Abstract
Primary cytomegalovirus (CMV) infection promotes oxidative stress and reduces nitric oxide (NO) bioavailability in endothelial cells. These events are among the earliest vascular responses to cardiovascular risk factors. We assessed the roles of NAD(P)H oxidase and NO bioavailability in microvascular responses to persistent CMV infection alone or with hypercholesterolemia. Wildtype (WT) or gp91phox (NAD(P)H oxidase subunit) knockout mice received mock-inoculum or 3×104PFU murine CMV (mCMV) IP 5wks before placement on normal or high cholesterol diet (−HC) for 4wks before assessment of arteriolar function and venular blood cell recruitment using intravital microscopy. Some WT groups received sepiapterin (a precursor of the nitric oxide synthase co-factor, tetrahydrobiopterin), or apocynin (NAD(P)H oxidase inhibitor/antioxidant). Endothelium-dependent vasodilation was impaired in mCMV vs. mock WT, regardless of diet. This was not affected by sepiapterin, and pharmacological inhibition of nitric oxide synthase reduced dilation similarly in Mock and mCMV mice. Apocynin or deficiency of total, but not only blood cell or vascular wall (tested using bone marrow chimeras), gp91phox protected against arteriolar dysfunction. Blood cell recruitment was induced by mCMV-HC. Sepiapterin, but not NAD(P)H oxidase deficiency/apocynin, reduced leukocyte accumulation, whereas platelet adhesion was reduced by sepiapterin, apocynin or total, platelet-specific or vascular wall gp91phox-deficiency. These data implicate activation of both hematopoietic and vessel wall NAD(P)H oxidase in mCMV-induced arteriolar dysfunction, and platelet and vascular NAD(P)H oxidase in the thrombogenic phenotype induced by mCMV-HC. In contrast, findings with sepiapterin suggest eNOS dysfunction, perhaps uncoupling, mediates venular, but not arteriolar, responses to mCMV-HC, thus indicating that NAD(P)H oxidase and eNOS differentially regulate microvascular responses to mCMV.
Keywords: Cytomegalovirus infection, cardiovascular disease, NAD(P)H oxidase, microvasculature, arteriolar vasodilation, platelet adhesion, eNOS
Introduction
Cytomegalovirus (CMV) is a β-herpesvirus that is contracted by >60% of the population, and normally establishes a mild lifelong persistent infection in immunocompetent individuals [1, 2]. Epidemiological studies have implicated CMV in the pathogenesis of cardiovascular diseases (CVD), such as atherosclerosis, myocardial infarction, carotid and coronary artery disease [3–6]. Data from animal studies support an active, rather than bystander, role for CMV in the development of CVD, most likely through the initiation and/or propagation of inflammation [7–10]. In fact, the induction of inflammatory pathways is integral to the survival strategy of the virus, in that infected blood monocytes act as vehicles for the virus and are recruited to tissues in order to disseminate the virus [11, 12]. Thus primary CMV infection can elicit inflammatory and thrombogenic responses in isolated microvascular endothelial cells [13–19]. We have shown that persistent CMV infection leads to impaired endothelium-dependent vasodilation responses in arterioles and mild inflammation in venules in vivo, which may help explain its contribution to CVD development [20]. Furthermore, CMV and hypercholesterolemia synergize to induce a exaggerated inflammatory and thrombogenic responses in venules [20, 21].
Mounting evidence indicates that one of the first changes seen in the vasculature in response to CVD risk factors is endothelial dysfunction characterized by reduced nitric oxide (NO) bioavailability and enhanced reactive oxygen species (ROS) generation [22]. NAD(P)H oxidase is an important source of superoxide (O2·−) that contributes to CVD [23–25]. It is present in many different cell types, including neutrophils, platelets, vascular smooth muscle cells and endothelial cells. NAD(P)H oxidase mediates the microvascular responses to hypercholesterolemia, with both vessel wall- and blood cell-associated NAD(P)H oxidase contributing to leukocyte recruitment in postcapillary venules [26, 27]. Hypercholesterolemia also causes tetrahydrobiopterin (BH4) depletion and increased O2·− release from eNOS [28, 29], although such changes have not been evaluated to date in response to CMV infection.
Primary CMV infection of different cell types invokes disparate responses in terms of ROS generation. Decreased O2·− has been reported in CMV-infected macrophages [30] and neutrophils [31], whereas this virus has been show to elevate ROS generation in monocytes [32] and vascular smooth muscle cells [33]. Furthermore, responses differ between endothelial cells, in that human umbilical vein endothelial cells do not have altered ROS release upon CMV infection [34], but microvascular endothelial cells show enhanced intracellular oxidative stress under basal conditions, and exacerbated DMNQ-induced O2·− generation [35]. A majority of this stimulated O2·− release was reversed by polyethylene glycol-conjugated superoxide dismutase (SOD). While evidence is limited on the exact ROS-generating enzymes affected by CMV, it was discovered that NAD(P)H oxidase activity is elevated in vascular smooth muscle cells during primary CMV infection [36]. Because of its expression in many cell types relevant to microvascular dysfunction, it is plausible that NAD(P)H oxidase in some blood cell populations and in endothelial cells and smooth muscle cells of microvessels may also be a target of CMV during persistent infection.
Here we examined the effects of CMV on arteriolar dilation and, in the presence of hypercholesterolemia, the inflammatory and thrombogenic characteristics of postcapillary venules in wild type and gp91phox-deficient mice to interrogate the contribution of vascular and blood cell-NAD(P)H oxidase in these processes. We also addressed the potential effects of CMV-induced eNOS uncoupling via BH4 depletion on microvascular function.
Methods
Animals
Wild-type C57BL/6J mice (WT), B6.129S6-Cybbtm1Din/J (gp91phox−/−) on a C57BL/6J background, and CD45 congenic B6.SJL-PTPRCPEP/BOY mice (which express CD45.1) were obtained from Jackson Laboratories, Bar Harbor, ME. Because exposure of humans to virus primarily occurs during childhood, and the fact that the microvasculature exhibits a dysfunctional phenotype upon exposure to CVD risk factors, and long before large vessel disease is seen, we used young mice in our study. Mice (3–5 wk old) were injected with mock inoculum or 3×104 plaque forming units (PFU) of the Smith strain of mCMV as previously described [20]. Based on our previous findings [20], intravital microscopy was performed at 9 wks post-infection (p.i.). Mice were further divided into those maintained on normal diet (ND) or mice placed on high cholesterol diet (HC) (Teklad 90221 containing 1.25% cholesterol, 15.8% fat and 0.125% choline chloride, Harlan Teklad, Madison, WI) at 5 wks post-infection until observation 4 wks later (2 wks of this diet induces arteriolar dysfunction, and venular blood cell recruitment, that resolves by 4 wks of feeding before developing again later [20, 26]). Separate groups of mCMV-ND and mCMV-HC mice were treated with 3 mM apocynin (EMD Chemicals Inc., Gibbstown, NJ) in their drinking water for 1 wk prior to the experiment, based on work by, and advice from, Professor Joseph L. Unthank, Indiana University School of Medicine [37]. Other mCMV-ND and mCMV-HC groups were injected with sepiapterin (Cayman Chemical, Ann Arbor, MI) for 3 days prior to observation (2.7 mg/kg on day -3 followed by 10 mg/kg body weight on days -2 & -1). This was based on a paper in a chronic murine model in which the observed leukocyte and platelet adhesion in postcapillary venules were abrogated by this treatment [38]. A small number of saline-injected mice were performed as controls for the sepiapterin groups. n=4–10 for all groups.
