Abstract
The rotation around the amide bond in N,N-diethyl-m-toluamide (m-DEET) has been studied extensively and often used in laboratory instructions to demonstrate the phenomenon of chemical exchange. Herein, we show that a simple modification to N,N-diethyl-o-toluamide (o-DEET) significantly alters the dynamics of the restricted rotation around the amide bond due to steric interactions between the ring methyl group and the two N-ethyl groups. This alters the classic two-site exchange due to restricted rotation around the amide bond, to a three-site exchange, with the third conformation trapped at a higher-energy state compared to the other two. This often overlooked phenomenon is elucidated using variable-temperature NMR, two-dimensional exchange spectroscopy and molecular modeling studies.
Keywords: Dynamic NMR, chemical exchange, restricted rotation
I. Introduction
Amides have been studied by nuclear magnetic resonance (NMR) spectroscopy more extensively than any other class of compounds. Such studies have included a range of molecular sizes from small molecules such as formamide to large ones such as polypeptides and proteins (1–4). One of the earliest applications of NMR spectroscopy to the study of hindered internal rotation was made with N,N-dimethylformamide (5–8). This was followed rapidly by the earliest application of NMR signal shape analysis to the quantitative determination of the rate of internal rotation (2,4,8). Chemical synthesis, variable-temperature 1H NMR studies, and molecular mechanics calculations on N,N-diethyl-m-toluamide (m-DEET) has been proposed as an experiment for the undergraduate physical-organic laboratory (9). Rotation about ortho-substituted N,N-diethylbenzamides was first described by Bedford et al (10), and subsequently extensively studied by others (4,11,12). In this paper, we demonstrate that steric interactions between the methyl group at the ortho position and the two N-ethyl groups in N,N-diethyl-o-toluamide (o-DEET) modulates the restricted rotation of the amide bond to introduce an additional higher-energy barrier. Over the temperature range studied (1 – 55 °C), the third site in the o-DEET does not go through coalescence, while the other two sites undergo classical exchange pattern similar to the m-DEET. The presence of the third site is demonstrated experimentally using two-dimensional exchange spectroscopy and molecular mechanics calculations further elucidates the complex conformational energy landscape for o-DEET in comparison with m-DEET.
II. Materials and Methods
Synthesis
m-DEET and its analogs were synthesized starting with the toluic acid (parent compound). The acid was first converted into the acid chloride by treatment with an excess of oxalyl chloride in tetrahydrofuran (THF) using a catalytic amount of dimethylformamide (DMF). The reaction was worked up and the resultant acid chloride was used directly for the next reaction without any purification. The secondary amine of choice was reacted with the acid chloride in the presence of triethylamine to yield the final amide. Each analog was purified by fractional crystallization or column chromatography. Approximately 10 mg of each sample was dissolved in CDCl3 (total volume of 600 L). Each NMR tube was glass-sealed by first applying the freeze-thaw technique using liquid nitrogen to expel any dissolved air/oxygen, followed by sealing them using a small propane torch.
NMR spectroscopy
All the NMR experiments were performed in a 400-MHz (1H resonance frequency) VNMRS spectrometer (Varian-Agilent) and using a one-NMR probe. The temperature of each sample temperature was calibrated using MeOH (13). The probe temperature was varied from 1°C to 55 °C (in steps of 3 °C). One-dimensional, variable-temperature experiments were performed with 16 transients over 16 K complex points after calibrating the 90° pulse at each temperature. Samples were equilibrated for 20 minutes at each temperature and a relaxation delay of 30s was used between the transients. NMR spectra were processed (no window function and Fourier transformed to 32K) and saved under NUTS format (Acorn NMR Inc., Livermore, CA) and were used in WinDNMR (14,15) to estimate the exchange rates (kex s−1). A two-site exchange model was used to fit the variable temperature line shape data assuming a constant spin-spin relaxation time T2 (linewidth of 20 Hz for both samples). This linewidth is a fitting parameter used in the WinDNMR program to account for the unresolved germinal and vicinal coupling contributing to the lineshape and does not reflect the observed linewidth that is much narrower (~2Hz) at coalescence. Only slight broadening of the lineshape of the third site was noted, which led to the assumption that a two-site exchange model would suffice to understand the restricted rotation with respect to the other two sites in o-DEET. A linear fit between the estimated exchange rates (natural logarithm scale) vs. inverse of temperature in K (1000/T) was used to estimate the activation energy of the Eyring plot.
