Abstract
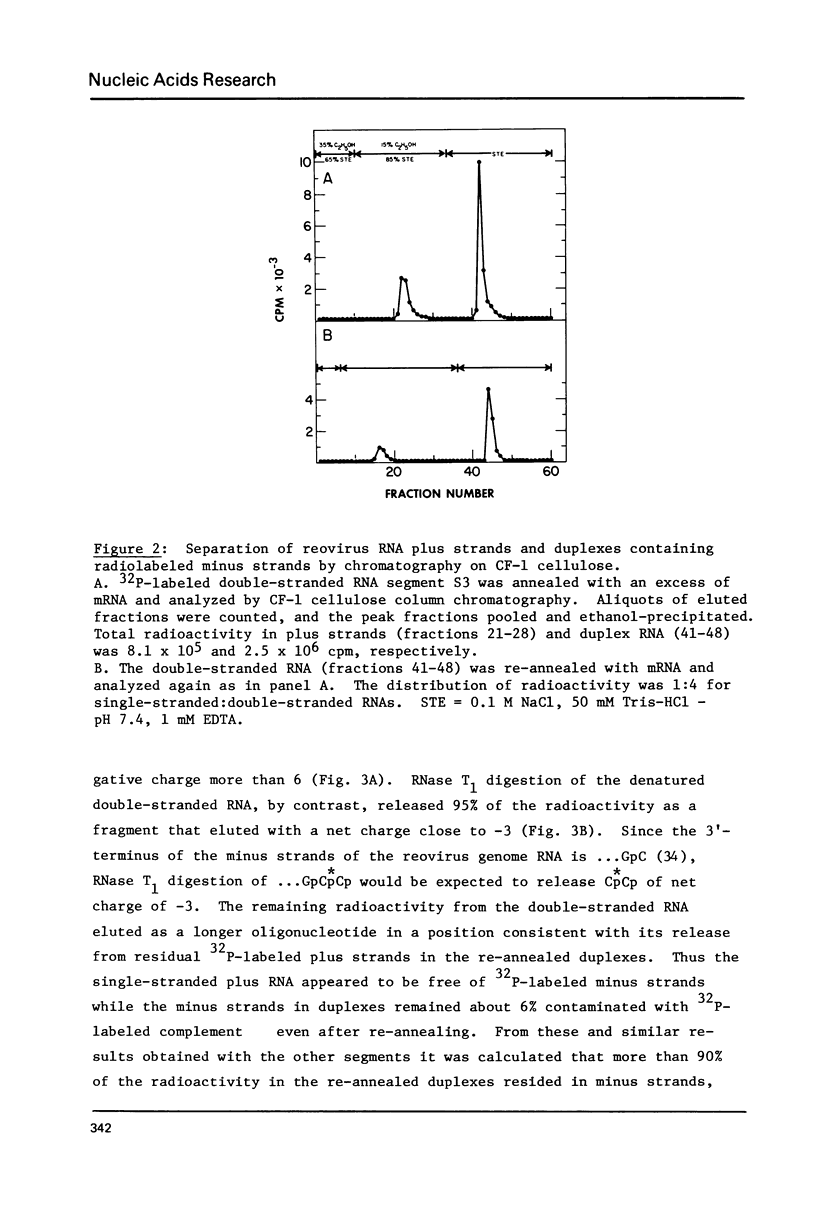
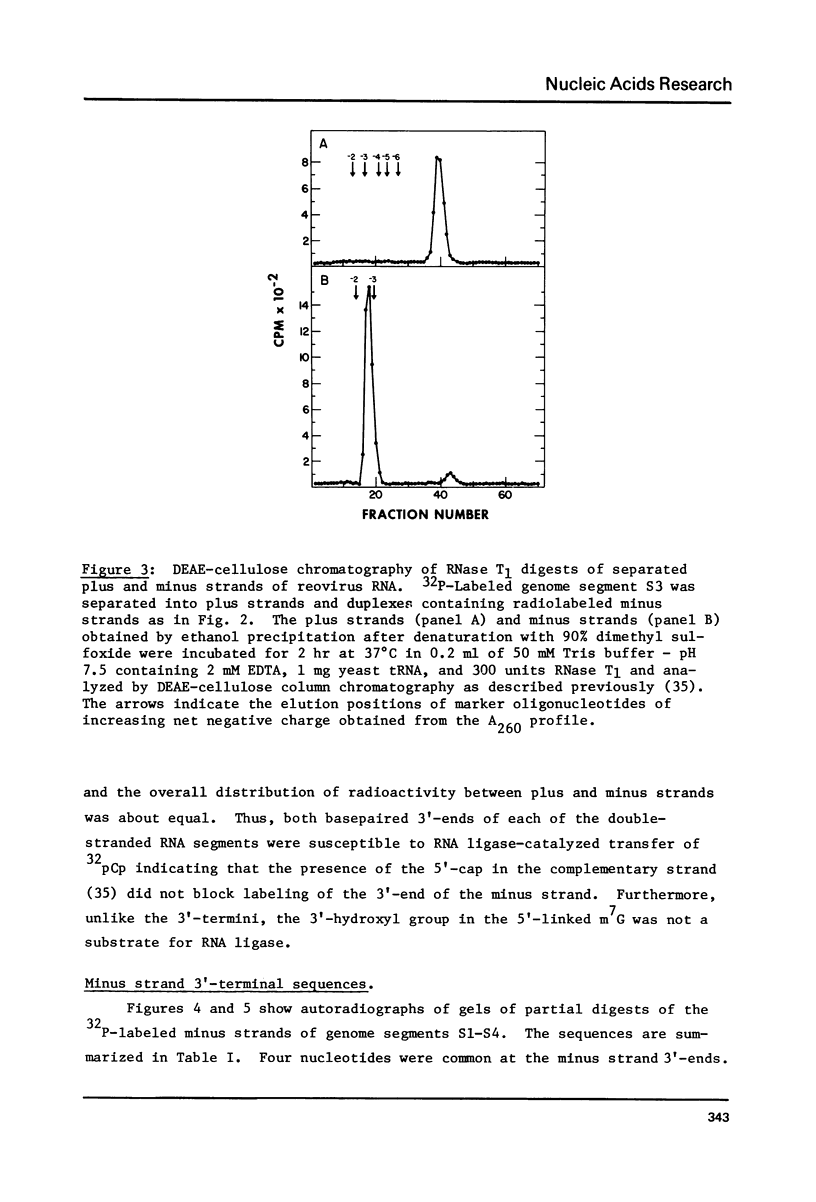
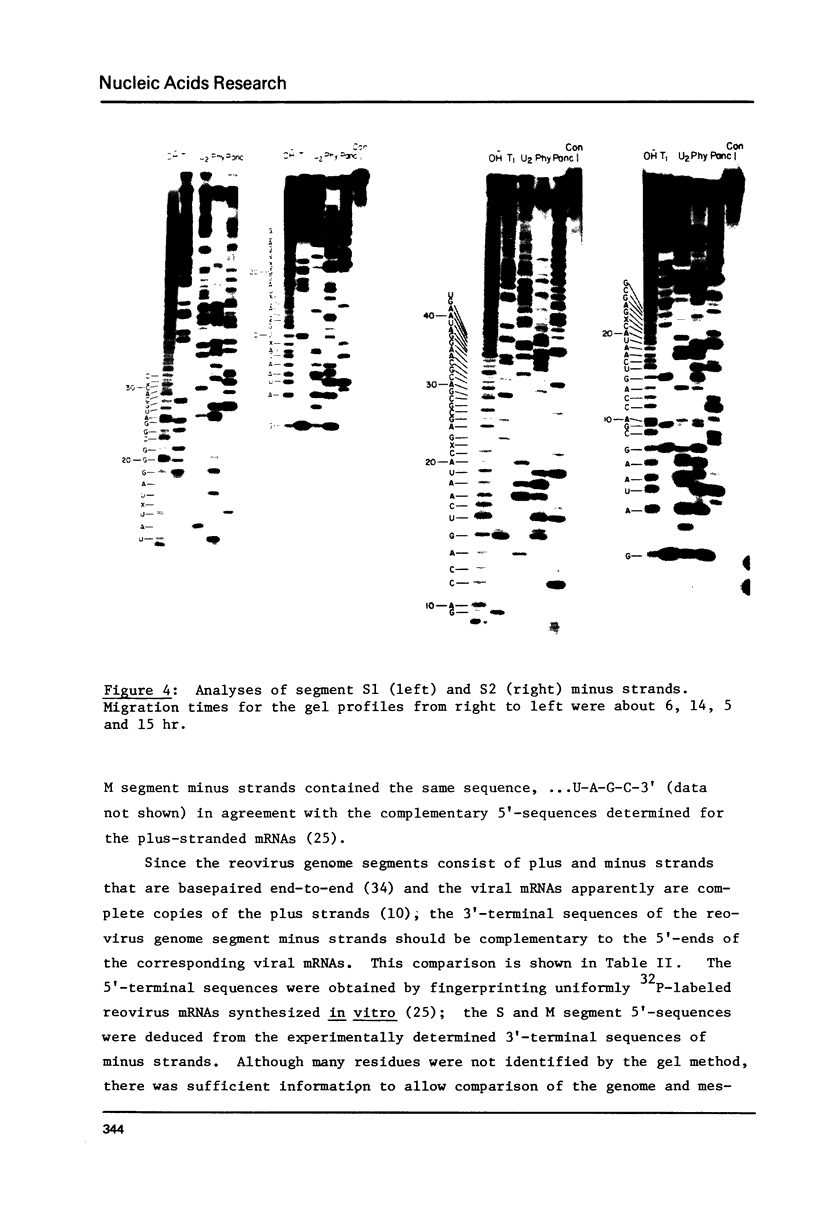
All ten reovirus genome RNA segments were radiolabeled at their 3'-termini by incubation with RNA ligase and 32pCp. The extent of radiolabeling was similar for each of the double-stranded RNAs in the genome segment mixture. Radioactivity was equally distributed between the separated plus and minus strands indicating that the 5'-cap in plus strands did not block 3'-end-labeling of minus strands. The 3'-termini of the four S and three M segments included the common sequences: ...U-A-G-C in minus strands and ...U-C-A-U-C in plus strands. By comparing the minus strand 3'-sequences with 5'-sequences of reovirus mRNAs, small-size genome segments S2, S3 and S4 were correlated with the previously sequenced initiation fragments s46, s45 and s54 derived from small class mRNAs. Medium-size genome segments M1, M2 and M3 similarly were correlated with fragments m30, m52 and m44, respectively. The N-terminal amino acid sequences deduced from the mRNA nucleotide sequences can now be assigned to the nascent chains of particular reovirus proteins.
Full text
PDF




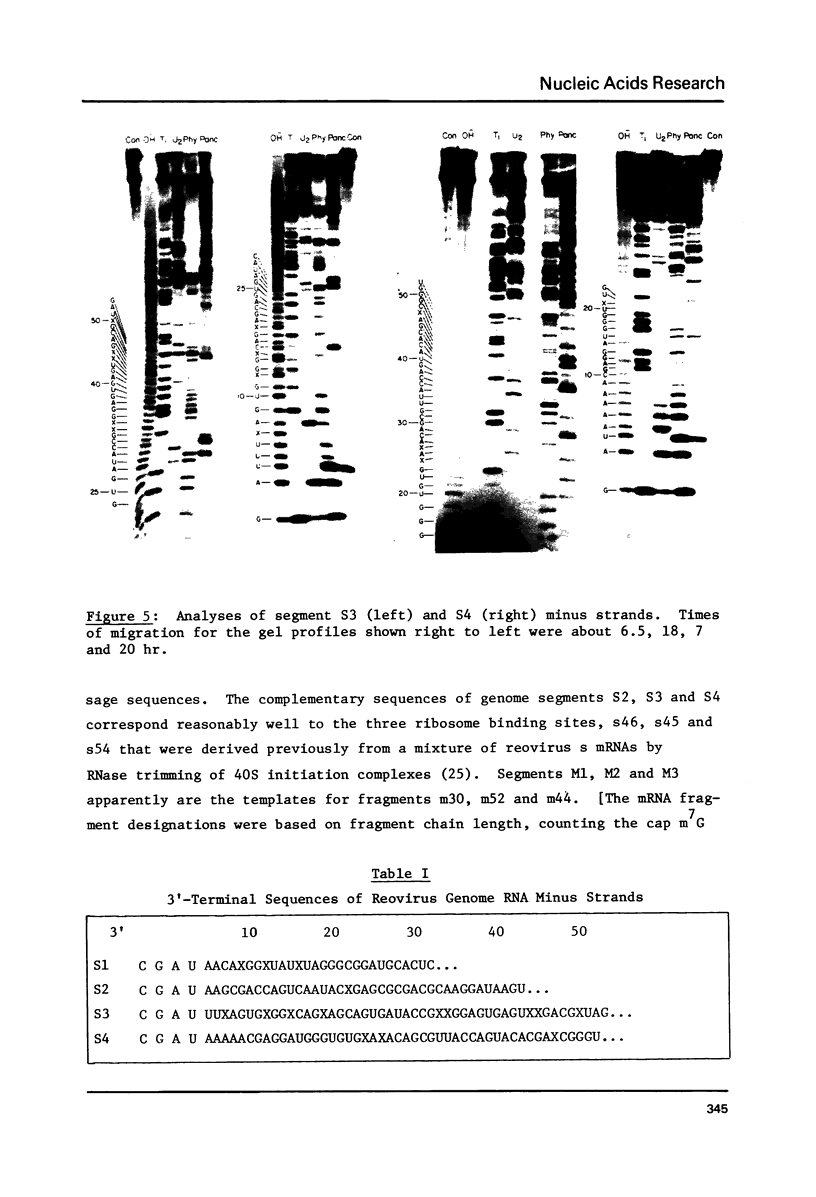