Bone Marrow Chimeras
Three combinations of chimeras were generated as previously described [27]. WT→WT chimeras were CD45 congenic WT mice that received bone marrow cells from WT animals, reserving NAD(P)H oxidase function (a small number of the reverse transplants were performed and exhibited comparable responses to mCMV and HC, therefore, for the sake of presentation, these were combined as the WT→WT group). The gp91phox−/−→WT chimeras, with NAD(P)H oxidase deficient blood cells, but normal vascular wall enzyme activity, were produced by transplanting bone marrow from a gp91phox−/− mouse into a CD45 congenic mouse. The WT→gp91phox−/− chimeras were generated by transplanting bone marrow cells from CD45 congenic mice into gp91phox−/− recipients, such that these mice expressed gp91phox in their circulating cells, but not in cells of the vessel wall. Flow cytometry was used to verify proper chimera reconstitution, as previously described [39]. Conversion was considered adequate when >90% of blood cells were of donor origin (i.e. CD45.1 for congenic bone marrow and CD45.2 for WT and gp91phox−/− donors). Once confirmed, chimeric mice were exposed to the infection and diet protocol described above.
Surgical Protocol
At the time of experimentation, mice were anesthetized with ketamine hydrochloride (150 mg/kg body weight, i.p.) and xylazine (7.5 mg/kg body weight, i.p.). The right jugular vein was canulated for administration of platelets to animals undergoing venule observation. Core body temperature was maintained at 35±0.5°C. Animal handling procedures were approved by the LSU Health Sciences Center Institutional Animal Care and Use Committee and were in accordance with the guidelines of the American Physiological Society.
Intravital Microscopy
The cremaster muscle was prepared for intravital microscopy as described previously [40], and postcapillary venules (20–40 µm diameter) with a wall shear rate (WSR) of ≥500/s were assessed. The venule with the least number of adherent and emigrated leukocytes at the end of 30 min stabilization was chosen for the study. 108 platelets (in a volume of 120 µl) (isolated from a donor mouse by a series of centrifugation steps as previously described [41]) were infused via the jugular vein over 5 min and allowed to circulate for a further 5 min. Platelet donors matched the source of the recipients in terms of infection and diet unless specified otherwise. One minute recordings of the leukocytes (light microscopy) followed by 1 min recordings of the platelets (fluorescent microscopy) were made of the first 100 µm of every 300 µm along the length of the unstimulated vessel, beginning as near to the source of the venule as possible. A leukocyte was considered adherent if it remained stationary for ≥30 s (#/mm2 vessel wall surface) and was measured throughout the observation period. Leukocyte emigration was measured online at the end of each 1 min observation period. Emigrated leukocytes were expressed as the number of interstitial leukocytes per mm2 of view adjacent to the segment under observation (#/mm2). Platelets were considered saltating if they paused transiently (for ≥2 s but <30s) on the vessel wall, and firmly adherent if they arrested for ≥30 s. Total adhesion was calculated as the sum of saltation and firm adhesion, and all platelet parameters were expressed as # platelets/mm2 vessel wall surface. The mean value of each variable within a single venule was calculated and comparisons were made between the experimental groups.
Once the venular data had been collected, the animals were allowed to stabilize for 20–30 min, and arterioles with diameters between 15–40 µm and WSR of ≥500/s were chosen for study. Diameter and Vrbc were measured in the chosen sections before and after superfusion with 10−5 M of the endothelium-dependent vasodilator, acetylcholine (ACh), for 5 min. Arteriolar vasorelaxation responses to ACh were expressed as the percentage diameter change versus baseline. Arteriolar diameters were allowed to return to baseline with bicarbonate-buffered saline superfusion, before papaverine was applied to test for maximal endothelium-independent vasodilation. Arterioles that were not responsive to papaverine were excluded from the study. In separate groups (n=3–5/group) of WT-Mock-ND, WT-mCMV-ND and WT-mCMV-HC mice, we performed experiments on arterioles only, in which responses to ACh were measured, vessels were allowed to return to baseline diameter, and then the preparation was superfused with the NOS inhibitor, NG-nitro-L-arginine (L-NNA; 10−3 M) for 30 min before L-NNA+ACh was superfused for 5 min. Diameters were measured before the vessel was once again allowed to return to baseline before final stimulation with papaverine.
Plasma Cholesterol Levels
Plasma was frozen for subsequent measurement of cholesterol levels using a spectrophotometric assay (Cholesterol LiquiColor® Test from Stanbio Laboratory, Boerne, Tx).
Blood Cell Counts
Blood was drawn from the heart at the end of the experiment for leukocyte (using crystal violet stain) and platelet counts (using the unopette system) with the aid of a hemocytometer.
BH4 Levels
These were quantified by HPLC with electrochemical detection as previously described [42], and concentrations were normalized to the respective protein content of tissue samples. Briefly, the cremaster muscles were harvested at the end of the experiment, and snap-frozen. Tissue was homogenized in 50 mM phosphate buffer pH 2.6 containing 0.2 mM diethylenetriaminepentaacetic acid and freshly added 1 mM dithiothreitol. Samples were spun down at 12,500 rpm for 10 min at 4°C, and supernatants were analyzed on an HPLC system (ESA Biosciences CoulArray® system Model 582 and 542) using a Synergi Polar-RP column (Phenomenex, 4 µm, 250×4.6 mm) eluted with argon saturated 50 mM phosphate buffer pH 2.6.
Statistical Analysis
All values are reported as mean±SEM. ANOVA with Bonferroni/Dunn post-hoc test was used for statistical comparison of experimental groups.
Results
mCMV Infection leads to Impaired Endothelium-Dependent Vasodilation
ACh-induced dilation was not altered at 4 wks of HC in Mock-inoculated mice, when compared to normocholesterolemic counterparts. In contrast, endothelium-dependent dilation was significantly impaired in mCMV-ND mice versus mock-ND controls (Figure 1A). The addition of HC to mCMV-infected mice did not worsen this. As we have found previously, papaverine-induced (endothelium-independent) dilation was similar in all groups (data not shown).
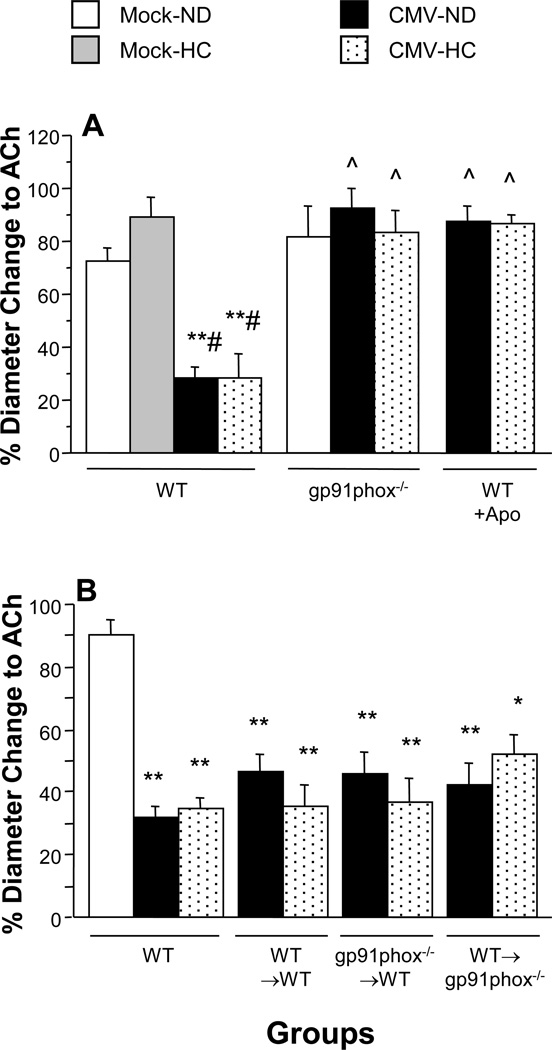
Figure 1.
Arteriolar vasodilation responses to ACh in untreated and apocynin (Apo)-treated WT mice, and gp91phox-deficient (gp91phox−/−) mice (Panel A) and in chimeric mice (donor→recipient) (Panel B) at 9 wks following mock-inoculation or infection with mCMV. Mice were placed on either a normal diet (ND) or a cholesterol-enriched diet (HC) for the final 4 weeks before observation. * P<0.0005 vs. Mock-ND; ** P<0.0001 vs. WT Mock-ND; # P<0.0001 vs. WT Mock-HC; ^ P<0.0001 vs. corresponding WT.
