Abstract
Background
Functional impairment of the orbital and medial prefrontal cortex underlies deficits in executive control that characterize addictive disorders, including alcohol addiction. Previous studies indicate that alcohol alters glutamate neurotransmission and one substrate of these effects may be through the reconfiguration of the subunits constituting ionotropic glutamate receptor (iGluR) complexes. Glutamatergic transmission is integral to cortico-cortical and cortico-subcortical communication, and alcohol-induced changes in the abundance of the receptor subunits and/or their splice variants may result in critical functional impairments of prefrontal cortex in the alcohol-addicted state.
Methods and results
The effects of chronic ethanol self-administration on glutamate receptor ionotropic NMDA (GRIN), as well as GRIN1 splice variant mRNA expression was studied in the orbitofrontal cortex (OFC; Area 13), dorsolateral prefrontal cortex (DLPFC; Area 46) and anterior cingulate cortex (ACC; Area 24) of male cynomolgus monkeys. Chronic ethanol self-administration resulted in significant changes in the expression of NMDA subunit mRNA expression in the DLPFC and OFC, but not the ACC. In DLPFC, the overall expression of NMDA subunits was significantly decreased in ethanol treated monkeys. Slight but significant changes were observed for synaptic associated protein 102 kD (SAP102) and neuronal nitric oxide synthase (nNOS) mRNAs. In OFC, the NMDAR1 variant GRIN1-1 was reduced while GRIN1-2 was increased. Furthermore, no significant changes in GFAP protein levels were observed in either the DLPFC or OFC.
Conclusion
Results from these studies provide the first demonstration of post-transcriptional regulation of iGluR subunits in the primate brain following long-term ethanol self-administration. Furthermore, changes in these transcripts do not appear to reflect changes in glial activation or loss. Further studies examining the expression and cellular localization of subunit proteins and receptor pharmacology would shed more light on the findings reported here.
Keywords: Ethanol, Glutamate, messenger RNA, Prefrontal Cortex, qPCR, Primate
Introduction
Chronic and excessive alcohol consumption can lead to changes in neuronal structure and function in several brain regions, including the frontal cortex. Previous studies in post-mortem tissue from alcoholics have reported decreased neuronal density in superior prefrontal cortex (Harper and Kril 1991) and cell soma atrophy in the superior frontal and anterior cingulate cortices (Harper and Kril 1989) that is accompanied by decreased dendritic arborization (Harper and Corbett 1990) and loss of large pyramidal neurons in these regions (Harper and Kril 1989). Neuroimaging studies in alcoholics have reported decreased gray and white matter volumes (Jernigan et al. 1991; Pfefferbaum et al. 1997; Pfefferbaum et al. 1998), decreased regional cerebral blood flow (Dally et al. 1988; Melgaard et al. 1990) as well as reduced glucose metabolism in the frontal cortex (Adams et al. 1993; Dao-Castellana et al. 1998).
These structural and metabolic changes are associated with deficits in selective attention, working memory, behavioral inhibition and attribution of stimulus salience (Thorpe et al. 1983; Volkow et al. 1996; Volkow et al. 1999; Elliott et al. 2000b; Elliott et al. 2000a; Goldstein and Volkow 2002; Goldstein et al. 2002). All of these functions are mediated by prefrontal cortex, with attention and working memory more associated with dorsolateral areas and behavioral inhibition more associated with ventral and medial fields. Based on this, we sampled individual fields from each of theses regions: area 46 from dorsolateral cortex, area 13 from orbital cortex and area 24a from medial cortex.
The findings described above were from human subjects with many decades of alcohol exposure and who lived in highly variable environments. It is not clear if the changes described above are due simply to alcohol or whether more subtle changes take place that are due just to alcohol and which may appear long before overt neuropathology is present. Because studies in rodents indicate that alcohol alters glutamatergic synaptic transmission and studies in rodents and humans suggest that the alterations in symaptic transmission are due at least in part to subunit reorganization and expression (Bruckner et al. 1997; Pickering et al. 2007; Raeder et al. 2008; Ridge et al. 2008), we sought to examine this systematically in our model of chronic ethanol self-administration.
Glutamate is the excitatory transmitter responsible for most communication within the nervous system. Even subtle alterations in glutamate-related signaling might therefore disrupt prefrontal cortical information processing. Glutamate signaling is mediated by both metabotropic and ionotropic receptors with the latter implicated in synaptic strength and maintenance as well as a variety of cellular activities including neuronal development, learning and memory, and the reinforcing and neuropathological effects of abused substances (Kalivas et al. 2003; Kalivas et al. 2005).
Ionotropic glutamate receptors (iGluRs) are divided into three classes based on pharmacology and subunit composition – NMDA, AMPA and kainate – each consisting of a combination of four subunits to form a functional ionophore. NMDA receptors require the expression of NR1 in combination with one or more NR2 (A-D) or NR3 (A-B) subunits. The NR1 subunit exists as one of eight splice variants distinguished by the inclusion or exclusion of a single N-terminal cassette (N1, exon 5) and/or two C-terminal cassettes, exons 21 and 22 (C1, C2 respectively). Additional biochemical diversity is provided by the existence of splice variants of the NMDAR1 subunit that confer different pharmacological and biochemical properties on the receptor, affect intracellular signaling (Hollmann et al. 1993; Traynelis et al. 1995; Koltchine et al. 1996; Dingledine et al. 1999) and affect receptor subunit trafficking (Horak and Wenthold 2009). These splice variants have been shown to be differentially regulated in various brain regions and during different stages of development, cellular activity, drug treatment and disease processes (Meshul et al. 1996; Le Corre et al. 2000; Loftis and Janowsky 2002; Guilarte and McGlothan 2003; Nagy et al. 2003; Hynd et al. 2004).
In addition to receptor subunit and NR1 splice variant expression, changes in subcellular localization and trafficking of subunits have been shown to contribute to alterations in receptor function (Kumar et al. 2003; Kumar et al. 2004). NMDA receptor localization and association with scaffolding proteins, such as those of the membrane-associated guanylate kinase (MAGUK) family including post-synaptic density 95 (PSD-95) and synaptic associated protein 102 (SAP102), play important roles in the control of downstream signals resulting from NMDA receptor activation (Elias and Nicoll 2007; Lau and Zukin 2007) and may well be affected by ethanol.
