inhibition above calculated) was determined using the MacSynergy™ II software (Prichard and Shipman, 1990). Mitoxantrone showed significant synergistic activity in combination with CDV (Fig. 3 ), with a peak volume of 324.06 μM2%. In vitro peak volumes above 100 μM2% are considered to be highly predictive of in vivo efficacy (MacSynergy™ II software manual).
Fig. 3.
In vitro synergistic activity of mitoxantrone and cidofovir against cowpox. Mitoxantrone was tested for synergistic activity with cidofovir against CPXV. Triplicate plates of BSC-1 cells were infected at an m.o.i. of one with CPXV-GFP in the presence of increasing concentrations of both drugs, either independently or in combination. The intensity of the GFP signal was measured 24 hrs post-infection, and data were analyzed using the MacSynergy™ II software. (A) Isobologram. (B) 3D representation of inhibition curves. Data are representative of three independent experiments. Peak volume of synergy was 324.06 μM2%; volumes above 100 μM2% are considered to be highly predictive of in vivo efficacy.
To determine if the significant synergistic activity against CPXV observed between MXN and CDV in tissue culture extended to in vivo models, MXN and CDV were tested in an established model of CDV synergy (Quenelle et al., 2007). BALB/c mice challenged intranasally with CXPV were treated 1 day post-challenge with MXN (0, 0.25, or 0.5 mg/kg) and CDV (0, 3, 10 mg/kg, 30, or 100 mg/kg). Log-rank tests were performed to compare the survival times between different treatment groups. Comparisons of the CDV-only groups were relative to the mock-treated animals. Within CDV groups, comparisons of CDV + MXN were made relative to those receiving CDV-only. Mitoxantrone exhibited a minimal effect on survival when used alone against CPXV (Table 1 ). The use of MXN in combination with CDV had a minimal effect on survival relative to the use of CDV alone. Fewer animals survived treatment with 100 mg/kg CDV and 0.25 or 0.5 mg/kg MXN compared to that dose of CDV alone. This trend was suggestive of MXN in combination with high doses of CDV being detrimental to animal survival, although the data did not meet our criteria for significance (p = 0.0135; Table 1). Overall, these data show that MXN has minimal in vivo synergistic activity in combination with CDV against CPXV in BALB/c mice.
Table 1.
In vivo synergistic activity of mitoxantrone against cowpox in BALB/c mice, with and without cidofovir.
| Treatmenta | No. of mice moribund/No. tested (%) | Median day of death | Log-rank p valuee |
|---|---|---|---|
| Mock | 5/5 (100) | 7 | |
| MXN 0.25 mg/kg | 5/5 (100) | 8 | 0.0027b |
| MXN 0.5 mg/kg | 5/5 (100) | 8 | 0.0027b |
| CDV 3 mg/kg | 5/5 (100) | 8 | 0.0027b |
| MXN 0.25 mg/kg | 5/5 (100) | 8 | NSc |
| MXN 0.5 mg/kg | 5/5 (100) | 9 | 0.0495c |
| CDV 10 mg/kg | 5/5 (100) | 9 | 0.0027b |
| MXN 0.25 mg/kg | 5/5 (100) | 8 | 0.0027c |
| MXN 0.5 mg/kg | 5/5 (100) | 11 | 0.0027c |
| CDV 30 mg/kg | 5/5 (100) | 11 | 0.0027b |
| MXN 0.25 mg/kg | 5/5 (100) | 11 | NSc |
| MXN 0.5 mg/kg | 5/5 (100) | 11 | NSc |
| CDV 100 mg/kg | 0/5 (0) | N/Ad | 0.0027b |
| MXN 0.25 mg/kg | 2/5 (40) | N/Ad | NSc |
| MXN 0.5 mg/kg | 4/5 (80) | 12d | 0.0135c |
BALB/c mice were anesthetized with isoflurane and inoculated intranasally with 1 × 106 PFU CPXV. One day post-infection, animals were treated intraperitoneally with MXN and CDV at the indicated concentrations. Drugs were administered in opposite sides of the peritoneal cavity.
p Value calculated by log-rank statistic relative to mock-treated animals. NS, not significant.
p Value calculated by log-rank statistic relative to CDV-only animals at the same dose. Values shown in italic indicate groups with survival times significantly lower than the matched CDV-only group. NS, not significant.
Survivors were euthanized on day 21 of the study.
To adjust for the 14 hypothesis tests, a Bonferroni adjustment to the significance level was applied. A p-value of less than 0.05/14 = 0.004 is considered statistically significant.
