Abstract
The ability to model aspects of human addictive behaviors in laboratory animals provides an important avenue for gaining insight into the biochemical alterations associated with drug intake and the identification of targets for medication development to treat addictive disorders. The intravenous self-administration procedure provides the means to model the reinforcing effects of abused drugs and to correlate biochemical alterations with drug reinforcement. In this chapter, we provide a detailed methodology for rodent intravenous self-administration and the isolation and preparation of proteins from dissected brain regions for Western blot analysis and high throughput proteomic analysis. Examples of cocaine-induced alterations in the abundances of ionotropic glutamate receptor subunits in reinforcement related brain regions are provided.
Keywords: Cocaine, self-administration, glutamate, reinforcement, infrared immunoblotting
1. Introduction
A generally accepted tenet in drug abuse research is that certain drugs can function as reinforcing stimuli which contribute to their abuse liability in humans and can be modeled in animal. The ingestion of drugs such as cocaine exerts maladaptive effects on various biochemical substrates in several brain regions which in turn lead to further intake, drug craving, binge administration and relapse. A significant amount of research investigating the neurobiology of drug abuse is conducted in animal models (rodent and non-human primate) which closely resemble characteristics of human drug intake. Various behavioral models have been developed and are commonly used for studying the effects of abused drugs on brain biochemistry including self-administration, place conditioning, locomotor activity and sensitization, and intracranial self-stimulation to name but a few. While each play important roles in helping us understand the effects of drugs on behavior and biochemistry, the self-administration paradigm provides the ability to directly link such changes directly to the reinforcing effects of the drug. Generally, the self-administration paradigm involves the emission of specific behavior(s) by the animal or human (e.g. lever-press; nose-poke) that is maintained by drug administration (e.g. intravenous, oral or intracranial). There are several advantages of the self-administration paradigm with including substances abused by humans can function as positive reinforcing stimuli under laboratory conditions, the ability to generate clear dose-effect curves (5,8) and the homology of brain regions between species (rodent, non-human primate and human) that contribute to the reinforcing effects of the drugs.
The advent of high-throughput screening technologies has produced a paradigm shift in the manner in which scientists are able to detect and identify molecular mechanisms related to disease. Proteomic analysis strategies allow the simultaneous assessment of thousands of genes and proteins of known and unknown function - thereby enabling a global biological view of addictive disorders. A major challenge in proteomic biology is to understand the function of proteins in the context of human disease, such as in addictive disorders. Broad scale evaluations of protein expression are well suited to the study of drug abuse given the multigenic nature of drug addiction, the anatomical and cellular complexity of the brain compared with other tissues (including the vast representation of expressed proteins in the brain), and the relatively limited knowledge of the molecular pathology of these disorders (16-18).
2. Materials
2.1 Intravenous catheter for rat self-administration
The majority of equipment for catheterization can be produced in the lab or is commercially available through Med Associates Inc., Coulbourne Instruments and other manufacturers.
Backplate and polyvinyl chloride tubing catheter (20,21). Catheters are composed of 25 ga polyethylene tubing with two-2 mm sections of 20 ga polyethylene tubing placed over the smaller tubing at 2.5 cm and 12 cm from the proximal end. Between the points, the smaller tubing is looped to prevent crimping. Two 10-cm strands of suture are tied at each of these points for anchoring the catheter to the muscle.
Spring leash to protect the catheter and provide points of connection with backplate and swivel.
Single channel swivel to enable side to side motion of the animal while connecting the syringe.
Syringe infusion pump (Razel Scientific Instruments). Investigators should take into account the motor speed of the syringe pump and the diameter of syringe in determining the volume of drug that will be delivered through the catheter.
Operant chamber with response lever(s), stimulus lights and tone generator. Commercially available from Med Associates , Inc. and Coulbourn Instruments.
Bacteriostatic heparinized saline (1.7 units/ml)
Drugs for anesthesia (pentobarbital and thiopental) as well as for self-administration require state and federal government licensure for the investigator.