Based on available data, we hypothesized that chronic ethanol self-administration would induce differential patterns of expression of NMDA receptor subunit transcripts and NR1 splice variants indifferent prefrontal cortical fields. Therefore, we examined the mRNA expression of NMDA subunits, six NR1 splice variants as well as synaptic proteins, PSD-95, SAP102 and nNOS, associated with the NMDA receptor complexes.
Results
Ethanol self-administration
Individual drinking patterns of the six monkeys included in this study have been reported previously (Monti et al. 2004; Hemby et al. 2006; Anderson et al. 2007). As previously reported, the average daily intakes for 12 months of self-administration ranged from 1.0 -3.33 g/kg ethanol and the average blood ethanol concentrations ranged from 68-188 g/kg (Anderson et al., 2007). Mean daily intake, total intake and blood ethanol concentrations are provided in Table 1. Estimations of these intakes in terms of human drink-equivalents is roughly 0.25 g/kg per drink, or a range of 6-15 drinks/day during the last 6 months of self-administration prior to the brain being harvested. These levels of drinking are considered moderately heavy to heavy in terms of human ethanol consumption (Vivian et al. 2001; Grant et al. 2008).
Table 1. Ethanol Consumption for monkeys used in this study.
Daily intake values and blood ethanol concentrations represent mean ± S.E.M.
| Subject | Daily Intake (g/kg) | Total Intake (g/kg) | Blood Ethanol Concentration (n) |
|---|---|---|---|
| 5404 | 3.33 ± 0.24 | 2289.8 | 188 ± 17 (33) |
| 5497 | 1.76 ± 0.10 | 1670.8 | 105 ± 11 (33) |
| 6101 | 1.85 ± .012 | 1524.2 | 100 ± 9 (28) |
| 6304 | 1.17 ± 0.13 | 897.8 | 63 ± 13 (30) |
| 6305 | 1.49 ± 0.08 | 1184.5 | 62 ± 10 (30) |
| 6306 | 2.70 ± 0.17 | 2757.7 | 173 ± 10 (35) |
NMDA subunit mRNA expression
DLPFC (Area 46)
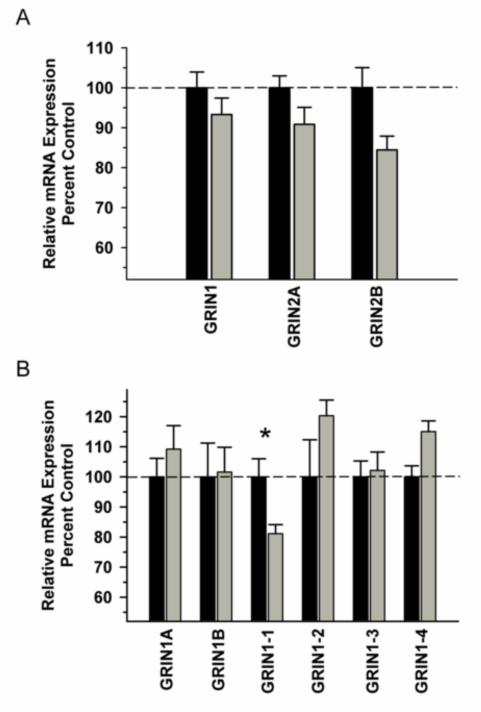
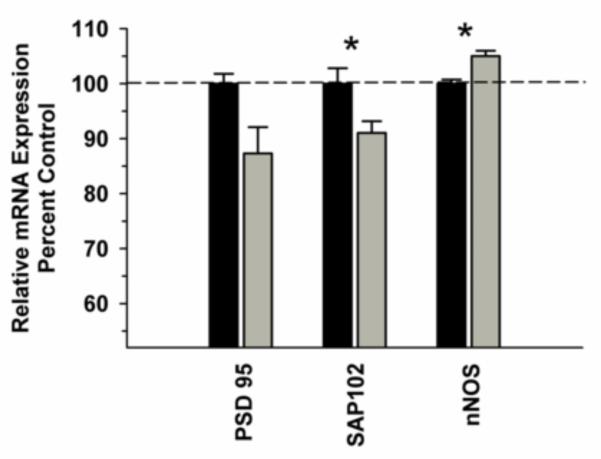
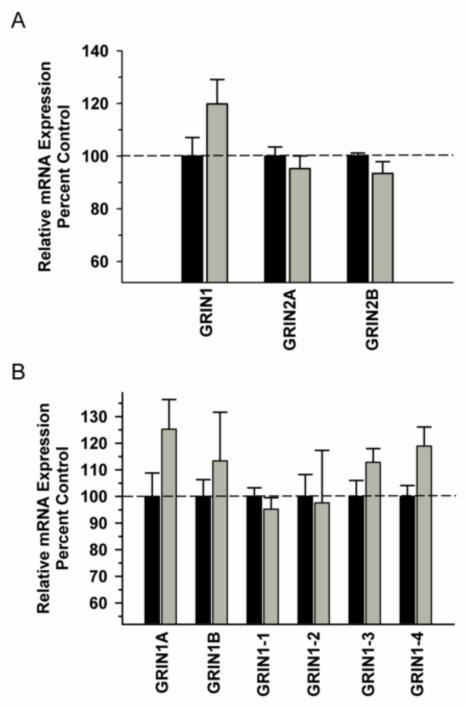
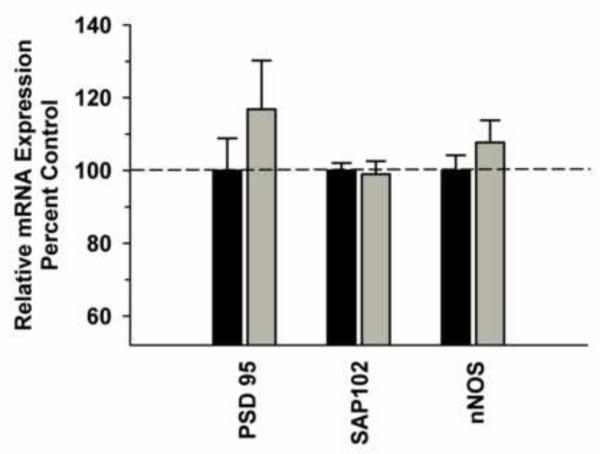
In the DLPFC, we observed a significant difference in NMDA receptor subunit mRNA (GRIN 1, GRIN 2A, GRIN 2B) expression between the groups [F(1,29)=11.486, P=0.002]; however, there was no significant Group x Subunit interaction [F(2,29)=0.688; P=0.512] (Figure 1A). Analysis of GRIN1 variants revealed a trend towards a significant Group x Variant interaction [F(5,59)=2.306; P=0.059] with post hoc analysis revealing a significant decrease in variant GRIN1-1 in the ethanol group (Figure 1B). Analysis of synaptic associated protein mRNAs in the DLPFC revealed increased nNOS [F91,9)=16.435, P=0.004) and decreased SAP102 [F(1,9)=8.234, P=0.021) as well as a trend towards decreased PSD 95 [F(1,9)=5.127, P=0.053) in the ethanol group (Figure 2). Correlational analysis revealed significant positive correlations between GRIN1A mRNA levels and average daily intake (r2=0.819, P=0.0462) and between PDS95 mRNA levels and average daily intake (r2=0.955, P=0.00294) and average blood ethanol concentrations (r2=0.913, P=0.0109) (Table 2).