In summary, we evaluated MXN, an anthracycline derivate that had previously been characterized as an in vitro inhibitor of VACV infection (Deng et al., 2007), for activity against CPXV and MPXV. Both viruses were sensitive to MXN in vitro (Fig. 1), and MXN increased the MDD of C57Bl/6 mice infected with a lethal dose of CPXV (Fig. 2). An in vitro screen for synergy between MXN and CDV indicated that MXN may be more effective in vivo in combination with CDV than when used alone (Fig. 3), but this was not observed in our animal study (Table 1). Mitoxantrone has demonstrated several immunomodulatory activities in addition to its anti-tumor proliferation activity, including the suppression of B- and T-cells and the promotion of a TH2-type cytokine response (Fidler et al., 1986a, Fidler et al., 1986b, Vogelgesang et al., 2010). Such immunomodulation can be beneficial for treating autoimmune disorders such as multiple sclerosis. Clearance of poxvirus infections, however, requires both B- and T-cell activity, and, at least in the case of ectromelia virus, a TH1 cytokine response (Chaudhri et al., 2004, Chaudhri et al., 2006, Xu et al., 2004). It is therefore possible that the immunomodulatory activity was enough to negate any in vivo synergistic activity between MXN and CDV.
In agreement with a previous report which found that MXN exhibited no in vivo activity against intranasal VACV infection in BALB/c mice (Deng et al., 2007), we observed no efficacy against intranasal CPXV infection in BALB/c mice. Mitoxantrone did, however, demonstrate efficacy when used to treat intraperitoneally-infected C57Bl/6 mice, suggesting that differences in the route of infection and in mouse strain susceptibility to infection may influence MXN’s efficacy. While related anthracenediones have been reported to have in vivo antiviral activity against viruses unrelated to poxviruses (Dang et al., 2009, Sill et al., 1974), to our knowledge this is the first report of limited in vivo antiviral activity by MXN against poxviruses.
Acknowledgments
The authors thank Grant McFadden (University of Florida) for the gift of the cowpox and cowpox-GFP viruses, Xiao Liu (NIAID) for assistance with statistics, and Russ Byrum, Isis Alexander, Bernardo Rosa, and Nicholas Oberlander for technical assistance. This work was supported, in part, by the NIAID Division of Intramural Research.
References
- Altmann S., Brandt C.R., Murphy C.J., Patnaikuni R., Takla T., Toomey M., Nesbit B., McIntyre K., Covert J., Dubielzig R., Leatherberry G., Adkins E., Kodihalli S. Evaluation of therapeutic interventions for vaccinia virus keratitis. J. Infect. Dis. 2011;203:683–690. doi: 10.1093/infdis/jiq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Americo J.L., Moss B., Earl P.L. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J. Virol. 2010;84:8172–8180. doi: 10.1128/JVI.00621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R.M., Owens G., Schriewer J., Melman L., Beadle J.R., Hostetler K.Y. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology. 2004;318:474–481. doi: 10.1016/j.virol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Chaudhri G., Panchanathan V., Bluethmann H., Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J. Virol. 2006;80:6339–6344. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri G., Panchanathan V., Buller R.M.L., van den Eertwegh A.J.M., Claassen E., Zhou J., de Chazal R., Laman J.D., Karupiah G. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc. Natl Acad. Sci. USA. 2004;101:9057–9062. doi: 10.1073/pnas.0402949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang S.S., Jia X.L., Song P., Cheng Y.A., Zhang X., Sun M.Z., Liu E.Q. Inhibitory effect of emodin and Astragalus polysaccharide on the replication of HBV. World J. Gastroenterol. 2009;15:5669–5673. doi: 10.3748/wjg.15.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 2002;55:1–13. doi: 10.1016/S0166-3542(02)00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L., Dai P., Ciro A., Smee D.F., Djaballah H., Shuman S. Identification of novel antipoxviral agents: mitoxantrone inhibits vaccinia virus replication by blocking virion assembly. J. Virol. 2007;81:13392–13402. doi: 10.1128/JVI.00770-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraffour S., Snoeck R., de Vos R., van Den Oord J.J., Crance J.M., Garin D., Hruby D.E., Jordan R., De Clercq E., Andrei G. Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures. Antivir. Ther. 2007;12:1205–1216. [PubMed] [Google Scholar]
