Abstract
The present retrograde labeling study was designed to determine the presence and pattern of projections from individual subdivisions of the central nucleus of the amygdala (CNA) to the nucleus pontis oralis (NPO), which is a critical brainstem site involved for the generation and maintenance of active (REM) sleep. Projections from the CNA were labeled with the retrograde tracer, cholera toxin B-subunit (CTB), which was injected, unilaterally, via microiontophoresis, into the NPO. Sections of the amygdala were immunostained in order to identify CTB-labeled CNA neurons and CNA neurons that contained CTB plus the vesicular glutamate transporter 2 (VGLUT2), which is a marker for glutamatergic neurons. Histological analyses revealed that retrogradely-labeled neurons that project to the NPO were localized, ipsilaterally, within the medial, lateral and capsular subdivisions of the CNA. In addition, a substantial proportion (24%) of all retrogradely-labeled CNA neurons also exhibited VGLUT2 immunoreactivity. The present study demonstrates that glutamatergic neurons, which are present within various subdivisions of the CNA, project directly to the NPO. These data lend credence to the hypothesis that NPO neurons that are involved in the control of active sleep are activated by glutamatergic projections from the amygdala.
Keywords: amygdalofugal projection, glutamate, cholera toxin B, retrograde, reticular formation
INTRODUCTION
It is well established that active (or rapid-eye-movement, REM) sleep (AS) occurs as a result of the activation of executive AS-on neurons that are located in and/or within the vicinity of the nucleus pontis oralis (NPO) (Baghdoyan et al., 1987; Xi et al., 1999; Jones, 2004; Chase and Morales, 2005; Siegel, 2005; Lu et al., 2006; Luppi et al., 2006; McCarley, 2007). In addition, there are abundant data demonstrating that these AS-Generator neurons are activated by excitatory cholinergic inputs from the laterodorsal and pedunculopontine tegmental nuclei (LDT/PPT) (Roth et al., 1996; Jones, 2004; Siegel, 2005; McCarley, 2007). However, there is a growing consensus that other sites may also be capable of directly inducing AS by activating neurons that constitute the AS-Generator (see below). Accordingly, we hypothesized that excitatory projections from specific nuclei within the central nucleus of the amygdala (CNA) activate neurons in the NPO which results in the generation of AS. To test the veracity of this hypothesis, which was based upon the preceding consensus and our recent electrophysiological data (Xi et al., 2009; 2010), we utilized retrograde and double immunohistochemical labeling techniques to determine (a) whether neurons in subdivisions of the CNA send projections directly to the NPO, and (b) whether these neurons constitute an excitatory glutamatergic CNA-NPO projection system.
There is a great deal of clinical as well as experimental data which provide support for the hypothesis that the CNA plays an important role in the production of AS. Functional imaging studies in humans have demonstrated an increase in regional cerebral blood flow, bilaterally, in the amygdala during AS, suggesting that the amygdala is activated during this state (Maquet et al., 1996). The concept that CNA neurons are active during AS is confirmed by presence of an increase in the discharge rate of CNA units in the transition from quiet sleep to AS in chronic rats (Jha et al., 2005). In addition, microinjections of the cholinergic agonist carbachol, into the CNA in chronic cats, induces a prolonged enhancement of episodes of AS (Calvo et al., 1996). While electrical stimulation of the CNA in rats results in an increase in the duration of AS (Smith and Miskiman, 1975), functional inactivation of the CNA using either the GABAA agonist muscimol (Sanford et al. 2002) or tetrodotoxin (Tang et al. 2005) decreases AS. Furthermore, our preliminary electrophysiological studies have demonstrated that NPO neurons are orthodromically activated following stimulation of the CNA (Xi et al., 2009). Anatomically, tract-tracing studies have revealed that descending CNA projecting neurons innervate, directly, with ipsilateral predominance, cells in the NPO (Hopkins and Holstege, 1978; Krettek and Price, 1978; Price and Amaral, 1981 Takeuki et al., 1988; Boissard et al., 2003; Yasui et al., 2004). Taken together, the preceding findings suggest that the CNA, via the ventral amygdalofugal pathway (Veening et al., 1984; Sanford et al., 2002; Sah et al., 2003), is capable of exciting the AS-Generator in the NPO, thereby promoting AS in a manner similar to, but distinctly separate from, the classical cholinergic activation of AS via inputs from the LDT/PPT to the NPO.
Since the neurotransmitters of CNA neurons are heterogeneous, consisting of inhibitory and excitatory molecules in addition to several neuropeptides (McDonald et al., 1989; Day et al., 1999; Poulin et al., 2008), the present study was designed to identifying the excitatory neurotransmitter basis for the control of the AS-Generator by CNA neurons that project directly to the NPO. Preliminary data have been presented in abstract form (Chase et al., 2009; Zhang et al., 2010).