A role for NAD(P)H oxidase in mCMV-Induced Arteriolar Dysfunction
In order to elucidate the role of NAD(P)H oxidase in mCMV-induced arteriolar dysfunction, we used mice deficient in the gp91phox subunit of this enzyme. Normal vasodilation responses to ACh in Mock-ND mice were not altered by the mutation (Figure 1A). Mice deficient in gp91phox exhibited complete protection against mCMV-induced arteriolar dysfunction. Similar protection was also noted in arterioles of gp91phox−/− mCMV-HC mice. Apocynin also restored normal vasodilation responses in both mCMV-ND and mCMV-HC groups.
In order to determine whether the NAD(P)H oxidase activation that led to arteriolar dysfunction in the mCMV-infected mice occurred primarily in the vessel wall or the circulating blood cells, we generated bone marrow chimeras that had gp91phox-deficient blood cells but intact vessel wall NAD(P)H oxidase, or vice versa. As would be expected the control WT→WT mCMV groups exhibited arteriolar dilation responses to ACh that were comparable to WT-mCMV groups, regardless of dietary regimen (Figure 1B). This impairment of endothelium-dependent vasodilation was not altered by the absence of gp91phox−/− in circulating cells in the gp91phox−/− →WT chimeric groups infected with mCMV. Similarly, deficiency of vessel wall gp91phox in the WT→gp91phox−/− chimeras did not confer any protection against mCMV-induced arteriolar impairment.
No Role for NAD(P)H oxidase in mCMV-HC-Induced Leukocyte Recruitment in Postcapillary Venules
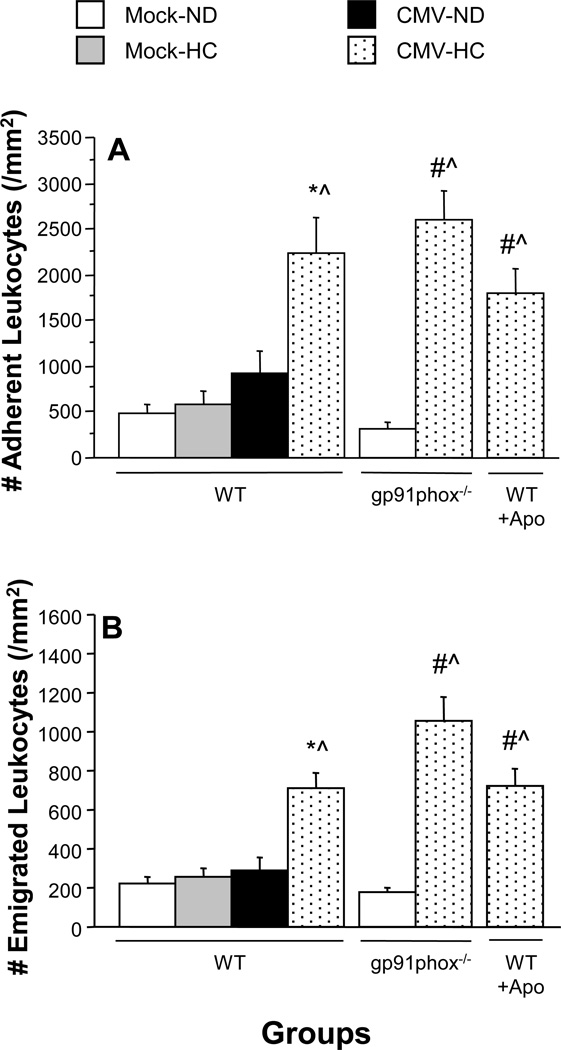
Leukocyte adhesion (Figure 2A) or emigration (Figure 2B) were not altered by either HC or mCMV alone. In contrast, exposure to both risk factors led to an approximately 4.5-fold increase in leukocyte adhesion (Figure 2A) and more than 3-fold enhancement of leukocyte emigration (Figure 2B). In gp91phox−/− mice, basal (mock-ND) levels of leukocyte recruitment were comparable to WT mice. The lack of gp91phox failed to protect against mCMV-HC-induced inflammation (Figure 2). Administration of apocynin for 1 week prior to observation, which was effective at reversing arteriolar dysfunction, also failed to confer protection against leukocyte recruitment in postcapillary venules of mCMV-HC mice (Figure 2).
Figure 2.
Leukocyte adhesion in postcapillary venules (Panel A) and emigration into the interstitum (Panel B) in untreated and apocynin (Apo)-treated WT mice and in gp91phox-deficient mice (gp91phox−/−) at 9 wks following mock-inoculation or infection with mCMV. Mice were fed either normal diet (ND) or high cholesterol diet (HC) for the final 4 weeks before measurement. * P<0.001 vs. all other mock groups; # P≤0.0005 vs. WT Mock-ND; ^ P<0.0001 vs. gp91phox−/− Mock-ND.
NAD(P)H oxidase Contributes to Platelet Adhesion in Postcapillary Venules of mCMV-HC Mice
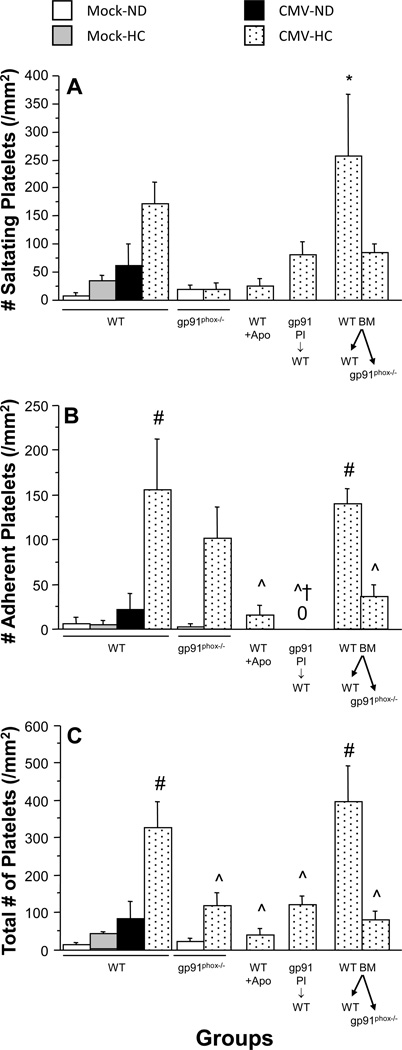
Similar to the leukocytes, the combination of mCMV infection and a cholesterol-enriched diet, but neither alone, led to elevated adhesion of matched exogenous platelets, due to both transient (saltation) and firm adhesion (Figure 3). In gp91phox−/− mice, platelet saltation (Figure 3A) was reduced to Mock-ND levels, and firm adhesion (Figure 3B) was moderately decreased. This translated into a significant attenuation of total platelet recruitment, when compared to WT counterparts (Figure 3C). Apocynin offered slightly better protection against the mCMV-HC-induced thrombogenic phenotype. When gp91phox−/− platelets were observed in WT recipients, both exposed to mCMV-HC, firm platelet adhesion was completely abrogated, although saltation was only partially reduced (Figure 3). Total adhesion of platelets derived from gp91phox−/− mice in WT recipients was comparable to that observed in gp91phox−/− recipients. When the role of vascular wall gp91phox was tested by assessing platelet adhesion in WT→gp91phox−/− chimeras using matched donors, it was shown that deficiency of vessel wall gp91phox was similar to lack of platelet gp91phox in reducing platelet recruitment (Figure 3). This was not a manifestation of the chimeric process as WT→WT mCMV-HC mice exhibited platelet recruitment responses that were comparable to WT counterparts.
Figure 3.
Recruitment of exogenous platelets in postcapillary venules of WT mice receiving normal or apocynin water (Apo), gp91phox-deficient (gp91phox−/−) mice and bone marrow chimeras (WT BM→recipient) at 9 wks post-injection with mock-inoculum or mCMV. Mice were placed on either a normal diet (ND) or a high cholesterol diet (HC) for the final 4 weeks before observation. The platelets were obtained from matched donors, except where indicated by gp91 Pl→WT, in which gp91phox−/− platelets were observed in WT recipients (both mCMV-HC). Platelet interactions were divided into the following categories: A) saltating (stationary for ≥2 s, but <30 s); B) firmly adherent (stationary for ≥30 s); C) total adhesion (derived from the sum of saltation and firm adhesion). “0” denotes no firm adhesion observed. * P≤0.001 vs. all groups except corresponding WT; # P≤0.0001 vs. Mock-ND, Mock-HC, and mCMV-ND WTs; ^ P<0.001 vs. WT mCMV-HC and WT BM→WT mCMV-HC; † P<0.001 vs. gp91phox−/− mCMV-HC.