2.2. Protein Isolation, Fractionation and Quantification (NOTE 1)
Tabletop centrifuge for 1.5 – 2.0 ml tubes
10X phosphate buffered saline
TSE buffer: 10mM Tris Ph=7.5, 300 mM sucrose, 1 mM EDTA
CLB buffer: 100 mM HEPES, 10 mM NaCl, 1mM KH2PO4, 5 mM NaHCO3, 1mM CaCl2, 0.5 mM MgCl2
Commercial protease inhibitor cocktail (e.g. Halt Protease Inhibitor Cocktail, Pierce Biotechnology/Thermo Scientific) or 10 mM HEPES, 10 mM NaCl, 1 mM KH2PO4, 5 mM NaHCO3, 1 mM CaCl2, 0.5 mM MgCl2, 5 mM EDTA, and the following protease inhibitors (PI): 1 mM phenylmethylsulfonylfluoride, 10 mM benzamidine, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 μg/ml pepstatin. Commercial phosphatase inhibitor (e.g. Halt Phosphatase Inhibitor Cocktail, Pierce Biotechnology/Thermo Scientific) (NOTE 2).
Spectrophotometer
2.3 Infrared Western Blotting
Pre-cast polyacrylamide gels (Ready Gel Tris-HCl; Bio-Rad, Hercules, CA). Store at 4°C.
Running buffer (1X TGS buffer): 25 mM Tris, pH 8.3, 192 mM glycine, 0.1% SDS. Store at room temperature.
Transfer buffer (1X TG buffer): 25 mM Tris, 192 mM glycine, 20% methanol. Store at room temperature.
Wash buffer: 1X PBS, 0.1% Tween-20. Store at room temperature.
Transfer membrane: Bio-Rad transblot-transfer membrane, pure nitrocellulose membrane (0.2 μm).
3MM chromatography paper
Primary antibodies: NR1: 1:300 mouse monoclonal (#MAB363, Chemicon: recognizes NR1-1a, NR1-1b, NR1-2a, NR1-2b splice variants); NR2A: 1:300 mouse monoclonal (#MAB1526, Chemicon); NR2B: 1:1000 rabbit polyclonal (07-351; Millipore) and neuronal tubulin: 1:1000 mouse monoclonal (#05-559, Millipore). Polyclonal antibodies for AMPA and kainate receptor subunits are purchased from Millipore and used at a dilution of 1:1000.
Secondary antibodies: IRdye 800 conjugated affinity purified (Rockland Immunochemicals, Gilbertsville, PA); Alexa Fluor 680 (Invitrogen, Carlsbad, CA). Store at 4°C.
LiCor Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE).
Blocking buffer: Odyssey blocking buffer (cat#:927-40000). Store at 4°C.
Pre-stained molecular markers: Precision Plus Protein Western C standards (Bio-Rad, Hercules, CA). Store at 4°C.
2.4. Protein processing for proteomics
Sample clean-up kits such as; 2D clean-up kit (cat. no. 80-6484-51; GE HealthCare); ReadyPrep 2-D Cleanup Kit (Bio-Rad) and 2-D Sample Prep Kits (Pierce Biotechnology).
Sample buffer (30 mM Tris-HCl, 2 M thiourea, 7 M urea and 4% CHAPS, pH 8.5). (NOTE 3).
2D-Quant kit (GE HealthCare).
CyDye DIGE Fluor minimal dyes 2, 3, and 5 (cat. no. 80-6484-51; GE HealthCare).
>99.5% pure dimethylformamide (DMF: USB Corporation) and 10 mM lysine.
Rehydration buffer (2 M thiourea, 7 M urea, 2% dithiothreitol (DTT), 4% CHAPS and 2% Pharmalyte, Destreak rehydration buffer (GE HealthCare)) (NOTE 3).
2.5 Two dimensional polyacrylamide gel electrophoresis (2D-PAGE)
EttanTM IPGphorTM apparatus (GE HealthCare); alternative equipment is PROTEAN IEF System (Bio-Rad), Multiphor II Horizontal Electrophoresis Unit (PerkinElmer) or UniPhor Horizontal Electrophoresis Unit(Sigma-Aldrich).