Figure 1.
Effect of chronic self-administration by cynomolgus macaques on NMDA subunit expression in DLPFC. Ethanol induced a significant decrease in NMDA receptor subunit expression; however, there was no Group x Subunit interaction (A). A trend towards a significant interaction was observed for GRIN1 splice variant expression, with post hoc analysis revealing a significant decrease GRIN1-1 in the ethanol group (B). Black bar – control subjects, gray bar – ethanol subjects. Asterisks indicate a significant difference compared to control subjects (P<0.05).
Figure 2.
Effect of chronic self-administration on PSD-95, SAP102 and nNOS mRNA expression in the DLPFC. Ethanol induced a significant decrease in SAP102 mRNA, a significant increase in nNOS mRNA and a slight decrease in PSD95 mRNA levels (P=0.053). Black bar – control subjects, gray bar – ethanol subjects. Asterisks indicate a significant difference compared to control subjects (P<0.05).
Table 2. Correlational analysis of gene expression with average daily intake, total intake and average blood ethanol concentrations.
Values expressed are Pearson correlation coefficients (r2) for the six male monkeys that consumed alcohol in the present study.
| DLPFC | OFC | ACC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Daily Intake |
Total Intake |
BEC | Daily Intake |
Total Intake |
BEC | Daily Intake |
Total Intake |
BEC | |
| GRIN1 | 0.381 | 0.539 | 0.376 | 0.288 | 0.402 | 0.222 | 0.326 | 0.451 | 0.405 |
| GRIN2A | 0.321 | −0.0166 | 0.120 | 0.680 | 0.596 | 0.587 | 0.654 | 0.521 | 0.544 |
| GRIN2B | 0.471 | 0.586 | 0.473 | 0.757 | 0.896 * | 0.768 | 0.528 | 0.742 | 0.551 |
| GRIN1-1 | 0.675 | 0.292 | 0.587 | 0.558 | 0.595 | 0.609 | −0.0258 | −0.0652 | −0.119 |
| GRIN1-2 | 0.112 | 0.101 | 0.00863 | 0.479 | 0.446 | 0.458 | 0.633 | 0.244 | 0.502 |
| GRIN1-3 | 0.0731 | −0.193 | 0.0216 | 0.278 | −0.0216 | 0.232 | 0.132 | −0.219 | −0.0453 |
| GRIN1-4 | −0.250 | −0.271 | −0.209 | 0.263 | −0.0647 | 0.240 | 0.737 | 0.384 | 0.643 |
| GRIN1A | 0.819 * | 0.595 | 0.770 | 0.663 | 0.642 | 0.588 | 0.459 | 0.420 | 0.447 |
| GRIN1B | −0.256 | −0.0326 | −0.248 | 0.0817 | 0.198 | 0.0337 | 0.511 | 0.368 | 0.437 |
| PSD95 | 0.955 ** | 0.765 | 0.913 * | 0.422 | 0.704 | 0.455 | 0.462 | 0.491 | 0.497 |
| SAP102 | 0.548 | 0.526 | 0.452 | 0.717 | 0.633 | 0.633 | 0.240 | 0.298 | 0.208 |
| nNOS | 0.142 | −0.180 | −0.0649 | 0.194 | 0.313 | 0.111 | 0.523 | 0.490 | 0.582 |
P<0.05
P<0.01
OFC (Areas 13a and 13m)
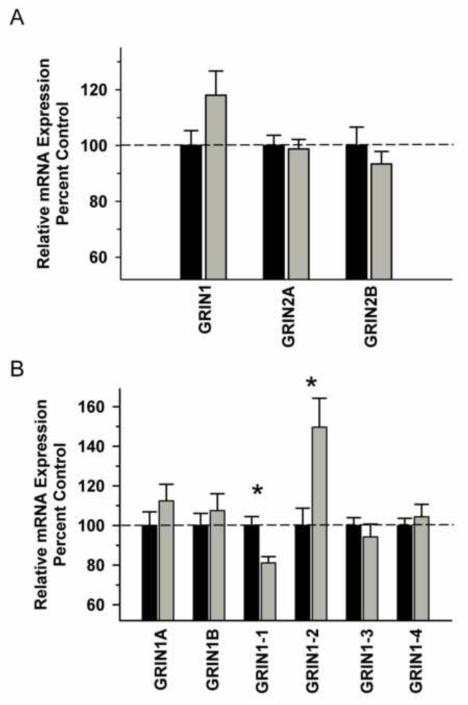
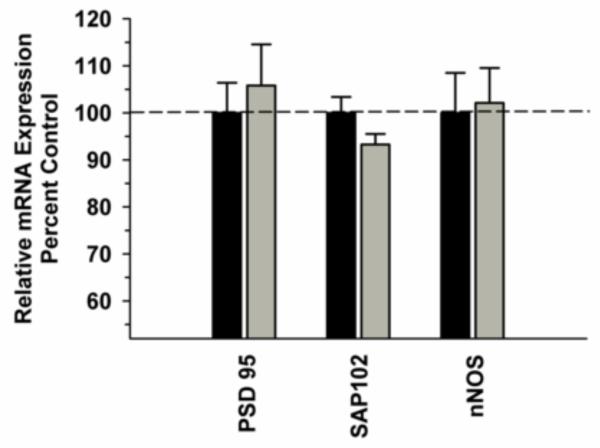
In the OFC, there was no significant difference in NMDA receptor subunit mRNAs between the Groups [F(1,29)=0.556, P=0.463] and no significant Group x Subunit interaction [F(2,29)=2.657, P=0.082] (Figure 3A). In contrast, there was a significant interaction between Group and GRIN variants, due to decreased GRIN 1-1 (P=0.035) and increased in GRIN 1-2 (P=0.001) in the ethanol group (Figure 3B). No significant changes in any of the synapse-associated proteins investigated were observed between the ethanol and control groups (Figure 4).Correlational analysis revealed a significant positive correlation between GRIN2B mRNA levels and total ethanol intake (r2=0.896, P=0.0158), but not for any of the other transcripts for this region (Table 2).