Sepiapterin Failed to Protect Against mCMV-Induced Arteriolar Dysfunction
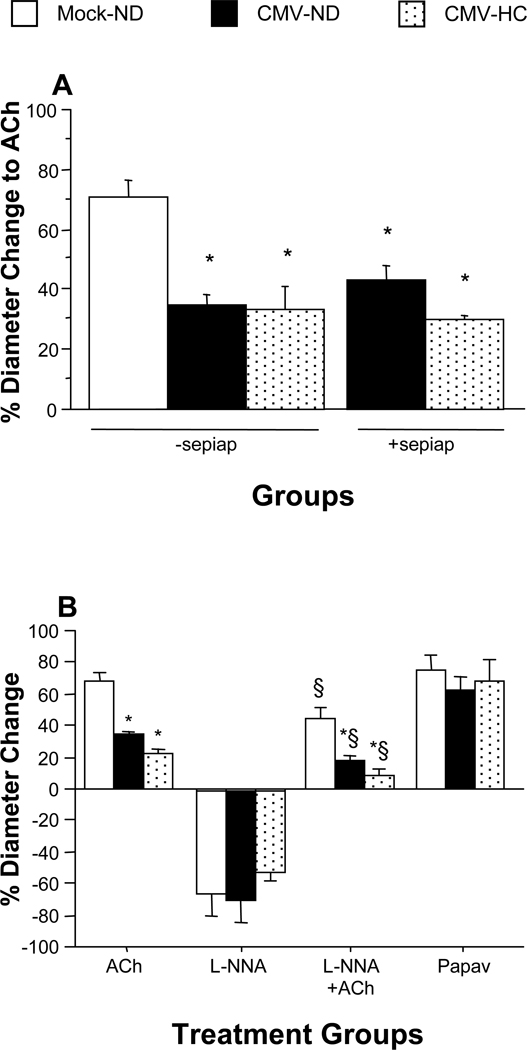
To test the possibility that eNOS dysfunction mediates the microvascular dysfunction induced by mCMV, we treated mice with sepiapterin, the BH4 precursor. For arteriolar vasodilation, mCMV-infected mice receiving sepiapterin responded to ACh at levels similar to saline-treated or untreated mCMV-ND mice (these control groups were comparable, and were combined for presentation) (Figure 4A). This suggests that eNOS does not become dysfunctional/uncoupled in arterioles in response to mCMV. A similar lack of protection was seen in sepiapterin-treated mCMV-HC mice. In a subset of WT mice (n=3–5/group) we performed experiments to determine if mCMV altered NO bioavailability as measured by arteriolar reactivity in the presence of a NOS inhibitor, L-NNA (Figure 4B). When arterioles of Mock-ND mice were exposed to L-NNA alone, a 67% reduction in resting diameter was observed. Basal diameter was similarly decreased following L-NNA treatment in mCMV-ND mice. Vasodilation responses to ACh were partly reduced by L-NNA in Mock-ND mice, indicating that NO contributed to ~33% of the ACh response. The absolute reduction of ACh-induced vasodilation in the mCMV-ND group by L-NNA was comparable (Figure 4B). Additional exposure of mCMV-infected mice to HC did not significantly alter any of these responses when compared to mCMV alone.
Figure 4.
Arteriolar vasodilation responses to ACh in WT mice at 9 wks following mock inoculation or infection with mCMV (Panel A). Mice were placed on either a normal diet (ND) or a cholesterol-enriched diet (HC) for the final 4 weeks before observation. A group of mCMV-HC mice received sepiapterin (sepiap) for 3 days prior to observation. * P<0.0005 vs. Mock-ND. Panel B shows arteriolar responses to ACh, L-NNA, ACh+L-NNA, and papaverine in WT mice exposed to mock or mCMV, and ND or HC. * P<0.05 vs. WT Mock-ND; # P<0.005 vs. WT Mock-HC; ^ P<0.0005 vs. WT CMV-ND; ^^ P<0.0001 vs. WT CMV-ND; † P<0.01 vs. WT CMV-HC; § P<0.05 vs. corresponding ACh treatment.
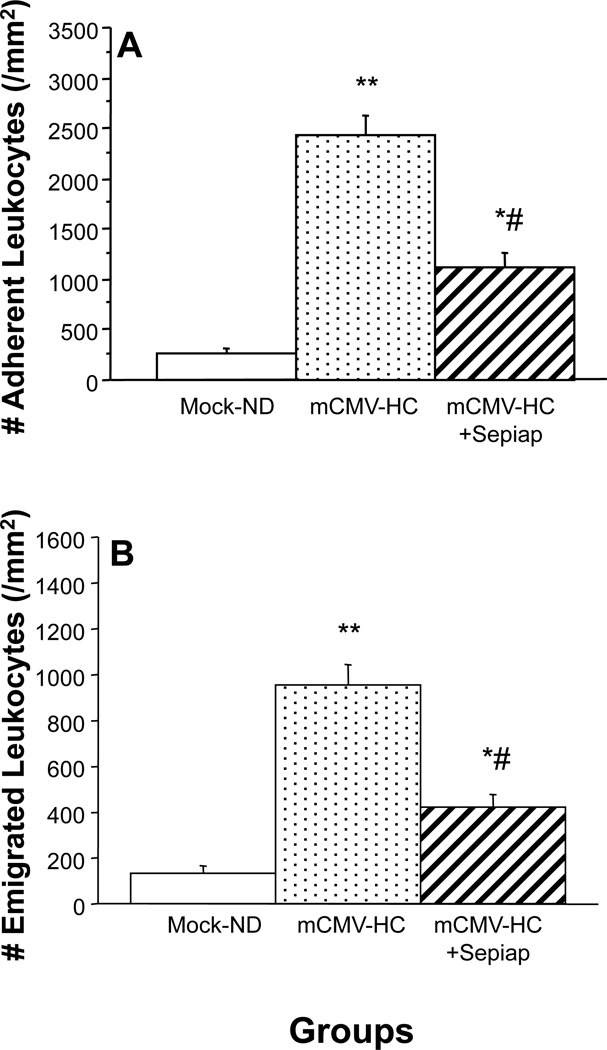
Sepiapterin Provides Protection against Venular Blood Cell Recruitment
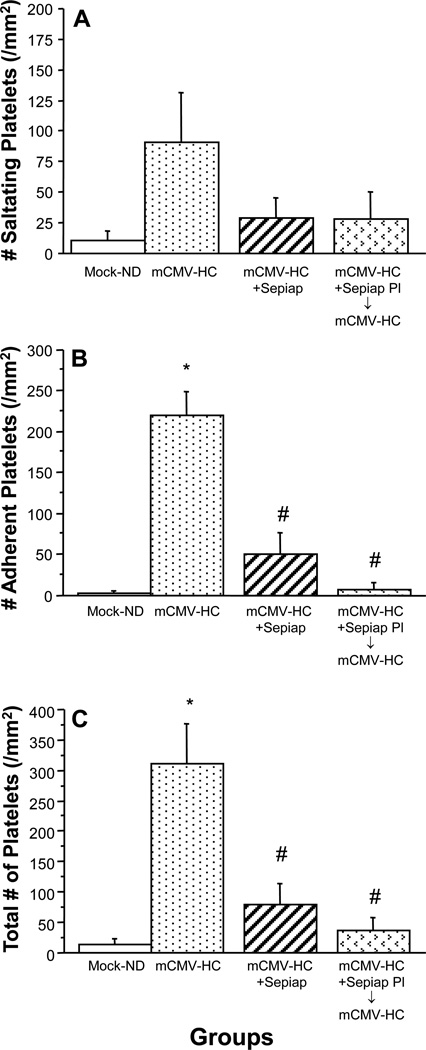
As before, leukocyte recruitment was elevated in mCMV-HC mice when compared with Mock-ND controls (Figure 5). In mCMV-HC mice that received the BH4 precursor, sepiapterin, both leukocyte adhesion (Figure 5A) and emigration (Figure 5B) were significantly reduced, although they remained above baseline. The increased platelet adhesion in postcapillary venules that was noted in mCMV-HC mice (Figure 6) was also significantly attenuated by treatment of the mice with sepiapterin (Figure 6C), with the sepiapterin being similarly effective against saltation (Figure 6A) and firm adhesion (Figure 6B). When platelets from sepiapterin-treated mCMV-HC donors were observed in untreated mCMV-HC recipients, platelet accumulation was attenuated to levels comparable to that seen when both donor and recipient were treated with sepiapterin.