Two-dimensional exchange spectroscopy (EXSY) (16) was performed at three different temperatures (1 °C, 25 °C and 55 °C) using a standard Nuclear Overhauser Effect Spectroscopy (NOESY) pulse sequence (17). The time domain data dimension of each EXSY experiment was 2048 x 128 (t2 x t1) complex points with an exchange mixing time of 200 ms. A total of 16 transients were collected at each t1 point with a relaxation delay of 30s between the transients. Two-dimensional data were processed using Mestrenova (Mestrelab Research, Santiago de Compostela, Spain) with a squared cosine apodization to a final spectral dimension of 2048 x 2048 points.
Molecular mechanics calculations were performed using the program Avogadro (an open-source molecular builder and visualization tool (v1.03); www.avogadro.openmolecules.net) as a function of two dihedral angles; (C-C-C-O / Aromatic-CO) and (O-C-N-C). The dihedral angle was changed from −180° to +180° in steps of 5°. For each value of so calculated, the dihedral angle was changed −180° to +180° in steps of 5°, leading a matrix of 33 x 33. All the calculations were performed on a dual-core Windows workstation.
III. Results
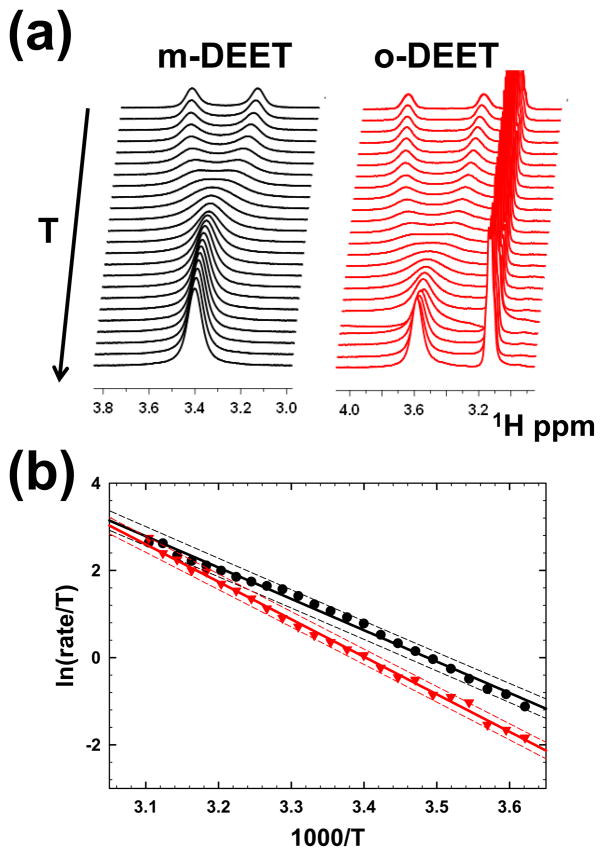
Variable-temperature NMR data are summarized in Figure 1. The temperature- dependence of the m-DEET spectrum follows the characteristic features corresponding to a two-site chemical exchange process (Fig. 1a). In the case of o-DEET, in addition to the characteristics two site exchange peaks (3.83 ppm and 3.36 ppm at 1°C; 3.61 ppm at 55 °C), there is an additional peak at 3.12 ppm (Fig. 1a). As noted earlier, both methyl groups of m-DEET are centered at 1.17 ppm and they exhibit line shape changes (with changing temperature) characteristic of a two-site exchange (data not shown) (9). Figure 1b shows the Eyring analysis plot (ln (kex/T) vs. 1000/T) for both m-DEET (black symbols) and o-DEET (red symbols). Linear fit (95% confidence prediction intervals plotted as dashed lines of Fig 1b) was used to determine the activated enthalpy (ΔH‡) and entropy (ΔS‡) of the exchange mechanism (ΔH‡ kJ/mol = −slope x 1000 /R, and ΔS‡ J/(mol.K) = (Y-intercept − ln(kB/h)) x R, where R, kB and h are gas, Boltzmann and Planks’ constants, respectively in SI units), leading to the following thermodynamic parameters: m-DEET, ΔH‡ =59.8 ± 1.1 kJ/mol and