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Shatkin A. J. 5'-Terminal m-7G(5')ppp(5')G-m-p in vivo: identification in reovirus genome RNA. Proc Natl Acad Sci U S A. 1975 Feb;72(2):742–745. doi: 10.1073/pnas.72.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Tomasz J., Shatkin A. J. Mechanism of formation of reovirus mRNA 5'-terminal blocked and methylated sequence, m7GpppGmpC. J Biol Chem. 1976 Aug 25;251(16):5043–5053. [PubMed] [Google Scholar]
- Gomatos P. J., Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. doi: 10.1073/pnas.49.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings K. E., Millward S. Nucleotide sequences at the 5' termini of reovirus mRNA's. J Virol. 1978 Nov;28(2):490–498. doi: 10.1128/jvi.28.2.490-498.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Joklik W. K. Demonstration that the same strand of reovirus genome RNA is transcribed in vitro and in vivo. Virology. 1971 May;44(2):450–453. doi: 10.1016/0042-6822(71)90276-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Nucleotide sequences of 5'-terminal ribosome-protected initiation regions from two reovirus messages. Nature. 1977 Sep 29;269(5627):391–394. doi: 10.1038/269390a0. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Characterization of ribosome-protected fragments from reovirus messenger RNA. J Biol Chem. 1976 Jul 25;251(14):4259–4266. [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Characterization of translational initiation regions from eukaryotic messenger RNAs. Methods Enzymol. 1979;60:360–375. doi: 10.1016/s0076-6879(79)60034-4. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Identification of features in 5' terminal fragments from reovirus mRNA which are important for ribosome binding. Cell. 1978 Jan;13(1):201–212. doi: 10.1016/0092-8674(78)90150-2. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Sequences and properties of two ribosome binding sites from the small size class of reovirus messenger RNA. J Biol Chem. 1977 Oct 10;252(19):6895–6908. [PubMed] [Google Scholar]
- Lhoas P. Mating pairs of Saccharomyces cerevisiae infected with double stranded RNA viruses from Aspergillus niger. Nat New Biol. 1972 Mar 22;236(64):86–87. doi: 10.1038/newbio236086a0. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Miura K., Watanabe K., Sugiura M., Shatkin A. J. The 5'-terminal nucleotide sequences of the double-stranded RNA of human reovirus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3979–3983. doi: 10.1073/pnas.71.10.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. Genetics of reovirus: identification of the ds RNA segments encoding the polypeptides of the mu and sigma size classes. Virology. 1978 Sep;89(2):594–604. doi: 10.1016/0042-6822(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Shatkin A. J. Reovirus genome RNA segments: resistance to S-1 nuclease. Virology. 1975 Mar;64(1):96–105. doi: 10.1016/0042-6822(75)90082-3. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Watanabe Y., Graham A. F. Defective virions of reovirus. J Virol. 