Immobiline DryStrips (240_3_0.5 mm, linear 4–7/6–9/3–10 pH; GE Healthcare); alternative sources are Bio-Rad, Sigma-Aldrich and Isogen Lifesciences.
Ettan Dalt II System (GE HealthCare); alternative equipment is PROTEAN Plus DodecaTM Cell (Bio-Rad).
Pre-cast 8–15% gradient SDS-PAGE (2,400_2,000_1 mm; Jule Inc.).
Mass spectrometer: Applied Biosystems 4700/4800 Proteomics Analyzer (MALDI-TOF/TOF); most other mass spectrometers are capable of analysis; however, MALDI-TOF-TOF has an advantage of higher throughput and provides detailed peptide sequence analysis.
3. Methods
Historically, the abuse liability of cocaine is attributed to the direct effects of the drug on dopamine uptake blockade yielding elevated extracellular dopamine concentrations that occur in discrete areas of the brain, specifically the nucleus accumbens (NAc), ventral tegmental area (VTA), prefrontal cortex – data from human subjects (2,11,12) as well as animal models (6-8,13,14,24). More recently, attention has focused on significant alterations in glutamatergic transmission in the VTA and NAc following cocaine administration in rodents and humans associated with the neuroplasticity of cocaine addiction (3,4,15,19,22,23). A further delineation of the neural contributions and alterations of addictive behaviors has been postulated recently which identifies the dysregulation of prefrontal glutamatergic projections to NAc as an essential component. Briefly stated, prefrontal cortical dopamine alterations lead to preferential responding for drug-related stimuli whereas accumbal- glutamatergic alterations underlie the unmanageable aspects of drug-seeking behaviors (9,10).
In many respects, substance abuse can be seen as a disease of synaptic dysregulation and pathology. Several studies have demonstrated significant morphological and electrophysiological disturbances in mesolimbic brain structures reflecting significant synaptic modification following cocaine administration (REFS) - effects that are mediated predominantly by ionotropic glutamate receptors. Since subunit composition determines the functional properties of ionotropic glutamate receptors (1), alterations in the abundance of ionotropic glutamate receptor subunits in specific brain regions are correlated with changes in neuronal excitability and synaptic strength underlying long term biochemical and behavioral effects of cocaine which, in turn, may affect subsequent drug intake.
Our groups and others have examined the effects of self-administered cocaine on glutamate dysregulation in rats, non-human primates and in post-mortem tissue from cocaine overdose victims. In this chapter, we provide an overview of methods used for rodent self-administration as well as biochemical procedures used to explore changes in glutamate receptor abundances in brain regions following self-administration.
3.1 Intravenous catheter implantation for rat
Rats are anesthetized by administration and surgery is undertaken using aseptic surgical procedures.
A 3 cm region is shaved on the right region of the underside of the neck along with a 5 × 5 cm region on the back 6 cm from the base of the neck proceeding caudally. Both areas are hand shaved in order to remove remaining fur and swabbed with tincture of iodine.
A 3 cm incision is made in the shaved area of the back, 1.5 cm lateral from the midline, and covered with sterile gauze. Following a 2.5 cm incision is made in the shaved area of the neck above the jugular vein. The jugular vein is exposed and cleared of fascia.
A trocar (3-mm diameter) is inserted at the base of the neck incision and is guided subcutaneously around the caudal region of the right forelimb to exit at the center of the back incision. The distal end of the polyethylene catheter is inserted through the trocar to exit at the back. Holding the proximal end of the catheter, the trocar is removed through the back incision.
The jugular vein is re-exposed and a 23-ga needle is used to puncture the vein to provide a point of entry for the proximal end of the catheter. The catheter is inserted as the needle is withdrawn and extends to just outside the right atrium. The catheter is anchored to muscle near the point of entry into the vein.