ΔS‡ = 10.9 ± 0.9 J/(mol.K); and o-DEET, ΔH‡ =71.5 ± 1.0 kJ/mol and ΔS‡ = 45.7 ± 1.9 J/(mol.K). One of the drawbacks of Eyring plot is that Y-intercept (used to determine ΔS‡) is normally a large extrapolation from the experimental data to high temperatures. In our case, the adjusted R2 values of the linear fit are good (> 0.98 for both the molecules) leading to reliable estimates ΔS‡. The increase entropic estimate in o-DEET in comparison with m-DEET by ~ 35 J/(mol.K) may be attributed the presence of the third site.
Figure 1.
(a) Temperature dependence of the NMR line shapes of the methylene protons in N,N-diethyl-m-toluamide (m-DEET) and N,N-diethyl-o-toluamide (o-DEET). (b) Eyring analysis between the natural logarithm of reaction rate to temperature (exchange rate/Temperature) derived from the lineshape analysis vs. inverse temperature. Black and red symbols represent data from m-DEET and o-DEET, respectively. The continuous line shows the linear best fit and the dashed lines correspond to prediction at 95% confidence.
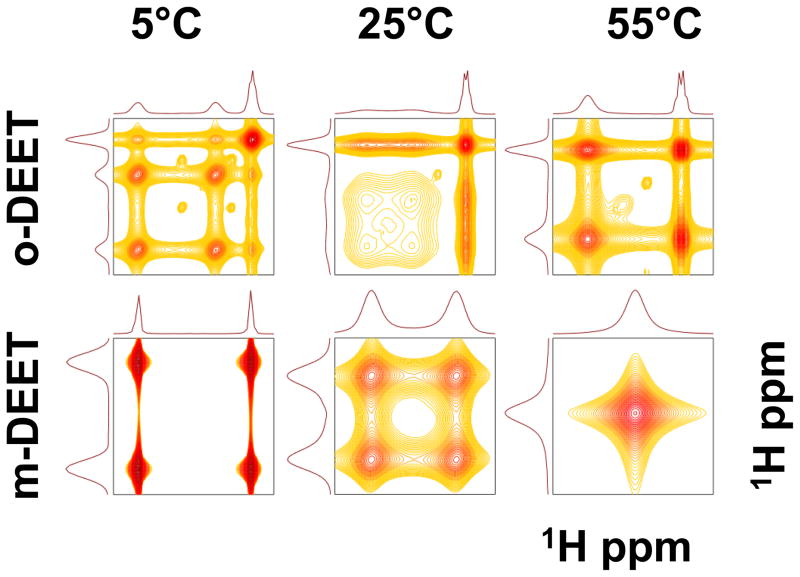
In order to demonstrate that the additional peak at 3.12 ppm (Fig. 1a) is part of the methylene resonances, we performed two-dimensional EXSY experiments (Figure 2). At a mixing time of 200 ms, no intramolecular NOEs between the protons are expected since o c≪ 1 ( o = spectrometer frequency in radians; c = rotational correlation time). Even if such NOEs are present, they are expected to be weak and their corresponding cross peaks would be of opposite sign to the diagonal peaks. The presence of strong cross peaks (of the same sign of the diagonal) in Figure 2 strongly suggests that cross peaks are predominantly due to a chemical exchange mechanism. Further the viscosity of chloroform does not change significantly over the temperature range (0.699 mPa·s at 0°C to 0.389 mPa·s at 60°C ) to induce a large change c in accordance with Stokes-Einstein-Debye equation where the rotational correlation time is directly proportional to solution viscosity. The possibility of zero-quantum peaks between the sites is also minimal because of a long mixing time (200 ms) and if present, they will have a multiplet structure reflecting the coupling pattern (18). Therefore it is clear that the cross peaks between the methylene protons are predominantly due to chemical exchange.