Figure 5.
Leukocyte adhesion in postcapillary venules (Panel A) and emigration (Panel B) into the tissue of WT mice, some of which received sepiapterin (sepiap). Mice were observed at 9 wks post-injection with mock-inoculum or mCMV. HC was given for 4 wks prior to experimentation. * P<0.05 vs. WT Mock-ND; ** P<0.0001 vs. Mock-ND; # P<0.0001 vs. WT Mock-HC.
Figure 6.
Saltation, firm adhesion and total adhesion of exogenous platelets from matched donors in postcapillary venules of recipients at 9 wks post-injection with mock-inoculum or mCMV. Mice were maintained on ND or placed on HC 5 wks later. Some mCMV-HC mice also received sepiapterin for 3 days prior to observation. In the final group, platelets from sepiap-treated donors were monitored in untreated recipients (both mCMV-HC). * P<0.0001 vs. WT Mock-ND; # P<0.0005 vs. mCMV-HC.
BH4 Levels
BH4 levels in the cremaster were not significantly altered by mCMV or mCMV-HC (Table 1). Sepiapterin increased the BH4 levels in the mCMV-ND cremaster by 49-fold, while elevating BH4 in the mCMV-HC cremaster by 36-fold, when compared with corresponding untreated groups.
Table 1.
BH4 levels in cremaster muscles of mice 9 wks following injection with mock inoculum or mCMV. Mice were either maintained on normal diet (ND) or placed on a high cholesterol diet (HC) at 5 wks post-injection. Groups of mCMV mice were treated with sepiapterin for 3 days prior to harvesting (+sepiap).
| Group | Cremaster BH4 (pmol/mg protein) |
|---|---|
| Mock-ND | 6.6±2.26 |
| mCMV-ND | 12.4±1.05 |
| mCMV-HC | 5.3±4.71 |
| mCMV-ND+sepiap | 611.4±127.88* |
| mCMV-HC+sepiap | 190.3±45.12 |
P<0.0005 vs. other groups.
Plasma Cholesterol, Venular Wall Shear Rate and Circulating Blood Counts
Plasma cholesterol levels in all HC groups were approximately 2-fold higher than corresponding normocholesterolemic controls (Mock-ND: 70±3.7 mg/dL; Mock-HC: 142±12.4 mg/dL (P<0.05)). mCMV, genetic mutation or the chimeric process did not alter the cholesterol levels when compared to WT counterparts (data not shown). The venular wall shear rates in all groups in which blood cell adhesion was measured were comparable, and did not explain the differences in venular leukocyte and platelet recruitment between mCMV-HC and mock-ND groups (data not shown). Circulating blood counts of leukocytes and platelets were also similar between groups.
Discussion
Cytomegalovirus has long been recognized as a risk factor in transplantation, and more recently evidence is growing for its role in CVD [3–6]. One hypothesis for how this virus contributes to CVD is that it activates inflammatory pathways important for the pathogenesis of the disease. In fact, primary CMV infection of isolated endothelial cells leads to oxidative stress, adhesion molecule upregulation and leukocyte and platelet adhesion [13–19, 35]. Less is known about the impact of persistent CMV on the vasculature, which is important because although a majority of the population becomes infected with CMV in childhood, this is a lifelong infection and CVD develops over decades. We have previously shown that, like other cardiovascular risk factors, persistent CMV infection can induce arteriolar dysfunction, and can synergize with another risk factor, hypercholesterolemia, to promote an inflammatory and thrombogenic phenotype in postcapillary venules [20]. There is some evidence that primary CMV infection can induce oxidative stress, which is one of the first events leading to such microvascular responses to HC, diabetes and other risk factors [22, 26], and here we show that activation of NAD(P)H oxidase in blood cells and the vessel wall, and impaired function of eNOS have distinct roles in arteriolar dysfunction, and venular inflammation, but that both share a common pro-thrombogenic impact in venules exposed to both mCMV and HC.
There are several pathways through which CMV infection may lead to impaired endothelium-dependent dilation. One such pathway is through the O2·−-generating enzyme, NAD(P)H oxidase, which is present in many cell types and has been shown to mediate the arteriolar dysfunction that occurs after 2 wks of high cholesterol feeding [26]. To test this possibility, we used mice deficient in gp91phox (also known as NOX-2), which is a membrane subunit of NAD(P)H oxidase. Our findings that gp91phox-deficient mice were completely protected against the impaired arteriolar dilation in both mCMV and mCMV-HC mice supports a role for a gp91phox-containing NAD(P)H oxidase in these responses. Apocynin has been widely used as a pharmacological inhibitor of NAD(P)H oxidase, although this compound acts as an antioxidant in non-leukocytic cell types [43]. Nonetheless, the conclusion derived from our mutant mice experiments that NAD(P)H oxidase is responsible for the arteriolar dysfunction was further corroborated by our experiments using apocynin, in which comparable protection was noted.
gp91phox is present in the dominant NAD(P)H oxidase responsible for O2·− generation in leukocytes [44] and platelets [45, 46], and is present in vascular endothelium [47], and in vascular smooth muscle cells from resistance vessels but not large arteries [48, 49]. We sought to determine whether circulating cells or cells of the vessel wall may be the primary cellular source of NAD(P)H oxidase that contributes to arteriolar dysfunction in our model by generating bone marrow chimeras with circulating cells deficient in gp91phox but with intact vascular gp91phox, or vice versa. Our results indicate that both cells of the vessel wall and circulating cells are responsible for the NAD(P)H oxidase-mediated arteriolar dysfunction during mCMV infection. In contrast to the gp91phox−/− mice, deficiency of gp91phox in only one or other of the vessel wall or blood cells did not prevent the impaired dilation responses, suggesting that NAD(P)H oxidase activation in either compartment was sufficient to induce the dysfunctional phenotype. These findings also support the possibility that apocynin targeted both the blood cells and the vessel wall.
Primary infection of endothelial cells with CMV leads to leukocyte and platelet adhesion [12, 16, 19], and we have previously shown that persistent mCMV infection can induce mild leukocyte and platelet adhesion responses in postcapillary venules that are transient in nature, whereas it prolongs and exacerbates HC-induced blood cell recruitment [20]. We focused on the latter and confirmed that mCMV-HC, but not either risk factor alone, promotes significant leukocyte and platelet accumulation at 9 wks post-infection, 4 wks HC. Lack of gp91phox offered no protection against the inflammatory response. This was somewhat surprising in that both gp91phox [26] and p47phox [22] deficiency attenuate the HC-induced leukocyte adhesion at 2 wks of diet, and here we are assessing the prolongation of this response by mCMV [20]. Whether mCMV alters the underlying mechanisms of leukocyte recruitment even at 2 wks HC, or whether the gp91phox-mediated phase of inflammation has passed and it does not govern the later leukocyte recruitment at 4 wks diet in mCMV-infected mice remains unclear. However, the fact that similar findings were obtained with apocynin further emphasize that there are distinct pathways leading to arteriolar dysfunction and venular inflammation in response to mCMV-HC.