1970 Aug;6(2):226–236. doi: 10.1128/jvi.6.2.226-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pett D. M., Vanaman T. C., Joklik W. K. Studies on the amino and carboxyl terminal amino acid sequences of reovirus capsid polypeptides. Virology. 1973 Mar;52(1):174–186. doi: 10.1016/0042-6822(73)90407-8. [DOI] [PubMed] [Google Scholar]
- Pilly D., Niemeyer A., Schmidt M., Bargetzi J. P. Enzymes for RNA sequence analysis. Preparation and specificity of exoplasmodial ribonucleases I and II from Physarum polycephalum. J Biol Chem. 1978 Jan 25;253(2):437–445. [PubMed] [Google Scholar]
- Robertson J. S. 5' and 3' terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979 Aug 24;6(12):3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Both G. W. Reovirus mRNA: transcription and translation. Cell. 1976 Mar;7(3):305–313. doi: 10.1016/0092-8674(76)90159-8. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., LaFiandra A. J. Transcription by infectious subviral particles of reovirus. J Virol. 1972 Oct;10(4):698–706. doi: 10.1128/jvi.10.4.698-706.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Shatkin A. J., Banerjee A. K. Absence of polyadenylic acid from reovirus messenger ribonucleic acid. J Biol Chem. 1973 Dec 10;248(23):7993–7998. [PubMed] [Google Scholar]
- Weiner H. L., Ramig R. F., Mustoe T. A., Fields B. N. Identification of the gene coding for the hemagglutinin of reovirus. Virology. 1978 May 15;86(2):581–584. doi: 10.1016/0042-6822(78)90099-5. [DOI] [PubMed] [Google Scholar]