MATERIALS AND METHODS
Animals
Adult guinea pigs (n = 6) of both sexes were used in the present study. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the VA Greater Los Angeles Healthcare System (VAGLAHS); all experimental procedures were conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, 1996). Animals were housed in a temperature (22±1°C) and humidity (50–70%) controlled environment with a 12:12 hour light/dark cycle (light on from 6:00–18:00); the animals had ad libitum access to food and water. Appropriate measures were taken to minimize pain, discomfort or stress of the animals and only the minimum number of animals were used that were necessary to produce reliable scientific data.
Delivery of the retrograde tracer (cholera toxin B-subunit) to the NPO
Prior to aseptic surgery, each animal was premedicated with antibiotic (Baytril, 5 mg/kg, s.c.), analgesic (Buprenorphine, 0.05 mg/kg, s.c.), and anti-inflammatory (Dexamethasone, 1 mg/kg, s.c.) drugs. Then, under isoflurane anesthesia (4% induction, 2.5% maintenance), the animal was positioned in a stereotaxic frame, the calvarium was exposed and a small hole (3 mm diameter) overlying the NPO (A 0.8 to 1.5, L 1.0 to 1.5, H −9 to −9.8; Rapisarda and Bacchelli, 1977) was made to permit entry of a glass microelectrode (30 μm tip size) containing a solution of cholera toxin B-subunit (CTB; 2% in 0.1M phosphate buffer (PB), pH 6; List Biological laboratories, Campbell, CA). CTB was injected via iontophoresis (+2 μA, 7 s on and 7 s off for a duration of 20 min), into the NPO, as described in previous publications (Morales et al., 1999; Fung et al., 2001; McGregor et al., 2005; Pose et al., 2005; Torterolo et al., 2009). Two injections of the CTB solution were carried out; they were separated by 500 μm along a single vertical penetration track within the NPO in each animal. After the glass microelectrode was withdrawn, the hole in the calvarium was sealed with bone wax, the skin incision sutured and the animal was allowed to recover.
Perfusion and immunohistochemical procedures
Following a survival period of 5 days, each animal was deeply anesthetized with sodium pentobarbital and perfused intracardially with heparinized saline followed by 500 ml of a fixative (4% paraformaldehyde, 15% picric acid, 0.25% glutaraldehyde in 0.1M PB at pH 7.4). After overnight post-fixation, the forebrain and brainstem were cryoprotected in a solution of sucrose (25%) in 0.1M PB at pH 7.4. Serial coronal sections were cut (at a thickness of 30 and 16 μm of the forebrain and brainstem, respectively) on a Leica CM3050 S cryostat.
Immunohistochemical processing to detect single-labeled neurons for CTB have been described, in detail, in previous publications (Morales et al., 1999; Fung et al., 2001; McGregor et al., 2005; Pose et al., 2005; Torterolo et al., 2009). In the present study, free-floating brain sections were incubated sequentially with: (1) a primary goat antibody directed against CTB (List Biological Laboratories, Campbell, CA) at 1:30,000 dilution in 0.1M phosphate buffered saline (PBS) with 0.3% Triton-X-100 (PBST) for overnight; (2) a secondary, biotinylated donkey anti-goat antibody (1:2000 dilution in PBST; Jackson Immunoresearch, West Grove, PA) for 90 min; and (3) reagents of the ABC Elite Kit (1:200; Vector Laboratories, Burlingame, CA) for 60 min. In order to visualize the black granules that indicate the presence of retrogradely-labeled CNA neurons and CTB injection sites, the sections were processed with a mixture of diaminobenzidine (DAB; 0.2%), 0.05% hydrogen peroxide, and 0.6% nickel ammonium sulphate in 50mM Tris buffer (pH 7.6).
Double immunohistochemical staining for CTB and the vesicular glutamate transporter 2 (VGLUT2) in the amygdala was carried out in order to demonstrate that retrogradely-labeled CNA neurons were glutamatergic. In previous reports, similar double immunostaining techniques were employed to demonstrate double-labeling with CTB and other antigens (Morales et al., 1999; Fung et al., 2001; McGregor et al., 2005; Pose et al., 2005; Torterolo et al., 2009). Accordingly, in the present study, initially CTB immunostaining procedures, as described above, were performed. The sections were then treated with 5% normal donkey serum with 2% bovine serum albumin for 1 hr in order to pre-block non-specific binding sites in the tissue. Subsequently, the sections were incubated with a mouse monoclonal antibody directed against VGLUT2 (MAB5504, 1:350; Millipore, Temecula, CA) in 3% normal donkey serum, PBST, and 0.1% sodium azide first at room temperature overnight and then at 4°C for 48 hours. The tissue sections were then incubated with a donkey anti-mouse IgG secondary antibody (1:400; Jackson ImmunoResearch Laboratories, Inc., Westgrove, PA) in PBST for 90 min at room temperature. To visualize VGLUT2 immunoreactivity, a similar ABC technique (1:400 in PBST; 1 hr) as described above was used except that a non-intensified (absence of nickel ions) DAB reaction was employed to produce brown VGLUT2 immunoreactivity.