The incision is sutured and treated topically with Neosporin antibiotic powder. The distal end of the catheter is guided through a Teflon shoulder harness (Med Associates, Inc.). The harness provides a point of attachment for a spring leash connected to a single channel swivel at the opposing end. The catheter is threaded through the leash for attachment to the swivel.
The fixed end of the swivel is connected to a syringe by polyethylene tubing. The syringe is placed in a computer controlled motor driven syringe pump. An infusion of thiopental is administered as needed to assess catheter patency.
Immediately following surgery, an analgesic should be administered. Rats should be monitored every 30 minutes after surgery until conscious and a minimum of three times per day for two days following surgery by the P.I. After this time, rats are observed a minimum of two times per day for the remainder of the experiment by the P.I. The health of the rats should be monitored according to the guidelines issued by the institutional animal care and use committee and the National Institute of Health.
3.2 Rat self-administration procedures
1. These instructions utilize the Med Associates Drug Self-Administration Chamber for rats but the general instructions can be adapted to other operant apparatus.
2. Rats are transferred from their home cage to an operant conditioning chamber enclosed in a sound attenuated box. Each chamber contains a response lever, a house light, a tone generator, a ventilation fan and a syringe pump located outside the sound attenuated box.
2. An IBM compatible computer is used for programming of the self-administration session and for data collection (Med Associates). Extraneous noise is masked by the ventilation fan and white noise generator in the room. Prior to each session, the swivel and catheter should be flushed with heparinized saline before connecting the catheter to the infusion pump via a 20 gauge Luer hub and 28 gauge male connector.
3. Responding is engendered normally engendered under a fixed ratio one (FR1) schedule of reinforcement whereby one response on the lever results in the infusion of the drug. Upon completion of the response requirement, a drug infusion is delivered and a time-out is in effect. During the time-out, the lever light is extinguished, the house light illuminated, and a tone generated. The end of the time-out is signaled by illumination of the lever light and extinguishing of the house light and tone. During the time-out period, responses on levers are recorded but have no scheduled consequence. Following stable responding, the schedule of reinforcement can be adjusted according to the study design.
4. It is noted that numerous studies have described self-administration in non-human primates but the behavioral and procedural complexity requires significant training, expertise and resources that are beyond the scope of this chapter.
3.3 Necropsy and Dissection
Following the completion of experimental studies, subjects should be euthanized according to the guidelines set forth by the institutional animal care and use committee. We recommend the most expedient and humane method of sedation. Please keep in mind that sedatives may affect certain proteins of interest (e.g. GABA receptors). Ensure complete sedation (unresponsive to tactile and painful stimuli). The following steps pertain to necropsy and dissection for both rat and monkey.
Following intracardial perfusion with ice cold PBS, craniotomy is performed exposing the brain. Following removal of bone and dura, the brain is removed, rinsed with 1X PBS (4°C) and placed immediately in a brain matrix at 4°C. (NOTE 4)
The brain is blocked in the plane of interest (e.g. coronally). From the rostral to caudal aspects, the brain blocks are removed and placed caudal side down on an aluminum plate. At this point, the regions of interest can be dissected immediately or the blocks can be stored at −80°C for later use.
Upon dissecting the regions of interest from rodent, monkey or human samples, tissue is pulverized using a metal mortar and pestal (kept on dry ice) in the presence of liquid nitrogen. Following evaporation of the liquid nitrogen, the pulverized tissue is stored in eppendorf tubes and kept at −80°C.
3.4 Protein Isolation
Pulverized tissue samples are dounce homogenized in the presence of RIPA lysis buffer and protease inhibitors. Crude protein homogenates can be used for analysis or processed further using a variety of fractionation protocols. Commonly, we use a fractionation protocol which yields membrane, cytosolic and nuclear protein fractions.
Homogenates are centrifuged at 7500 RPM for 5 min. The supernatant is removed and the pellet (nuclei and debris) are resuspended in 20 mM Tris HCl, 1 mM EDTA (pH=8.0) with the protease inhibitor cocktail and centrifuged at 7500 RPM for 5 min.
Repeat twice and resuspend pellet in the solution and store at −20°C (nuclear fraction).