It is now well accepted that platelets not only mediate thrombus formation but also are important components of the inflammatory cascade in CVD and other diseases. Similar to the platelet recruitment in response to 2wk HC alone [26], we found that deficiency of gp91phox or treatment with apocynin also reduced the platelet adhesion in mCMV-HC mice. By observing gp91phox-deficient platelets in WT mice, we were able to show that platelet gp91phox played a major role in this thrombogenic response. This could have important implications in hypercholesterolemic patients in whom platelet NAD(P)H oxidase is activated and mediates the associated upregulation of CD40L [50], because persistent CMV infection may enhance or prolong these platelet responses. Furthermore, experiments with chimeras implicated a role for vascular wall NAD(P)H oxidase in the thrombogenic phenotype in mCMV-HC mice, which is also in agreement with previous findings for hypercholesterolemia [26]. CMV can increase O2·− generation in microvascular endothelial cells [35], and increases NAD(P)H oxidase activity in arterial vascular smooth muscle cells [36] during primary infection. Whether the vascular NAD(P)H oxidase is located in the endothelial and/or smooth muscle cells in our model remains to be elucidated. The fact that both platelet and vascular wall NAD(P)H oxidase contributed to platelet adhesion, but that leukocyte recruitment was not mediated by this enzyme, suggests that if platelets contribute to the inflammatory phenotype during mCMV infection, this must be via a pathway other than NAD(P)H oxidase.
The disassociation of leukocyte and platelet recruitment, despite vascular NAD(P)H oxidase activation, is somewhat surprising, because vascular NAD(P)H oxidase has been previously shown to mediate both in venules of hypercholesterolemic mice [27]. However, perhaps mCMV-HC leads to levels of vascular NAD(P)H oxidase-derived ROS that are sufficient to induce platelet, but not leukocyte, adhesion, or mCMV-HC activates other pathways that overcome any anti-inflammatory effect of gp91phox deficiency or apocynin treatment. There are conflicting reports about the induction of ROS by CMV in leukocytes [30–32], albeit these data were obtained during primary infection, and our data would suggest that if ROS are generated by leukocytes in response to mCMV-HC, NAD(P)H oxidase is not the primary source. The possibility remains that other oxidant-generating enzymes or dysregulation of antioxidant defenses in the vessel wall and/or circulating blood cells may mediate the venular inflammation in mCMV-HC mice. Under inflammatory conditions associated with oxidative stress, the levels of the essential co-factor for eNOS, BH4, can be decreased through both oxidation and reduced de novo generation, leading to uncoupling of eNOS. This results in O2·− generation from the oxygenase domain of the enzyme. Sepiapterin, a BH4 precursor, has proven protective in models of atherosclerosis and hypercholesterolemia [51, 52]. Our finding that arteriolar dysfunction was not altered by sepiapterin, but was by gp91phox-deficiency, was somewhat surprising in light of previous findings that have shown a link between NAD(P)H oxidase and uncoupling of eNOS [53]. This did not appear to be due to a lack of sepiapterin reduction to BH4, because BH4 levels were elevated 49- and 36-fold in mCMV-ND and mCMV-HC mice respectively by sepiapterin, although we appreciate this may not specifically reflect what is occurring in arterioles. Superfusion of sepiapterin directly onto the cremaster muscle (an approach that is effective in other models [52]), also failed to offer protection (data not shown). Although it is plausible this is tissue-specific because ACh only partly acts through nitric oxide to stimulate smooth muscle cell relaxation in cremasteric arterioles [54], we were able to show that ~30% of the vasodilation was due to NO, and this remained unaltered by mCMV, indicating normal eNOS function. This is also in agreement with observations in humans where L-NMMA-induced responses in the forearm were similar in uninfected and CMV-infected individuals [55]. Taken together with our NAD(P)H oxidase data, it is likely mCMV is impairing endothelium-dependent arteriolar vasodilation through an NAD(P)H oxidase-mediated alteration of non-eNOS pathways such as cyclooxygenase or endothelium-derived hyperpolarizing factors.
Despite the lack of effect of sepiapterin in arterioles, this treatment conferred protection against both the leukocyte and platelet recruitment that were observed in mCMV-HC mice. Although it is well-established that reduced NO and increased ROS promote blood cell adhesion, and that eNOS becomes uncoupled in inflammatory conditions, much of the focus of the impact of sepiapterin has been on arterial function and there are only a limited number of studies showing a beneficial effect of sepiapterin on leukocyte and platelet recruitment [38, 56], one of which was used as a basis for our dosing regimen [38]. This has been attributed not only to recoupling of NOS [38], but also to immune modulation via reduction of IL-2 levels [56]. Our findings that BH4 levels in the cremaster were greatly enhanced by sepiapterin would suggest BH4 may at least be partly involved in the protection. Although BH4 levels were not attenuated by CMV infection, regardless of diet, BH4 levels in the mCMV-HC+sepiapterin group only rose to less than one third of the levels achieved in the mCMV-ND+sepiap group. This would suggest that oxidative stress may be higher in the mCMV-HC group, which could cause oxidation of BH4 to BH2. Such a scenario would also be consistent with leukocyte and platelet adhesion occurring in the mCMV-HC group, but not the mCMV-ND mice, and with our findings from platelet experiments that implicate activation of both platelet and vessel wall NAD(P)H oxidase in the thrombogenic phenotype. There is evidence that the BH4:BH2 ratio is more important than absolute BH4 levels [57, 58], and we have preliminary evidence supporting this possibility in that sepiapterin enhanced the BH4:BH2 ratio in mCMV-HC mice from 0.2±0.01 to 4.0±3.6. Nonetheless, we cannot exclude other alternatives including that sepiapterin reductase is less functional in the mCMV-HC mice versus the mCMV-ND group, or that sepiapterin has other targets such as the immune system, and this warrants further investigation. Treatment of the platelet donor, but not recipient, with sepiapterin was also sufficient to reduce platelet adhesion, which suggests that uncoupling of eNOS in the platelets may play an important role in the thrombogenic phenotype we observed in these venules. While very little is known about such a response in platelets, a study by Dixon et al. supports such a possibility [59]. Alternatively, sepiapterin may have had an indirect anti-thrombogenic effect on the platelets within the donor, resulting in lower recruitment in the non-treated recipient. These possibilities require further clarification.
There is some evidence from primary infection of isolated cells that CMV can activate NAD(P)H oxidase in vascular smooth muscle cells from coronary arteries [36], and dysregulate eNOS in endothelial cells [34, 60]. We have extended these findings to show different functional roles for these enzymes during persistent infection in the intact microvasculature. A gp91phox-containing NAD(P)H oxidase contributed to endothelium-dependent arteriolar vasodilation during persistent mCMV infection and, in the presence of hypercholesterolemia, platelet adhesion. In addtion, eNOS mediated venular inflammatory and thrombogenic responses induced by mCMV-HC. Although it is possible that these responses are specific to the cremaster muscle, primary CMV infection invokes an inflammatory phenotype in endothelial cells from several vascular sources [61]. Furthermore, hypercholesterolemia has been shown to have similar effects in other tissues such as the brain [62] and small bowel [63] as are observed in the cremaster [26, 27, 40], therefore such an explanation appears unlikely, but requires confirmation. Further study will also determine whether these findings are gender-related. Unlike much of the data on hypercholesterolemia alone, in which the arteriolar and venular leukocyte recruitment share most of the same underlying mechanisms, these appear to be somewhat (but not completely [21]) disconnected during the prolongation of venular inflammation by CMV, which is an important consideration in the translation of findings from animal studies of cardiovascular risk factors to the clinic, where many patients are CMV-positive. This may be particularly important in individuals at risk for stroke or heart attack in which events in the microvasculature determine the level of tissue damage, and patient outcome.
Highlights.
> Cytomegalovirus (CMV) infection impairs arteriolar dilation. > This is mediated by blood cell- and vessel wall- associated NAD(P)H oxidases. > CMV and hypercholesterolemia synergize to induce venular blood cell recruitment. > Platelet and vascular NAD(P)H oxidases mediate the thrombogenic phenotype. > eNOS dysfunction underlies both the leukocyte and platelet adhesion in these venules.