Figure 3.
Effect of chronic self-administration by cynomolgus macaques on NMDA subunit expression in OFC. Ethanol self-administration did not affect NMDA receptor subunit mRNA expression in this region (A). A significant interaction was observed for GRIN1 splice variant expression, with post hoc analysis revealing a significantly decreased GRIN1-1 expression and increased GRIN1-2 expression in the ethanol group (B). Black bar – control subjects, gray bar – ethanol subjects. Asterisks indicate a significant difference compared to control subjects (P<0.05).
Figure 4.
Effect of chronic self-administration on PSD-95, SAP102 and nNOS mRNA expression in the OFC. No significant differences were observed between the groups for PSD-95, SAP102 and nNOS expression. Black bar – control subjects, gray bar – ethanol subjects.
ACC (Areas 24a and24b)
In contrast to the other prefrontal regions, no significant Main Effect or interactions were observed for any of the GRIN subunits tested (Figure 5 A and B). No significant differences were detected between ethanol and control groups with regard to GRIN1 variant expression in this field. In addition, there were no significant differences in any of the synapse-associated proteins investigated (Figure 6). No significant correlations between transcript levels and average daily intake, total intake or average blood ethanol concentrations were observed (Table 2).
Figure 5.
Effect of chronic self-administration by cynomolgus macaques on NMDA subunit expression in ACC. Ethanol induced a significant decrease in NMDA receptor subunit expression; however, there was no Group x Subunit interaction (A). A trend towards a significant interaction was observed for GRIN1 splice variant expression, with post hoc analysis revealing a significant decrease GRIN1-1 in the ethanol group (B). Black bar – control subjects, gray bar – ethanol subjects. Asterisks indicate a significant difference compared to control subjects (P<0.05).
Figure 6.
Effect of chronic self-administration on PSD-95, SAP102 and nNOS mRNA expression in the ACC. Ethanol induced a significant decrease in PSD-95 and SAP102 mRNAs and a slight increase in nNOS expression. Black bar – control subjects, gray bar – alcohol subjects. Asterisks indicate a significant difference compared to control subjects (P<0.05).
GFAP Western Blot Analysis
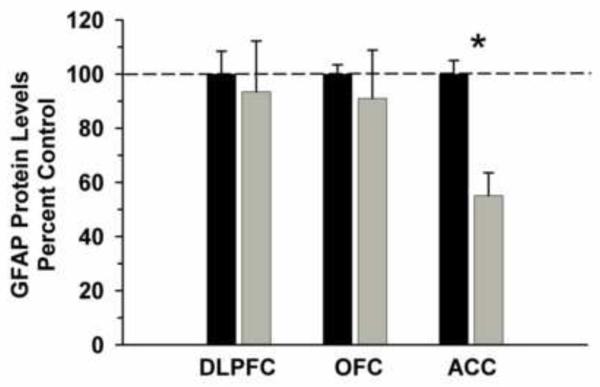
We examined GFAP protein levels in cytosolic fractions as a marker of glial cell loss and a measure of possible brain damage that was not visible either in the gross brain specimens or Nissl stained sections. . Ethanol consumption resulted in significantly decreased levels of GFAP in the ACC (t=-7.147, df=8, P<0.01), but not in the DLPFC (t=-0.313, df=8, P=0.762) or OFC (t=-0.581, df=5, P=0.577). For the ACC, there was no significant correlation between GFAP protein levels and daily intake (r2=-0.026, P=0.961), total intake (r2=-0.239, P=0.649) or BEC (r2=-0.107, P=0.840).
Discussion
The current findings are the first demonstration in any primate species that chronic ethanol self-administration can lead to areal specific changes in the expression of iGluR subunit mRNAs in the prefrontal cortex. This provides a possible molecular basis for how glutamatergic transmission is altered by chronic ethanol consumption (Ward et al. 2009). Ethanol self-administration resulted in significant changes in the DLPFC and OFC, but not the ACC – a field-specific distribution of effects like the one we previously reported for changes in GABAA receptor subunit mRNAs (Hemby et al. 2006).
In DLPFC, NMDA receptor subunit expression was significantly decreased and slight but significant changes were observed for SAP102 and NOS1 mRNA expression as well. In the OFC, the NMDAR1 variant GRIN1-1 was decreased and GRIN1-2 was increased. Because GFAP, a marker of astrocytic activation and glial content was not significantly altered in these fields, it is reasonable to suggest that the observed changes in NMDA receptor subunit expression were not due to neurodegeneration or glial cell loss.A significant decrease in GFAP was detected in the ACC. These data support and expand the results of studies in humans implicating glutamate dysregulation in alcohol abuse (Gass and Olive 2008; Ridge et al. 2008; Schumann et al. 2008; Ridge and Dodd 2009) and provide a potential molecular marker of prefrontal dysregulation in alcoholics (Dao-Castellana et al. 1998; O’Neill et al. 2001; Goldstein et al. 2004).
Alcohol abuse can be characterized in part by persistent drug-seeking and - taking behaviors that are mediated in part by the DLPFC and OFC. For example, OFC damage leads to deficits in decision-making strategies that depend on the analysis of likely outcome values to arrive at the most profitable outcome (Bechara et al. 1994; Bechara et al. 2000; Pears et al. 2003; Pickens et al. 2005).Alcoholics who who routinely consume large quantities of alcohol despite obvious long-term negative consequences exhibit a similar behavioral deficit (Bechara et al. 2001; Bechara and Damasio 2002; Bechara 2003). The DLPFC, OFC and ACC are commonly reported areas of cue-induced activation by abused substances, including alcohol (Wilson et al. 2004). Previous studies have reported that alcohol craving is associated with increased functional activity of the DLPFC (George et al. 2001; Wrase et al. 2002; Tapert et al. 2004; Olbrich et al. 2006) and OFC (Wrase et al. 2002; Tapert et al. 2003; Myrick et al. 2004).