Centrifuge supernatant at 25,000 RPM for 30 min at 4°C. Following, the supernatant containing the cytosolic fragment is removed and stored at −20°C (cytosolic fraction).
Resuspend pellet in 10 mM Tris (pH=7.5), 300 mM sucrose, 1 mM EDTA (pH=8.0), 0.1% NP40 and PIs and centrifuged at 5000 RPM for 5 min at 4°C. The supernatant is discarded and the pellet is resuspended in the buffer and washed three times before re-suspension in the buffer and PIs and storing the samples at −20°C (membrane fraction).
Protein concentrations of samples are calculated using the Bicinochoninic Acid (BCA) Protein Assay Kit (Pierce, Rockford, IL).
Mix reagents and prepare serial dilutions of standards 0-2 μg/μL as described in the BCA Assay protocol and incubate 30 min at 37°C.
Samples are quantified using spectrophotometer and concentrations are determined according to the standard curve.
3.5 SDS-polyacrylamide gel electrophoresis
These instructions use the BioRad Ready Gel System but are easily adaptable to other formats. Similarly, these instructions are specific for infrared immunoblotting using the LiCor Odessey imaging system. However, visualization of proteins using chemiluminescence can also be performed.
Dilute samples in 1.5 ml centrifuge tubes with Laemmeli sample buffer to achieve the final protein concentrations. Place tubes in a heat block (95°C) for 5 minutes and then immediately on ice. After cooling, centrifuge samples briefly to collect contents in bottom of tube.
Prepare a Ready Gel Tris-HCl of the percent polyacrylamide according to the protein(s) of interest. Carefully remove and discard the adhesive film on the bottom of the gel and the inserted comb.
Add 1X Running Buffer to the chamber covering the wells. Load identical concentrations of proteins along with pre-stained molecular weight marker.
Connect the assembly to a power supply. The gel can be run at 25-30 V or at 90-100 V if a cooling unit is available. Bromophenol blue will be the leading dye front and gel electrophoresis should be stopped when it reaches the anodic end of the gel.
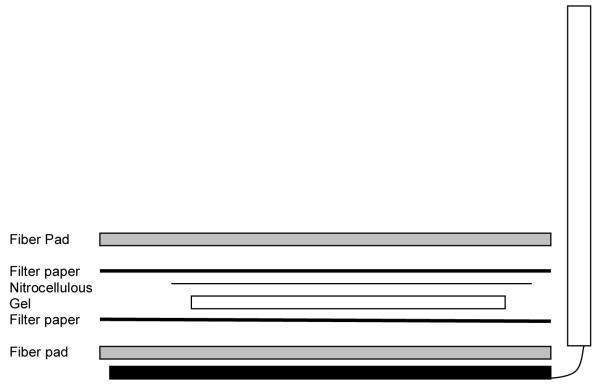
A tray containing transfer buffer needs to be of sufficient size to lay out the transfer cassette with the requisite foam and two pieces of 3MM Whatman paper. One fiber pad is placed on each side of the open cassette followed by a piece of 3MM paper. A section of nitrocellulose membrane cut slightly larger than the size of the separating gel is saturated with transfer buffer. The gel is removed from the gel unit and placed on top of one sheet of the saturated 3MM paper. The saturated nitrocellulose membrane is placed on top of the gel followed by the remaining piece of 3MM paper. A long thin cylinder (such as a pipette) is rolled over the 3MM paper to remove any bubbles. Afterwards, the remaining fiber pad is placed in top of the 3MM paper and the cassette is closed.
The transfer cassette is placed into the transfer tank with the nitrocellulous membrane between the gel and the anode. This is an extremely important as the incorrect orientation will result in the proteins being electrophoresed into the buffer instead of being transferred onto the nitrocellulose.
A refrigerated circulating water bath is used to maintain temperature between 5-10°C.
The lid to the transfer tank is secured and the power supply is activated at 25 V for 12 hours or 65 V for 3 hr.