Acknowledgments
This work was supported by grants from the American Heart Association (AHA 0565285B & 0730294N) and by COBRE Center grant 5P20-RR018724 from the National Center for Research Resources of the National Institutes of Health to KYS, and from the National Institutes of Health (HL067244) to JV-V.
The authors wish to thank Ms. Eunice Johnson and Ms. Candiss Hamric for their help with this study. Our appreciation to Professor Joseph L. Unthank, Ph.D., Indiana University School of Medicine, for his advice on the use of apocynin.
List of Abbreviations
- ACh
acetylcholine
- Apo
apocynin
- BH4
tetrahydrobiopterin
- CMV
cytomegalovirus
- eNOS
endothelial nitric oxide synthase
- gp91phox−/−
gp91phox knockout mice
- HC
high cholesterol diet
- iNOS
inducible nitric oxide synthase
- L-NNA
NG-nitro-L-arginine
- mCMV
murine CMV
- ND
normal diet
- O2·−
superoxide
- ROS
reactive oxygen species
- Sepiap
sepiapterin
- WT
Wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jarvis MA, Nelson JA. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr Opin Microbiol. 2002;5:403–407. doi: 10.1016/s1369-5274(02)00334-x. [DOI] [PubMed] [Google Scholar]
- 2.Sinzger C, Jahn G. Human cytomegalovirus cell tropism and pathogenesis. Intervirology. 1996;39:302–319. doi: 10.1159/000150502. [DOI] [PubMed] [Google Scholar]
- 3.Blum A, Giladi M, Weinberg M, Kaplan G, Pasternack H, Laniado S, Miller H. High anti-cytomegalovirus (CMV) IgG antibody titer is associated with coronary artery disease and may predict post-coronary balloon angioplasty restenosis. Am J Cardiol. 1998;81:866–868. doi: 10.1016/s0002-9149(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 4.Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Victor A, Hafner G, Prellwitz W, Schlumberger W, Meyer J. Impact of infectious burden on progression of carotid atherosclerosis. Stroke. 2002;33:2581–2586. doi: 10.1161/01.str.0000034789.82859.a4. [DOI] [PubMed] [Google Scholar]
- 5.Gabrylewicz B, Mazurek U, Ochala A, Sliupkas-Dyrda E, Garbocz P, Pyrlik A, Mroz I, Wilczok T, Tendera M. Cytomegalovirus infection in acute myocardial infarction. Is there a causative relationship? Kardiologia Polska. 2003;59:283–292. [PubMed] [Google Scholar]
- 6.Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561–3566. [PubMed] [Google Scholar]
- 7.Burnett MS, Durrani S, Stabile E, Saji M, Lee CW, Kinnaird TD, Hoffman EP, Epstein SE. Murine cytomegalovirus infection increases aortic expression of proatherosclerotic genes. Circulation. 2004;109:893–897. doi: 10.1161/01.CIR.0000112585.47513.45. [DOI] [PubMed] [Google Scholar]
- 8.Hsich E, Zhou YF, Paigen B, Johnson TM, Burnett MS, Epstein SE. Cytomegalovirus infection increases development of atherosclerosis in Apolipoprotein-E knockout mice. Atherosclerosis. 2001;156:23–28. doi: 10.1016/s0021-9150(00)00608-0. [DOI] [PubMed] [Google Scholar]
- 9.Vliegen I, Stassen F, Grauls G, Blok R, Bruggeman C. MCMV infection increases early T-lymphocyte influx in atherosclerotic lesions in apoE knockout mice. J Clin Virol. 2002;25(Suppl 2):S159–S171. doi: 10.1016/s1386-6532(02)00095-1. [DOI] [PubMed] [Google Scholar]
- 10.Vliegen I, Duijvestijn A, Grauls G, Herngreen S, Bruggeman C, Stassen F. Cytomegalovirus infection aggravates atherogenesis in apoE knockout mice by both local and systemic immune activation. Microbes Infect. 2004;6:17–24. doi: 10.1016/j.micinf.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Yurochko AD, Huang ES. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J Immunol. 1999;162:4806–4816. [PubMed] [Google Scholar]
- 12.Smith MS, Bentz GL, Alexander JS, Yurochko AD. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J Virol. 2004;78:4444–4453. doi: 10.1128/JVI.78.9.4444-4453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns LJ, Pooley JC, Walsh DJ, Vercellotti GM, Weber ML, Kovacs A. Intercellular adhesion molecule-1 expression in endothelial cells is activated by cytomegalovirus immediate early proteins. [see comment] Transplantation. 1999;67:137–144. doi: 10.1097/00007890-199901150-00023. [DOI] [PubMed] [Google Scholar]
- 14.Dengler TJ, Raftery MJ, Werle M, Zimmermann R, Schonrich G. Cytomegalovirus infection of vascular cells induces expression of pro-inflammatory adhesion molecules by paracrine action of secreted interleukin-1beta. Transplantation. 2000;69:1160–1168. doi: 10.1097/00007890-200003270-00022. [DOI] [PubMed] [Google Scholar]
- 15.Knight DA, Waldman WJ, Sedmak DD. Cytomegalovirus-mediated modulation of adhesion molecule expression by human arterial and microvascular endothelial cells. Transplantation. 1999;68:1814–1818. doi: 10.1097/00007890-199912150-00030. [DOI] [PubMed] [Google Scholar]
- 16.Grundy JE, Lawson KM, MacCormac LP, Fletcher JM, Yong KL. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J Infect Dis. 1998;177:1465–1474. doi: 10.1086/515300. [DOI] [PubMed] [Google Scholar]
- 17.Ricotta D, Alessandri G, Pollara C, Fiorentini S, Favilli F, Tosetti M, Mantovani A, Grassi M, Garrafa E, Dei Cas L, Muneretto C, Caruso A. Adult human heart microvascular endothelial cells are permissive for non-lytic infection by human cytomegalovirus. Cardiovasc Res. 2001;49:440–448. doi: 10.1016/s0008-6363(00)00258-3. [DOI] [PubMed] [Google Scholar]
- 18.Shahgasempour S, Woodroffe SB, Garnett HM. Alterations in the expression of -1, ICAM-1 and VCAM-1 after in vitro infection of endothelial cells with a clinical isolate of human cytomegalovirus. Microbiol Immunol. 1997;41:121–129. doi: 10.1111/j.1348-0421.1997.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 19.Rahbar A, Soderberg-Naucler C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol. 2005;79:2211–2220. doi: 10.1128/JVI.79.4.2211-2220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoretonenko MV, Leskov IL, Jennings SR, Yurochko AD, Stokes KY. Cytomegalovirus Infection Leads to Microvascular Dysfunction and Exacerbates Hypercholesterolemia-Induced Responses. Am J Pathol. 2010;177:2134–2144. doi: 10.2353/ajpath.2010.100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senchenkov E, Khoretonenko MV, Leskov IL, Ostanin DV, Stokes KY. P-selectin mediates the microvascular dysfunction associated with persistent cytomegalovirus infection in normocholesterolemic and hypercholesterolemic mice. Microcirculation. 2011;18:452–462. doi: 10.1111/j.1549-8719.2011.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stokes K, Cooper D, Tailor A, Granger D. Hypercholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Radic Biol Med. 2002;33:1026–1036. doi: 10.1016/s0891-5849(02)01015-8. [DOI] [PubMed] [Google Scholar]
- 23.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 24.Warnholtz A, Nickenig G, Schulz E, Macharzina R, Brasen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Bohm M, Meinertz T, Munzel T. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin- angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 25.Muller G, Morawietz H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal. 2009;11:1711–1731. doi: 10.1089/ars.2008.2403. [DOI] [PubMed] [Google Scholar]
- 26.Stokes KY, Russell JM, Jennings MH, Alexander JS, Granger DN. Platelet-associated NAD(P)H oxidase contributes to the thrombogenic phenotype induced by hypercholesterolemia. Free Radic Biol Med. 2007;43:22–30. doi: 10.1016/j.freeradbiomed.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokes KY, Clanton EC, Russell JM, Ross CR, Granger DN. NAD(P)H oxidase-derived superoxide mediates hypercholesterolemia- induced leukocyte-endothelial cell adhesion. Circ Res. 2001;88:499–505. doi: 10.1161/01.res.88.5.499. [DOI] [PubMed] [Google Scholar]