- Essbauer S., Pfeffer M., Meyer H. Zoonotic poxviruses. Vet. Microbiol. 2010;140:229–236. doi: 10.1016/j.vetmic.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler J., DeJoy S., Gibbons J. Selective immunomodulation by the antineoplastic agent mitoxantrone. I. Suppression of B lymphocyte function. J. Immunol. 1986;137:727–732. [PubMed] [Google Scholar]
- Fidler J., DeJoy S., Smith F., Gibbons J. Selective immunomodulation by the antineoplastic agent mitoxantrone. II. Nonspecific adherent suppressor cells derived from mitoxantrone-treated mice. J. Immunol. 1986;136:2747–2754. [PubMed] [Google Scholar]
- Fox E.J. Mechanism of action of mitoxantrone. Neurology. 2004;63:S15–18. doi: 10.1212/wnl.63.12_suppl_6.s15. [DOI] [PubMed] [Google Scholar]
- Hutson C.L., Olson V.A., Carroll D.S., Abel J.A., Hughes C.M., Braden Z.H., Weiss S., Self J., Osorio J.E., Hudson P.N., Dillon M., Karem K.L., Damon I.K., Regnery R.L. A prairie dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J. Gen. Virol. 2009;90:323–333. doi: 10.1099/vir.0.005108-0. [DOI] [PubMed] [Google Scholar]
- Lin Y.-C.J., Li J., Irwin C.R., Jenkins H., DeLange L., Evans D.H. Vaccinia virus DNA ligase recruits cellular topoisomerase II to sites of viral replication and assembly. J. Virol. 2008;82:5922–5932. doi: 10.1128/JVI.02723-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard M.N., Shipman C., Jr. A three-dimensional model to analyze drug–drug interactions. Antiviral Res. 1990;14:181–205. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- Quenelle D.C., Prichard M.N., Keith K.A., Hruby D.E., Jordan R., Painter G.R., Robertson A., Kern E.R. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob. Agents Chemother. 2007;51:4118–4124. doi: 10.1128/AAC.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., Blumberg S., Thomassen H.A., Pike B.L., Fair J.N., Wolfe N.D., Shongo R.L., Graham B.S., Formenty P., Okitolonda E., Hensley L.E., Meyer H., Wright L.L., Muyembe J.J. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sill A.D., Andrews E.R., Sweet F.W., Hoffman J.W., Tiernan P.L., Grisar J.M., Fleming R.W., Mayer G.D. Bis-basic-substituted polycyclic aromatic compounds. New class of antiviral agents. 5. Bis-basic ethers of anthraquinone and bisalkamine esters of anthraquinone dicarboxylic acids. J. Med. Chem. 1974;17:965–968. doi: 10.1021/jm00255a011. [DOI] [PubMed] [Google Scholar]
- Smee D.F., Sidwell R.W. A review of compounds exhibiting anti-orthopoxvirus activity in animal models. Antiviral Res. 2003;57:41–52. doi: 10.1016/S0166-3542(02)00199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.K., Self J., Weiss S., Carroll D., Braden Z., Regnery R.L., Davidson W., Jordan R., Hruby D.E., Damon I.K. Effective antiviral treatment of systemic orthopoxvirus disease: ST-246 treatment of prairie dogs infected with monkeypox. J. Virol. 2011 doi: 10.1128/JVI.02173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R.B., Watts D.M., Sbrana E., Siirin M., Popov V.L., Shu-Yuan X. Experimental infection of ground squirrels (Spermophilus tridecemlineatus) with monkey pox virus. Emerg. Infect. Dis. 2004;10:1563–1567. doi: 10.3201/eid1009.040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelgesang A., Rosenberg S., Skrzipek S., Bröker B.M., Dressel A. Mitoxantrone treatment in multiple sclerosis induces TH2-type cytokines. Acta Neurol. Scand. 2010;122:237–243. doi: 10.1111/j.1600-0404.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- Vora S., Damon I., Fulginiti V., Weber, Stephen G., Kahana M., Stein, Sarah L., Gerber, Susan I., Garcia-Houchins S., Lederman E., Hruby D., Collins L., Scott D., Thompson K., Barson, John V., Regnery R., Hughes C., Daum, Robert S., Li Y., Zhao H., Smith S., Braden Z., Karem K., Olson V., Davidson W., Trindade G., Bolken T., Jordan R., Tien D., Marcinak J. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 2008;46:1555–1561. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- Xu R., Johnson A.J., Liggitt D., Bevan M.J. Cellular and humoral immunity against Vaccinia virus infection of mice. J. Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- Yang G., Pevear D.C., Davies M.H., Collett M.S., Bailey T., Rippen S., Barone L., Burns C., Rhodes G., Tohan S., Huggins J.W., Baker R.O., Buller R.L.M., Touchette E., Waller K., Schriewer J., Neyts J., DeClercq E., Jones K., Hruby D., Jordan R. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]