Following completion of the transfer, the cassette is removed and disassembled by removing the top fiber pad and 3MM paper. The nitrocelluose membrane is removed, placed in a small tray and rinsed with transfer buffer for 5 minutes. The colored molecular markers should be clearly visible on the membrane.
The nitrocellulose membrane is incubated with 50%LiCor Buffer/50% PBS or one hour at room temperature on a rocking platform.
The blocking buffer is discarded and the membrane is incubated with the desired primary antibody/antibodies diluted in 50% LiCor Buffer/50% Wash Buffer for 12-15 hours at 4°C on a rocking platform. In addition to the antibody of interest, we also include a neuronal tubulin antibody to ensure equal protein loading and efficiency of transfer (NOTE 5).
- The primary antibody is removed and the membrane is rinsed with Wash Buffer four times for five minutes each to remove excess primary antibody. (NOTE)
- Add secondary antibody in 50% Odyssey buffer/50% Wash buffer. Monoclonal antibodies are visualized with goat anti-mouse AlexFluor680 conjugated secondary antibody (#A21076, Molecular Probes) and polyclonal antibodies were visualized with goat anti-rabbit IRDye800 conjugated secondary antibody (#610-132-121, Rockland Immunochemicals) diluted 1:15,000.Incubate for two hours at room temperature on a rocking platform. Be sure to cover the incubating tray with aluminum foil to prevent bleaching of the conjugated fluorophore on the secondary antibody.
The incubation buffer is discarded and the membrane is rinsed three times for fifteen minutes each with Wash Buffer followed by two rinses with 1X PBS two times for fifteen minutes each. The membrane is scanned on the LiCor Odyssey infrared scanner or stored at 4°C for later analysis. Membrane should be protected for light as noted above.
Membranes are scanned with the Licor Odyssey infrared scanner and signals are quantified with Odyssey version 1.2 software. Signal intensities for proteins of interest were reported as percent control relative to tubulin.
3.7 Protein processing for proteomics
Precipitate the protein sample using the 2D clean-up kit according to the manufacturer’s recommendations at 20° C overnight.
The next day, pellet the sample by centrifugation at 13,400g for 5 min at 4 1C and air-dry the pellet for 2 min.
Determine the protein concentration using the 2D-Quant kit which is compatible with the reagent concentration in the sample buffer. The protein concentration should be in the range of 5–10 mg/ml (adequately concentrate or dilute the samples).
Check the pH of all the samples and make sure that it is between 8.0 and 9.0 during cyanine dye labeling. Add adequate rehydration buffer to make the volume of the samples up to 450 ml and add 50 ml of Destreak rehydration buffer (NOTE 6 and 7).
3.8 Two Dimensional Polyacrylamide Gel Electrophoresis (2D-PAGE)
Perform the IEF according to GE Healthcare setup using the 24-cm pH 4–7 NL Immobiline DryStrips on an EttanTM IPGphorTM apparatus. The protocol can be adapted on most isoelectric focusing equipment (NOTE 8).
Equilibrate the IEF DryStrips to reduce the disulfide bonds by gently rocking them in 10 ml of reducing buffer/strip for 10 min. Immediately after this, alkylate the -SH groups of proteins by gently rocking the proteins in 10 ml of alkylating buffer/strip for 10 min. The SDS in the buffers also helps the proteins to acquire a negative charge, which drives their migration under the electrical current. Before proceeding to the next step, rinse the IEF strip in the SDS electrophoresis running buffer (NOTE 9).
Overlay 0.6% agarose solution on the top of the glass plate of a pre-cast 8–15% gradient SDS-PAGE. Place the IEF strip between the glass plates and push it with a thin plastic spacer ensuring that the IEF strip rests on the SDS-PAGE. The proteins are then separated on the basis of their molecular weight at 4 W overnight until the bromophenol blue dye front reaches the bottom of the gel (NOTE 10).
The gels can be scanned by varied scanners; Typhoon 9400TM scanner (GE Healthcare); FLA 5100 Imaging System (FUJIFILM) or Ettan DIGE imager (GE HealthCare) (NOTE 11).