Figure 2.
Two-dimensional exchange spectroscopy (EXSY) spectra at three different temperatures: Methylene region of EXSY spectra of o-DEET (top row) and m-DEET (bottom row) at 5°C, 25°C and 55°C. The 1H NMR spectra were acquired at 400 MHz with a mixing time of 200 ms. The small peaks are due to unidentified impurities.
At each of the three temperatures, the third peak at 3.12 ppm shows a cross peak with the other two groups. This third peak continues to show an exchange peak with those of the other methylene protons, but does not undergo a typical two- or three-site exchange process in the temperature range studied. The continued presence of the exchange cross peaks between the third resonance and the other two at each of the three temperatures studied, and the lack of any significant change in its lineshape (in the o-DEET only), is clearly indicative of the fact that the third site is induced due to the steric hindrance of the ortho-methyl group. Moving the methyl to either the meta position on the ring (Figure 2) or replacing the N,N-diethyl group with N,N-dimethyl does not show a similar modulation (19).
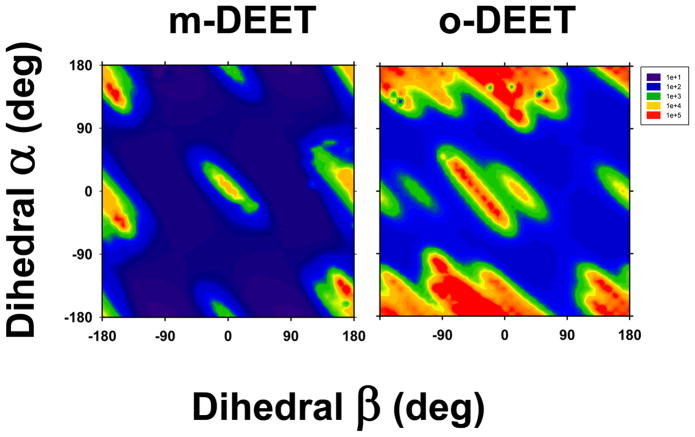
The results of the molecular mechanics calculations, performed as a function of the two independent dihedral angles and , are presented in Figure 3. The m-DEET molecule shows high energy conformations with both and close to 0° or 180° (red patches of Fig.3, left panel), suggesting a low energy conformations at ±90°. However, a more complex conformational space emerges for the o-DEET. With reference to in o-DEET, high-energy conformations are found between -90° and 0° (Figure 3, right panel), which is unlike the symmetric pattern of such conformations found in m-DEET. Though the variation in conformational energies with respect to in o-DEET is similar to that in m-DEET, the relative energies are much higher. This further suggests that an ortho-methyl group on the ring affects the conformational dynamics to a lesser extent around the dihedral angle as compared to same effect around the dihedral angle (directly connected to the amide bond).
Figure 3.
Molecular mechanics calculations of m-DEET (left) and o-DEET (right). Calculations were performed as a function of two dihedral angles C-C-C-O / Aromatic-CO) and (O-C-N-C). The contour plots represent the relative energy as a function of and . A heatmap color scheme is adopted; blue to red, corresponds to low to high energy, respectively.