Monkey are an advantageous model in which to perform these kinds of studies both because their drinking patterns mimic human patters because the monkey prefrontal cortex shares structural similarities with humans that are not present in rodents. In the rat, the PFC is composed only of a set of agranular fields (e.g., dorsal and ventral anterior cingulate, prelimbic and infralimbic fields). Monkey and human brains, by contrast, contain all of these agranular fields as well as dysgranluar and granular fields not seen in the rodent (Carmichael and Price 1994; Ongur and Price 2000).
The mechanisms which mediate the functional dysregulation of prefrontal cortex following alcohol administration are not well understood, but likely involve glutamatergic mechanisms. Studies in rodent cortex indicate either no change or increased NMDA receptor subunit mRNA expression (Morrow et al. 1994; Follesa and Ticku 1995; Snell et al. 1996; Kalluri et al. 1998; Hardy et al. 1999; Pickering et al. 2007; Raeder et al. 2008) depending on route of administration, duration of drinking and time of tissue collection. In humans, NMDA receptor subunit mRNA expression (NR1, 2A and 2B) is decreased in superior frontal and primary motor cortex of cirrhotic alcoholics when subjects were portioned according to the 5HTTLPR allele (Ridge et al. 2008). Because of the neurotoxic nature of chirrhosis, it is not clear what the causes of these changes may have been. Our current report of a significant decrease in NMDA receptor subunit mRNAs as well as decreased NR1-1 variants in the DLPFC and decreased NR1-1 and increased NR1-2 variant mRNA levels in the OFC following chronic ethanol self-administration, suggest that the changes seem in human subjects are indeed due to acohol.
In addition to changes in receptor subunit mRNA expression, other proteins such as PSD95, SAP102 and nNOS interact with NMDA receptors and contribute to synaptic strength and plasticity. PSD-95 and SAP102 are members of the membrane associated guanylate kinase family of proteins, which couple NMDA receptors to signaling complexes that regulate activity-dependent changes in synaptic strength and participate in NMDA receptor-dependent forms of synaptic plasticity such as long-term potentiation (LTP) and long-term depression (Migaud et al. 1998; Beique and Andrade 2003; Stein et al. 2003; Yao et al. 2004; Beique et al. 2006; Cuthbert et al. 2007). Previous studies have shown that nNOS contributes to NMDA receptor induced neuroplasticity as well as cerebral blood flow changes induced by NMDA receptor activation (Iadecola and Nedergaard 2007; Garthwaite 2008). Nitric oxide (NO) is synthesized via nNOS which is linked to the NR2 subunit of the NMDAR via PSD95 (Brenman and Bredt 1997; Christopherson et al. 1999). The biological relevance of the slight, but statistically significant, increase in nNOS mRNA expression in the DLPFC following chronic alcohol exposure deserves further investigation.
The present findings provide additional important insight into potential mechanisms of prefrontal cortical dysregulation following chronic alcohol use. Previously, using the same subjects examined in the present study, we reported regulation of specific GABA-A subunit mRNA expression in the DLPFC and OFC, but not the ACC, (Hemby et al. 2006). Thus, changes in both NMDA and GABA-A receptor configuration in DLPFC and OFC may underlie the decision making deficits resulting from chronic alcohol abuse (Bechara and Damasio 2002; Goldstein and Volkow 2002; Bechara 2003). Examination of the effects of ethanol on protein levels of receptor subunit expression, the class of neurons that are primarily affected, and the expression of related trafficking and anchoring proteins and their genes within these prefrontal regions will be fundamental to ascertaining the molecular pathology of prefrontal dysregulation in alcoholism.
Experimental Procedures
Subjects and ethanol self-administration
Ten adult male cynomolgus monkeys (Macaca fascicularis; 5.5 – 6.5 years at beginning of experiment) were subjects for the present experiments (n=6 ethanol group, n=4 control group). The drinking model and the animals behavior has been extensively analyzed elsewhere. Six adult male cynomolgus monkeys were quarantined for 2 months and then transferred to the laboratory primate housing room and assigned a cage within a quadrant rack as described previously. Attached to one wall of each monkey’s home cage was an operant panel that allowed access to all fluid and food requirements. Monkeys were trained to operate the drinking panel in daily 60-minute sessions and then induced to drink water and later ethanol (4% w/v in water). Following 120 days of induction, scheduled pellet delivery was discontinued. For 6 months, ethanol and water were available ad libitum and food was available in meals during daily 16-hour sessions. Following that, the monkeys underwent ethanol abstinence for 12 months. After the abstinence period, the monkeys were again allowed access to 4% ethanol, water, or food from the panel for 22 h/d for a range of 558 to 595 consecutive days (approximately 18 months). During the ad libitum period monkeys were allowed to self-administer 4% (w/v) ethanol daily, with the volume and rate of alcohol consumed determined solely by the monkey. Once the animals are given free access to ethanol, the onset of daily 22-hour sessions are signaled by the illumination of amber stimulus lights above both drinking spouts. Both 4% (w/v) alcohol and water were available at all times during the daily 22-hour sessions. In addition, a “meal structure” was imposed so monkeys were required to eat the daily allotment of food in no less than three “meals,” with at least two hours between each meal.
Experimentally naïve control subjects (n=4) were the same as those described previously (Ivester et al. 2007). Following 2 months of quarantine, monkeys were placed on the same diet as the ethanol-drinking animals (Vivian et al. 2002) and had very similar daily routines as the ethanol-drinking animals. Control individuals remained in the laboratory for 6 months prior to euthanasia.
Blood samples (20 μL) for the analysis of blood ethanol concentrations (BEC) were taken from the saphenous vein every fifth day from every monkey just before the lights turning off in the room and approximately seven hours following the onset of the session. Blood samples were sealed in air-tight vials containing 500 μl of distilled water and 20 μL of isopropanol (10%; internal standard) and stored at −4°C until assay using a gas chromatograph (Hewlett-Packard 5890 Series II, Avondale, PA) equipped with a headspace autosampler, flame ionization detector, and a Hewlett Packard 3392A integrator. All behavioral studies were conducted in the laboratory of Dr. Grant at Wake Forest University. The care of the animals and euthanasia procedures in this study were performed according to the National Institutes for Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Wake Forest University.