The gel images can be analyzed by DeCyderTM (GE HealthCare); Progenesis SameSpots (Nonlinear Dynamics)—the most automated of the softwares available for DIGE analysis—or Delta2D (DECODON)
The differentially regulated protein spots are analyzed by mass spectrometry for protein identification.
Figure 1.
Schematic of immunoblotting preparation showing location of fiber pad, Whatmann paper, nitrocellulose and gel.
Figure 2.
Representative Western blot analysis of proteins isolated from the nucleus accumbens of monkeys following eighteen months of cocaine self-administration compared to controls. Protein was isolated from membrane fractions and levels of ionotropic glutamate receptor subunit proteins GluR1, GluR2/3 and GluR5 were evaluated (5 μg) following separation on 10% SDS-PAGE. Data are expressed as mean (± S.E.M.) of the percent of control values per amount of protein loaded. Asterisks indicate a significant difference (P<0.05). B. Representative bands from two cocaine self-administration monkeys (+) and two control subjects (−) for each subunit are shown. Reprinted with permission from the Journal of Neurochemistry.
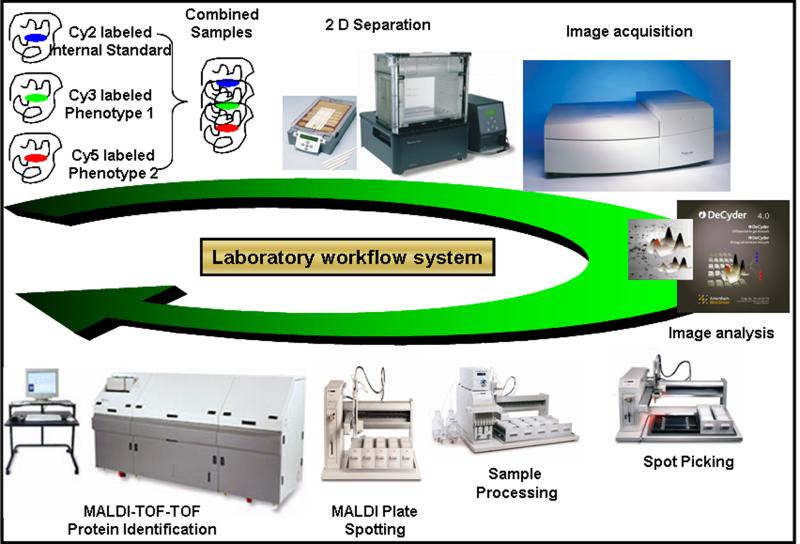
Figure 3. 2D-DIGE work-flow for comparative expression-proteomics.
Samples are labeled with fluorescent dyes (Cy 2, 3 and 5) and combined together prior to the IEF. An optimized protocol based on the tissue of interest is used for the IEF and second dimension separation of proteins. The three gel images are scanned by Typhoon™ scanner (GE Healthcare) so that the maximum pixel intensities are within the linear dynamic range and at the same time are consistent across the entire set of gels in the experiment. The differentially regulated protein spots, after the image analyses by DeCyder™ analysis software, are picked by robotic picker. These spots are robotically processed, including in-gel digestion, and prepared for acquiring mass spectra (MS and MS/MS) by MALDI-TOF-TOF. The acquired mass spectra are searched against the protein database, using MASCOT search engine, for the species of interest to obtain protein identification.
Acknowledgements
The preparation of this chapter was funded in part by DA012498, DA06634 and DA03628 (SEH).
Notes
All solutions should be prepared in double distilled water with resistivity up to 18.2 megohm·cm and total organic content less than 1 part per billion or HPLC grade water.
Different combinations of protease inhibitors can be used depending on the proteins and moieties of interest.
Confirm that all the solutions containing urea are prepared recently and also not heated above 37 °C to prevent protein carbamylation and subsequent formation of charge trains on the 2D gel.
The determination of the appropriate plane of dissection and the width of the sections are based on the region(s) of interest. Commercially available brain matrices for rodents and monkeys provide consistency of sectioning.