IV. Discussion & Conclusions
The changes in line shape observed in the NMR spectra of organic compounds, due to restricted rotation, are often explained in terms of atropisomerism and diastereotopicity. In the case of both o-DEET and m-DEET, there are two barriers to rotation as defined by dihedral angles and (Figure 3). Due to restricted rotation due to the partial double-bond (C=N) of the amide bond, , the barrier around is much smaller than that of , and therefore often negligible. A conventional one-dimensional NMR spectrum is interpreted to reflect the presence of two enantiomeric, atropisomeric conformers. At low temperatures, slow rotation around manifests itself as diastereotopicity of the CH2 group and as the temperature increases the rotation becomes rapid leading to a coalescence of the peaks (9). The EXSY spectrum of m-DEET (Fig. 2, lower panels) as well as the conformational sampling (Fig. 3) supports with this conclusion. However, in the case of o-DEET (top panels of Fig.2), the two downfield peaks (centered at 3.6 ppm) show the classic coalescence pattern similar to the m-DEET, while the peak at ~3.1 ppm does not follow the coalescence process. Furthermore, the observation of exchange cross peaks (Figure 2, top panels) suggest at the possibility of the “trapping” of the third conformation at a high-energy barrier. One-dimensional NMR experiments performed on o-DEET at −40 °C (J S Nowick, Unpublished results, personal communication) show three distinct peaks.
We have shown that simple alterations in the geometry can alter the energy landscape of conformational preferences sampled by the dynamics of the restricted rotation around the amide bond. One of the two methylene groups present in the ethyl group of o-DEET (N-CH2CH3) is trapped into a high-energy barrier due to the steric interactions between the diethyl groups and the methyl group at the ortho position of the aromatic ring. The NMR characterization via the variable-temperature line-shape studies suggest that the energy barrier is much higher than the thermal energy provided by the increase in temperature in the range (1 – 55° C) investigated. The activation enthalpy between the other two conformations of o-DEET is higher (~ 11 kJ/mol) than that for its constitutional isomer, m-DEET, when a two-site exchange model is assumed. As the lineshape of the third peak undergoes relatively little change over the temperature range studied, a two-site model may be considered valid. The fact that the third peak is, indeed, part of the methylene group is validated by the 2D EXSY experiments (Figure 2). For small molecules such as the ones considered here, cross peaks at a mixing time of 200 ms suggest the presence of exchange peaks and negligible transfer through the nuclear Overhauser effect (NOE). Molecular mechanics calculations (Figure 3) show drastic changes in the conformational preferences between the o-DEET and m-DEET. As expected for both molecules, the conformational changes with respect to the dihedral angle ‘ ’ are small. However, with respect to the dihedral angle , one of the low-energy conformations was found to be close to 90°C for o-DEET, while in m- DEET such conformations were found to be available at ±90°.
Chemical exchange studies provide provides fundamental knowledge on the physical chemistry of the molecules and it is an area continuing interest to the research community (2,20–23). For example in a recent study Bain and co-works (24) add valuable information of the effect of solvent on the dynamics of push-pull ethylene (25). These approaches provide a close correspondence between the predicted electronic structure calculations with experimental NMR measurements as well as various thermodynamic parameters . In spite of the well-studied restricted rotations around the amide bonds over the years, simple systems such as o-DEET and m-DEET, do provide surprising results. One possible reason that the conformational trapping mechanisms were not noted in the earlier literature could be due to the fact that the NMR experiments were restricted to much lower spectrometer field strengths and the traditional one-dimensional experiments.
Highlights.