Necropsy
Following euthanasia, the frontal cortex was dissected from the rest of the brain and the orbitofrontal cortex (OFC; Area 13), anterior cingulate cortex (ACC; Area 24) and dorsolateral prefrontal cortex (DLPFC; Area 46) were dissected as described previously (Hemby et al. 2006). The OFC dissection included the medial and lateral aspects of the medial orbital sulcus located rostral to the rostral end of the corpus callosum, and therefore included Areas 13a and 13m (Carmichael and Price 1994). The ACC was dissected from the rostral pole of the corpus callosum and included tissue from the cingulate gyrus up to but not including the lower bank of the cingulate sulcus, and therefore contained fields 24a and 24b. The DLPFC (Area 46) was dissected from the banks of the principle sulcus midway along its length.
Messenger RNA abundance
RNA Isolation and cDNA synthesis
Total RNA was isolated using Trizol (Sigma-Aldrich, St. Louis, MO) followed by chloroform extraction/isopropanol precipitation and stored at −80°C. 2 μg of total RNA from each sample, as well as a pool of total RNA combined from the 10 cynomologus monkeys, was reverse transcribed using random primers and SuperScript III kits (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Resulting cDNA product was diluted 1:100 with RNAse-free water for samples, and cDNA from the pooled samples was serially diluted in 2-fold dilutions from 1:20-1:640 for use as standards.
Real-time quantitative PCR
Standard Taqman assays were purchased for measurement of specific NMDA receptor subunit levels (GRIN1: Hs00609557_m1; GRIN2A: Hs00168219_m1; GRIN2B: Hs00168230_m1, PSD95: Hs00176354_m1; SAP102: Hs01020278_m1; nNOS: Hs00167223_m1, Applied Biosystems, Foster City, CA). Custom Taqman assays were designed for six NR1 splice variants in collaboration with Applied Biosystems (Foster City, CA). Two assays distinguish between the N-terminal variants (exon 5), NR1A and NR1B, while four assays distinguish between the C-terminal variants (exons 21 and 22), NR1-1, NR1-2, NR1-3 and NR1-4. All assays used FAM reporter dye and NFQ quencher and were designed such that the Taqman probe spanned the exon-exon junction (or included exon) that is specific to the splice variant being measured. Sequence information for these assays is listed in Table 2.
Using a 384 well format with the ABI Prism 7900HTS real-time detector, 0.5 l aliquots of Taqman Expression Assay (20X), 5.0 l 2X Absolute QPCR ROX PCR Mastermix (Abgene), and 4.5μl diluted cDNA (either sample or pooled standard) were mixed together and pipetted into single wells of the PCR plate. For no template controls (NTC) for each gene tested, water was added in lieu of cDNA. Each sample, including NTC was run in triplicate. Thermocycling conditions: 1) one cycle 2 min at 50°C, 2) one cycle 15 min at 95°C, and 3) 40 cycles 15 sec at 95°C and 1 min at 60°C. Fluorescence was measured during the 60°C step for each cycle. Reactions were quantified by the standard curve method using SDS2.1 software generating a mean quantity value (Qty mean) for each sample from the triplicates of that sample for each gene of interest. Endogenous controls were selected from a set of seven candidate reference transcripts: -actin (ACTB), ribosomal protein 18S (18S), TATA box binding protein (TBP), hypoxanthine phosphoribosyltransferase 1 (HPRT1), peptidylprolyl isomerase A (cyclophilin A) (PPIA), -glucuronidase (GUSB), and phosphoglycerate kinase 1 (PGK1) using geNorm software. geNorm is a collection of VBA macros for Microsoft Excel which allows the determination of the most stable reference genes from a given test panel of genes. By computing the average pairwise variation (V) for each control gene paired with all other tested control genes, geNorm calculates the gene expression stability measure (M) (Vandesompele et al. 2002). This allows for the selection of the most stably expressed control genes in a given sample set, minimizing any bias in the data as a result of normalization (see Supplementary Data). The gene expression normalization factor is calculated based on the geometric mean of a user-defined number of reference genes (Vandesompele et al. 2002). Three candidate genes (PGK1, Hs99999906_m1; TBP, Hs99999910_m1; and HPRT, Hs99999906_m1) were selected to serve as endogenous controls. Data for each gene of interest was expressed as Qty mean for the gene of interest/geometric mean of Qty mean values for the selected endogenous control genes. Normalized values are expressed as percent control.
Western Blot analysis
Cytoslic fractions were collected from tissue samples as described previously in (Tang et al. 2004; Hemby et al. 2005a; Hemby et al. 2005b). Protein concentrations were calculated using the bicinochoninic acid protein assay kit (Pierce, Rockford, IL) and diluted in Laemmli sample buffer to achieve the equivalent final protein concentrations. Sample buffer is added to 10 μg of protein from each sample and heated to 95°C for 5 minutes. Proteins were separated on pre-cast 10% Tris-HCl SDS-PAGE gels (Biorad) and transferred to nitrocellulose membranes. Membranes were blocked an incubated with the anti-GFAP antibody (1:5000; Synaptic Systems; Goettingen, Germany) overnight in Odyssey blocking buffer (Licor, Lincoln, NE). Visualization was accomplished with AlexFluor680 and IRDye800 labeled secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA and Molecular Probes, Eugene, OR). Blots were scanned with a Licor Odyssey infrared scanner and signals quantified with Odyssey version 1.2 software. Equal protein loading and efficiency of transfer were confirmed by probing blots with an anti-neuronal tubulin antibody (1:15,000; Millipore, Billerica, MA). Background-subtracted intensity values for each sample were calculated.
Currently available NR1 splice variant antibodies will detect the N1, C1, C2 and C2′ cassettes, respectively, but will not differentiate splice variants NR1-1 from NR1-2 (both contain the C2 cassette), NR1-1 from NR1-3 (both contain the C1 cassette) or NR1-3 from NR1-4 (both contain the C2′ cassette) and therefore would not enable correlative analysis of protein levels for the current study.