Antibodies should be tested across a range of protein concentrations to determine linearity of antigen to signal for each species as well as each brain region of interest.
The optimal labeling of the sample of interest should be achieved by a preliminary study. The goal is to achieve labeling for less abundant proteins at the same time maintaining the most abundant proteins in the linear dynamic range for quantitative analyses. A range of ratios for protein concentration: CyDye amount (50 mg: 100 pmol to 50 mg: 400 pmol) should be tested.
Prepare the rehydration buffer by freshly adding DTT and IPG buffer.
The length of the pH strip, its pH range as well as the IEF setup should be empirically determined to provide the best possible resolution for your sample of interest.
Ensure fresh DDT and iodoacetamide in the reducing and alkylating buffers, respectively. To minimize the protein loss do not exceed the stipulated times of alkylation and reduction.
Make certain that low-fluorescence glass plates with a reference marker are used. This is critical for the background pixel values of the scanned images to be as low as possible. To avoid variability in the second dimensional fractionation of proteins ensure that all the precast gels are from the same batch.
The gels should be scanned at an appropriate PMT value to bring all the protein spots in the linear dynamic range. It is essential that the maximum pixel intensities of all images do not differ from each other by more than 10,000–15,000. This is crucial to obtain significant quantitative comparison between the gel images.
References
- [1].Borges K, Dingledine R. Molecular pharmacology and physiology of glutamate receptors. In: Herman BH, Frankenheim J, Litten RZ, Sheridan PH, Weight FF, Zukin SR, editors. Glutamate and addiction. Humana Press; Totawa, NJ: 2002. pp. 3–22. [Google Scholar]
- [2].Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- [3].Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. Journal of Neurochemistry. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- [4].Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. Journal of Neuroscience. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hemby SE. Recent advances in the biology of addiction. Curr Psychiatry Rep. 1999;1:159–165. doi: 10.1007/s11920-999-0026-9. [DOI] [PubMed] [Google Scholar]
- [6].Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999;288:274–280. [PubMed] [Google Scholar]
- [7].Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- [8].Hemby SE, Johnson BA, Dworkin SI. Neurobiological basis of drug reinforcement. In: Johnson BA, Roache JD, editors. Drug Addiction and Its Treatment: Nexus of Neuroscience and Behavior. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 137–169. [Google Scholar]
- [9].Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D. Glutamate transmission and addiction to cocaine. Ann N Y Acad Sci. 2003;1003:169–175. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- [10].Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- [11].Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- [12].Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- [13].Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- [14].Pettit HO, Pan HT, Parsons LH, Justice JB., Jr. Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. Journal of Neurochemistry. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- [15].Tang W-X, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. Journal of Neurochemistry. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tannu N, Mash DC, Hemby SE. Cytosolic proteomic alterations in the nucleus accumbens of cocaine overdose victims. Mol Psychiatry. 2007;12:55–73. doi: 10.1038/sj.mp.4001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tannu NS, Hemby SE. Methods for proteomics in neuroscience. Prog Brain Res. 2006;158:41–82. doi: 10.1016/S0079-6123(06)58003-3. [DOI] [PubMed] [Google Scholar]
- [18].Tannu NS, Hemby SE. Two-dimensional fluorescence difference gel electrophoresis for comparative proteomics profiling. Nat Protoc. 2006;1:1732–1742. doi: 10.1038/nprot.2006.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- [20].Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- [21].Weeks JR. Long-term intravenous infusions. In: Myers RD, editor. Methods in Psychobiology. Vol. 2. Academic Press; New York: 1972. pp. 155–168. [Google Scholar]
- [22].White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. Journal of Pharmacology & Experimental Therapeutics. 1995;273:445–454. [PubMed] [Google Scholar]
- [23].Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. Journal of Pharmacology & Experimental Therapeutics. 1997;281:699–706. [PubMed] [Google Scholar]
- [24].Zito KA, Vickers G, Roberts DC. Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacology, Biochemistry & Behavior. 1985;23:1029–1036. doi: 10.1016/0091-3057(85)90110-8. [DOI] [PubMed] [Google Scholar]