Steric induced modulation of restricted amide rotation
Two-site chemical exchange to a three-site exchange mechanism
Variable temperature NMR, 2D exchange NMR spectroscopy and molecular mechanics calculations
Acknowledgments
The authors acknowledge Dr. J. S. Nowick (University of California, Irvine) for many insightful discussions and Dr. S. Attar (California State University, Fresno) for critical reading of the manuscript. This work was in part supported by a RIMI (Research Infrastructure for Minority Institutions) grant (P20MD002732).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allerhand A, Gutowsky HS. Journal of Chemical Physics. 1965;42:4203–12. doi: 10.1063/1.1696165. [DOI] [PubMed] [Google Scholar]
- 2.Bain AD. Progress in Nuclear Magnetic Resonance Spectroscopy. 2003;43:63–103. doi: 10.1016/j.pnmrs.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Palmer AG. Encyclopedia of nuclear magnetic resonance. In: Grant HDM, RK, editors. Advances in NMR. Wiley; Chichester: 2002. pp. 344–53. [Google Scholar]
- 4.Stewart WE, Siddall TH. Chemical Reviews. 1970;70:517–51. [Google Scholar]
- 5.Drakenbe T, Forsen S. Journal of Physical Chemistry. 1970;74:1–7. [Google Scholar]
- 6.Forsen S, Hoffman RA. Journal of Chemical Physics. 1963;39:2892–901. [Google Scholar]
- 7.Gutowsky HS, Vold RL, Wells EJ. Journal of Chemical Physics. 1965;43:4107–25. [Google Scholar]
- 8.Wiberg KB, Rablen PR, Rush DJ, Keith TA. Journal of the American Chemical Society. 1995;117:4261–70. [Google Scholar]
- 9.Jensen BL, Fort RC. Journal of Chemical Education. 2001;78:538–40. [Google Scholar]
- 10.Bedford GR, Greatban D, Rogers DB. Chemical Communications. 1966. pp. 330–31.
- 11.Mannschr A, Mattheus A, Rissmann G. Journal of Molecular Spectroscopy. 1967;23:15–31. [Google Scholar]
- 12.Siddall TH, Stewart WE. Journal of Physical Chemistry. 1971;74:3580–83. [Google Scholar]
- 13.Van Geet AL. Analytical Chemistry. 1970;42:679–80. [Google Scholar]
- 14.Bampos N, Vidal–Ferran A. Journal of Chemical Education. 2000;77:130–33. [Google Scholar]
- 15.Reich HJ. WinDNMR: Dynamic NMR Spectra for Windows. JCE Software; 1995. [Google Scholar]
- 16.Jeener J, Meier BH, Bachmann P, Ernst RR. Journal of Chemical Physics. 1979;71:4546–53. [Google Scholar]
- 17.Anilkumar, Ernst RR, Wüthrich K. Biochemical and Biophysical Research Communications. 1980;95:1–6. doi: 10.1016/0006-291x(80)90695-6. [DOI] [PubMed] [Google Scholar]
- 18.Macura S, Huang Y, Suter D, Ernst RR. Journal of Magentic Resonance. 1981;43:259–81. [Google Scholar]
- 19.Maitra S, Krishnan VV, Thomson W. Conformational analysis of analogs of DEET focusing on the restricted rotation of the C-N bond using molecular modeling and VT NMR study. Abstracts of Papers of the American Chemical Society. 2011 [Google Scholar]
- 20.Bain AD. In: Annual Reports on Nmr Spectroscopy. Webb GA, editor. Vol. 63. 2008. pp. 23–48. [Google Scholar]
- 21.Perrin CL, Dwyer TJ. Chemical Reviews. 1990;90:935–67. [Google Scholar]
- 22.Pons M, Millet O. Progress in Nuclear Magnetic Resonance Spectroscopy. 2001;38:267–324. [Google Scholar]
- 23.Szalay Z, Rohonczy J. Progress in Nuclear Magnetic Resonance Spectroscopy. 2010;56:198–216. doi: 10.1016/j.pnmrs.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Ababneh-Khasawneh M, Fortier-McGill BE, Occhionorelli ME, Bain AD. J Phys Chem A. 2011;115:7531–7. doi: 10.1021/jp201885q. [DOI] [PubMed] [Google Scholar]
- 25.Sandstrom J. Topics in Stereochemistry. 1983;14:83–181. [Google Scholar]