Statistical Analysis
Levels of gene expression were calculated using SDS 2.1 software (Applied Biosystems, Foster City, CA) to interpolate the Ct values for each well onto a standard curve generated from the Ct values of a dilution series of standards. These quantity values were then averaged across triplicates after removal of outliers and expressed relative to the quantity value for the mean of the endogenous control genes measured in the same sample on the same plate. This relative value (gene of interest Qty mean/endogenous control Qty mean) was used for subsequent statistical analysis. Experiments determining relative gene expression for each candidate gene were run independently. For each gene, two-way analysis of variance was employed with Group (Ethanol and Control) and Subunit (per receptor class) as the Main factors and mRNA abundance as the dependent variable. All subunits for a receptor class were analyzed for each brain regions separately. Bonferroni’s test was used for post hoc analyses. For Western blot analysis of GFAP, data were analyzed using a one way ANOVA with hybridization intensity as the dependent variable. Post-hoc analysis was performed using Tukey’s test. Null hypotheses were rejected when P<0.05.
Supplementary Material
Supplemental Figure: Average expression stability (M) values of the seven selected control genes for the DLPFC (A), OFC (B) and ACC (C). As explained in the Methods section, the GeNorm algorithm provides a gene-stability measure to determine the expression stability of control genes based on non-normalized expression levels such that genes with the lowest M values have the most stable expression. The algorithm uses a stepwise exclusion of the genes with the highest M values (least stable) and recalculating new M values for the remaining genes (Vandesompele et al., 2002).
Figure 7.
GFAP protein levels in prefrontal cortical brain regions of ethanol consuming and control cynomolgus monkeys. Cytosolic fractions were isolated and separated on 10% SDS-PAGE. Data are expressed as mean (± S.E.M.) of the percent of control values per amount of protein loaded. Asterisks indicate a significant difference (P<0.05).
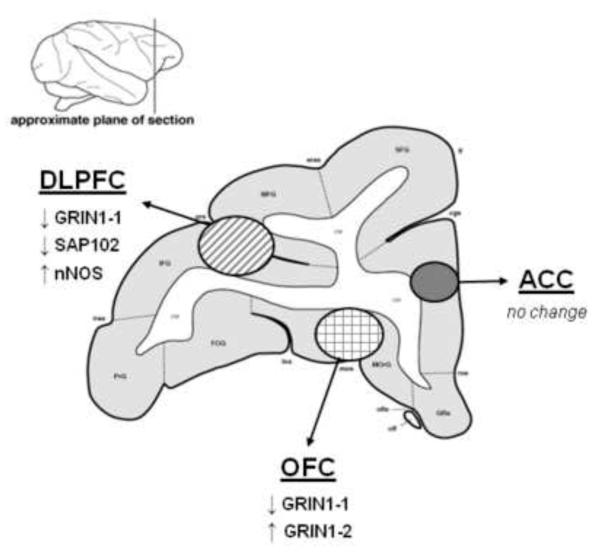
Figure 8.
Summary of changes in NMDA receptor subunit and NMDAR1 variants in vulnerable prefrontal cortical regions following ethanol consumption in cynomolgus monkeys.
Table 3.
Sequence information for custom-designed NR1 splice variant TaqMan assays
| Gene | Probe Sequence | Forward Primer | Reverse Primer | Size |
|---|---|---|---|---|
| NR1a | TCTGCCTTGGACTCAGG | CGACGACCACGAGGGC | GACCCGGGCCTCCAG | 151 |
| NR1b | AACCTCGACCAACTGTCC | TCAGCGACGACCACGAG | GCACCTTCTCTGCCTTGGG | 147 |
| NR1-1 | CCGGTGCTCGTGTCTT | CGGAAGAACCTGCAGGATAGAAAG | CGGCAGCACTGTGTCTTTT | 180 |
|
| ||||
| NR1-2 | CCGGTGCTCTGCAGGTT | AGCGGCACAAGGATGCT | GGCCCTCCTCCCTCTCAATAG | 159 |
| NR1-3 | ATGGTACTGCGTGTCTTT | GAAGAACCTGCAGGATAGAAAGAGT | TGCTGACCGAGGGATCTGA | 182 |
| NR1-4 | ACTGCTGCAGGTTCTT | GGCCGGGATCTTCCTGATTT | GCTGACCGAGGGATCTGAGA | 172 |
Acknowledgements
Research contained in this manuscript was supported in part by NIAAA grants P20 AA011997, U01 AA013510 (KAG) and the Wake Forest University Health Sciences (SEH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, Berent S, Kroll PD. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003;19:23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beique JC, Andrade R. PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex. J Physiol. 2003;546:859–867. doi: 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Bredt DS. Synaptic signaling by nitric oxide. Curr Opin Neurobiol. 1997;7:374–378. doi: 10.1016/s0959-4388(97)80065-7. [DOI] [PubMed] [Google Scholar]
- Bruckner MK, Rossner S, Arendt T. Differential changes in the expression of AMPA receptors genes in rat brain after chronic exposure to ethanol: an in situ hybridization study. J Hirnforsch. 1997;38:369–376. [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O’Dell TJ, Grant SG. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27:2673–2682. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dally S, Luft A, Ponsin JC, Girre C, Mamo H, Fournier E. Abnormal pattern of cerebral blood flow distribution in young alcohol addicts. Br J Addict. 1988;83:105–109. doi: 10.1111/j.1360-0443.1988.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000a;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000b;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Follesa P, Ticku MK. Chronic ethanol treatment differentially regulates NMDA receptor subunit mRNA expression in rat brain. Brain Res Mol Brain Res. 1995;29:99–106. doi: 10.1016/0169-328x(94)00235-7. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Chang L, Wang GJ, Fowler JS, Depue RA, Gur RC. The orbitofrontal cortex in methamphetamine addiction: involvement in fear. Neuroreport. 2002;13:2253–2257. doi: 10.1097/01.wnr0000044215.09266.bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL. Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Brain Res Mol Brain Res. 2003;113:37–43. doi: 10.1016/s0169-328x(03)00083-4. [DOI] [PubMed] [Google Scholar]
- Hardy PA, Chen W, Wilce PA. Chronic ethanol exposure and withdrawal influence NMDA receptor subunit and splice variant mRNA expression in the rat cerebral cortex. Brain Res. 1999;819:33–39. doi: 10.1016/s0006-8993(98)01340-7. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. J Neurol Sci. 1989;92:81–89. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Harper C, Corbett D. Changes in the basal dendrites of cortical pyramidal cells from alcoholic patients--a quantitative Golgi study. J Neurol Neurosurg Psychiatry. 1990;53:856–861. doi: 10.1136/jnnp.53.10.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J. If you drink your brain will shrink. Neuropathological considerations. Alcohol Alcohol Suppl. 1991;1:375–380. [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res. 2005a;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Tang W, Muly EC, Kuhar MJ, Howell L, Mash DC. Cocaine-induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non-human primates. J Neurochem. 2005b;95:1785–1793. doi: 10.1111/j.1471-4159.2005.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, O’Connor JA, Acosta G, Floyd D, Anderson N, McCool BA, Friedman D, Grant KA. Ethanol-induced regulation of GABA-A subunit mRNAs in prefrontal fields of cynomolgus monkeys. Alcohol Clin Exp Res. 2006;30:1978–1985. doi: 10.1111/j.1530-0277.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S. Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron. 1993;10:943–954. doi: 10.1016/0896-6273(93)90209-a. [DOI] [PubMed] [Google Scholar]