- 28.Vasquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol. 2002;22:1655–1661. doi: 10.1161/01.atv.0000029122.79665.d9. [DOI] [PubMed] [Google Scholar]
- 29.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engels W, Grauls G, Lemmens PJ, Mullers WJ, Bruggeman CA. Influence of a cytomegalovirus infection on functions and arachidonic acid metabolism of rat peritoneal macrophages. J Leukoc Biol. 1989;45:466–473. doi: 10.1002/jlb.45.5.466. [DOI] [PubMed] [Google Scholar]
- 31.Yourtee EL, Bia FJ, Griffith BP, Root RK. Neutrophil response and function during acute cytomegalovirus infection in guinea pigs. Infect Immun. 1982;36:11–16. doi: 10.1128/iai.36.1.11-16.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki S, Kameoka M, Nakaya T, Kimura T, Nishi N, Hirai K, Ikuta K. Superoxide generation by monocytes following infection with human cytomegalovirus. Immunopharmacology. 1997;37:185–190. doi: 10.1016/s0162-3109(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 33.Speir E. Cytomegalovirus gene regulation by reactive oxygen species. Agents in atherosclerosis. Ann N Y Acad Sci. 2000;899:363–374. doi: 10.1111/j.1749-6632.2000.tb06200.x. [DOI] [PubMed] [Google Scholar]
- 34.Bouwman JJ, Visseren FL, Bevers LM, van der Vlist WE, Bouter KP, Diepersloot RJ. Azithromycin reduces Chlamydia pneumoniae-induced attenuation of eNOS and cGMP production by endothelial cells. Eur J Clin Invest. 2005;35:573–582. doi: 10.1111/j.1365-2362.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- 35.Weis M, Kledal TN, Lin KY, Panchal SN, Gao SZ, Valantine HA, Mocarski ES, Cooke JP. Cytomegalovirus infection impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine in transplant arteriosclerosis. Circulation. 2004;109:500–505. doi: 10.1161/01.CIR.0000109692.16004.AF. [DOI] [PubMed] [Google Scholar]
- 36.Dhaunsi GS, Kaur J, Turner RB. Role of NADPH oxidase in cytomegalovirus-induced proliferation of human coronary artery smooth muscle cells. J Biomed Sci. 2003;10:505–509. doi: 10.1007/BF02256111. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X, Bohlen HG, Miller SJ, Unthank JL. NAD(P)H oxidase-derived peroxide mediates elevated basal and impaired flow-induced NO production in SHR mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H1008–H1016. doi: 10.1152/ajpheart.00114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood KC, Hebbel RP, Lefer DJ, Granger DN. Critical role of endothelial cell-derived nitric oxide synthase in sickle cell disease-induced microvascular dysfunction. Free radic biol med. 2006;40:1443–1453. doi: 10.1016/j.freeradbiomed.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 39.Tomita Y, Sachs DH, Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939–948. [PubMed] [Google Scholar]
- 40.Stokes KY, Gurwara S, Granger DN. T-cell derived interferon-gamma contributes to arteriolar dysfunction during acute hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2007;27:1998–2004. doi: 10.1161/ATVBAHA.107.146449. [DOI] [PubMed] [Google Scholar]
- 41.Cooper D, Chitman KD, Williams MC, Granger DN. Time-dependent platelet-vessel wall interactions induced by intestinal ischemia-reperfusion. Am J Physiol - Gastrointest Liver Physiol. 2003;284:G1027–G1033. doi: 10.1152/ajpgi.00457.2002. [DOI] [PubMed] [Google Scholar]
- 42.Whitsett J, Picklo MJ, Sr, Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler thromb vasc biol. 2007;27:2340–2347. doi: 10.1161/ATVBAHA.107.153742. [DOI] [PubMed] [Google Scholar]
- 43.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 44.Babior BM. The leukocyte NADPH oxidase. Isr Med Assoc J. 2002;4:1023–1024. [PubMed] [Google Scholar]
- 45.Pignatelli P, Sanguigni V, Lenti L, Ferro D, Finocchi A, Rossi P, Violi F. gp91phox-dependent expression of platelet CD40 ligand. Circulation. 2004;110:1326–1329. doi: 10.1161/01.CIR.0000134963.77201.55. [DOI] [PubMed] [Google Scholar]
- 46.Plumb RD, El-Sherbeeny NA, Dixon LJ, Hughes SM, Devine AB, Leahey WJ, McVeigh GE. NAD(P)H-dependent superoxide production in platelets: the role of angiotensin II and protein kinase C. Clin Biochem. 2005;38:607–613. doi: 10.1016/j.clinbiochem.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 48.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 49.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pignatelli P, Sanguigni V, Lenti L, Loffredo L, Carnevale R, Sorge R, Violi F. Oxidative stress-mediated platelet CD40 ligand upregulation in patients with hypercholesterolemia: effect of atorvastatin. J Thromb Haemost. 2007;5:1170–1178. doi: 10.1111/j.1538-7836.2007.02533.x. [DOI] [PubMed] [Google Scholar]
- 51.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 52.Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W. Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation. 2000;102:2172–2179. doi: 10.1161/01.cir.102.18.2172. [DOI] [PubMed] [Google Scholar]
- 53.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J clin invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hungerford JE, Sessa WC, Segal SS. Vasomotor control in arterioles of the mouse cremaster muscle. Faseb J. 2000;14:197–207. doi: 10.1096/fasebj.14.1.197. [DOI] [PubMed] [Google Scholar]
- 55.Grahame-Clarke C, Chan NN, Andrew D, Ridgway GL, Betteridge DJ, Emery V, Colhoun HM, Vallance P. Human cytomegalovirus seropositivity is associated with impaired vascular function. Circulation. 2003;108:678–683. doi: 10.1161/01.CIR.0000084505.54603.C7. [DOI] [PubMed] [Google Scholar]
- 56.Pieper GM, Ionova IA, Cooley BC, Migrino RQ, Khanna AK, Whitsett J, Vasquez-Vivar J. Sepiapterin decreases acute rejection and apoptosis in cardiac transplants independently of changes in nitric oxide and inducible nitric-oxide synthase dimerization. J Pharmacol Exp Ther. 2009;329:890–899. doi: 10.1124/jpet.108.148569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol. 2008;294:H1530–H1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dixon LJ, Morgan DR, Hughes SM, McGrath LT, El-Sherbeeny NA, Plumb RD, Devine A, Leahey W, Johnston GD, McVeigh GE. Functional consequences of endothelial nitric oxide synthase uncoupling in congestive cardiac failure. Circulation. 2003;107:1725–1728. doi: 10.1161/01.CIR.0000066283.13253.78. [DOI] [PubMed] [Google Scholar]
- 60.Shen YH, Zhang L, Utama B, Wang J, Gan Y, Wang X, Chen L, Vercellotti GM, Coselli JS, Mehta JL, Wang XL. Human cytomegalovirus inhibits Akt-mediated eNOS activation through upregulating PTEN (phosphatase and tensin homolog deleted on chromosome 10) Cardiovasc Res. 2006;69:502–511. doi: 10.1016/j.cardiores.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Bentz GL, Jarquin-Pardo M, Chan G, Smith MS, Sinzger C, Yurochko AD. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J Virol. 2006;80:11539–11555. doi: 10.1128/JVI.01016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishikawa M, Stokes KY, Zhang JH, Nanda A, Granger DN. Cerebral microvascular responses to hypercholesterolemia: roles of NADPH oxidase and P-selectin. Circ Res. 2004;94:239–244. doi: 10.1161/01.RES.0000111524.05779.60. [DOI] [PubMed] [Google Scholar]
- 63.Tailor A, Granger DN. Hypercholesterolemia promotes leukocyte-dependent platelet adhesion in murine postcapillary venules. Microcirculation. 2004;11:597–603. doi: 10.1080/10739680490503393. [DOI] [PubMed] [Google Scholar]