- Horak M, Wenthold RJ. Different roles of C-terminal cassettes in the trafficking of full-length NR1 subunits to the cell surface. J Biol Chem. 2009;284:9683–9691. doi: 10.1074/jbc.M807050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Selective loss of NMDA receptor NR1 subunit isoforms in Alzheimer’s disease. J Neurochem. 2004;89:240–247. doi: 10.1111/j.1471-4159.2003.02330.x. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Ivester P, Roberts LJ, 2nd, Young T, Stafforini D, Vivian J, Lees C, Young J, Daunais J, Friedman D, Rippe RA, Parsons CJ, Grant KA, Cunningham C. Ethanol self-administration and alterations in the livers of the cynomolgus monkey, Macaca fascicularis. Alcohol Clin Exp Res. 2007;31:144–155. doi: 10.1111/j.1530-0277.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D. Glutamate transmission and addiction to cocaine. Ann N Y Acad Sci. 2003;1003:169–175. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- Koltchine VV, Anantharam V, Bayley H, Treistman SN. Alternative splicing of the NMDAR1 subunit affects modulation by calcium. Brain Res Mol Brain Res. 1996;39:99–108. doi: 10.1016/0169-328x(96)00012-5. [DOI] [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O’Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Le Corre S, Harper CG, Lopez P, Ward P, Catts S. Increased levels of expression of an NMDARI splice variant in the superior temporal gyrus in schizophrenia. Neuroreport. 2000;11:983–986. doi: 10.1097/00001756-200004070-00017. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Cocaine treatment- and withdrawal-induced alterations in the expression and serine phosphorylation of the NR1 NMDA receptor subunit. Psychopharmacology (Berl) 2002;164:349–359. doi: 10.1007/s00213-002-1209-9. [DOI] [PubMed] [Google Scholar]
- Melgaard B, Henriksen L, Ahlgren P, Danielsen UT, Sorensen H, Paulson OB. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta Neurol Scand. 1990;82:87–93. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Bunker GL, Mason JN, Allen C, Janowsky A. Effects of subchronic clozapine and haloperidol on striatal glutamatergic synapses. Journal of Neurochemistry. 1996;67:1965–1973. doi: 10.1046/j.1471-4159.1996.67051965.x. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Monti PM, Tidey J, Czachowski CL, Grant KA, Rohsenow DJ, Sayette M, Maners N, Pierre P. Building bridges: the transdisciplinary study of craving from the animal laboratory to the lamppost. Alcohol Clin Exp Res. 2004;28:279–287. doi: 10.1097/01.alc.0000113422.04849.fa. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Devaud LL, Bucci D, Smith FD. GABAA and NMDA receptor subunit mRNA expression in ethanol dependent rats. Alcohol Alcohol Suppl. 1994;2:89–95. [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Nagy J, Kolok S, Dezso P, Boros A, Szombathelyi Z. Differential alterations in the expression of NMDA receptor subunits following chronic ethanol treatment in primary cultures of rat cortical and hippocampal neurones. Neurochem Int. 2003;42:35–43. doi: 10.1016/s0197-0186(02)00062-1. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Paris C, Hagenbuch F, Ebert D, Juengling FD. Brain activation during craving for alcohol measured by positron emission tomography. Aust N Z J Psychiatry. 2006;40:171–178. doi: 10.1080/j.1440-1614.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [Review] [115 refs] [DOI] [PubMed] [Google Scholar]
- Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J Neurosci. 2003;23:11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Gallagher M, Holland PC. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav Neurosci. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering C, Avesson L, Lindblom J, Liljequist S, Schioth HB. Identification of neurotransmitter receptor genes involved in alcohol self-administration in the rat prefrontal cortex, hippocampus and amygdala. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:53–64. doi: 10.1016/j.pnpbp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Raeder H, Holter SM, Hartmann AM, Spanagel R, Moller HJ, Rujescu D. Expression of N-methyl-d-aspartate (NMDA) receptor subunits and splice variants in an animal model of long-term voluntary alcohol self-administration. Drug Alcohol Depend. 2008;96:16–21. doi: 10.1016/j.drugalcdep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Dodd PR. Cortical NMDA Receptor Expression in Human Chronic Alcoholism: Influence of the TaqIA Allele of ANKK1. Neurochem Res. 2009 doi: 10.1007/s11064-009-9941-8. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Ho AM, Innes DJ, Dodd PR. The expression of NMDA receptor subunit mRNA in human chronic alcoholics. Ann N Y Acad Sci. 2008;1139:10–19. doi: 10.1196/annals.1432.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, Rietschel M, Rujescu D, Lourdusamy A, Clarke TK, Krause K, Dyer A, Depner M, Wellek S, Treutlein J, Szegedi A, Giegling I, Cichon S, Blomeyer D, Heinz A, Heath S, Lathrop M, Wodarz N, Soyka M, Spanagel R, Mann K. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40:71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Stein V, House DR, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268:873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. Characterization of the discriminative stimulus effects of N-methyl-D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey ( Macaca fascicularis) Psychopharmacology (Berl) 2002;162:273–281. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res. 1996;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Lallemand F, de Witte P. Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or ‘binge drinking’ alcohol abuse. Alcohol Alcohol. 2009;44:128–135. doi: 10.1093/alcalc/agn100. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Grusser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Average expression stability (M) values of the seven selected control genes for the DLPFC (A), OFC (B) and ACC (C). As explained in the Methods section, the GeNorm algorithm provides a gene-stability measure to determine the expression stability of control genes based on non-normalized expression levels such that genes with the lowest M values have the most stable expression. The algorithm uses a stepwise exclusion of the genes with the highest M values (least stable) and recalculating new M values for the remaining genes (Vandesompele et al., 2002).