Following all immunostaining procedures, coronal brain sections were mounted serially, dehydrated, and then cover-slipped with Permount. To aid in the anatomical localization of the amygdala and the NPO, one series of coronal sections comprising these two regions from each animal was counterstained with Pyronin-Y before dehydration. The nomenclature used to describe various sub-nuclei of the amygdala in the guinea pig was adapted from two atlases of the brain (Rapisada and Bacchelli, 1977; Paxinos and Watson, 1998).
The presence of the VGLUT2 protein or mRNA (as revealed by immunohistochemical labeling and in situ hybridization, respectively) within neuronal somata is a reliable marker for glutamatergic neurons (Fremeau et al., 2001; Herzog et al., 2001; Hrabovszky et al., 2006a, b). Previous studies in the rat have confirmed the specificity of VGLUT2 immunolabeling within individual hypothalamic neurons based upon: (1) confocal imaging for the co-detection of VGLUT2 by employing the same anti-VGLUT2 that we employed (MAB5504) in combination with another VGLUT2 antibody in order to conduct dual-immunofluorescence labeling studies (Hrabovsky et al., 2006a, b) and (2) in situ hybridization for the detection of VGLUT2 mRNA (ibid.). Thus, these and other studies have shown that both neuronal somata as well as terminals contain immunoreactivity for VGLUT2 (Blaesse et al., 2005; Ponzio et al., 2006). In addition, to ascertain the specificity of immunostaining, we carried out additional control immunohistochemical staining procedures that consisted of the omission of the primary mouse anti-VGLUT2 antibody from the aforementioned double immunolabeling procedures.
Data analysis
Light microscopy was employed to identify: (1) the site of CTB deposits in each animal, (2) retrogradely-labeled CNA projection (CTB+) neurons, and (3) glutamatergic CNA projection (CTB+/VGLUT2+) neurons. In order to estimate the population of glutamatergic projecting neurons from the CNA to the NPO, all double-labeled (CTB+/VGLUT2+) and single-labeled (CTB+) projection neurons were counted in every 6th section (which were separated by 180 μm) throughout the rostrocaudal CNA.
RESULTS
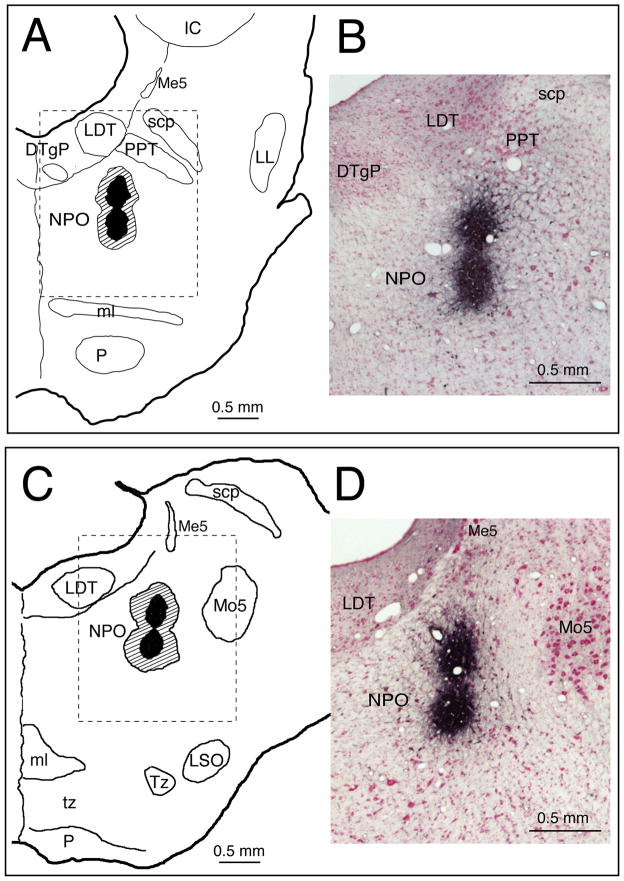
Figure 1 consists of injection sites for CTB in two representative guinea pigs; the centers of the injections were between the rostral (Figure 1, A–B) and caudal poles of the NPO (Figure 1, C–D). The CTB deposits were located in a similar region in the NPO in the remaining 4 animals. When observed under light microscopy, intense black granules representing CTB-immunoreactivity were present at the centers of the CTB injection sites (dark areas in Figure 1). The centers of the CTB injections were surrounded by peripheral regions of scattered immunoreactive CTB granules (hatched areas, Figure 1, A and C), indicating the extent of dispersion of the CTB tracer.
Figure 1.
Representative injection sites of CTB, in 2 guinea pigs, are shown in the rostral (A) and caudal levels (C) of the NPO. Photomicrographs B and D correspond to the dashed areas indicated in the rostral (A) and caudal (C) levels of the NPO, respectively. Each coronal section includes the injection site (darken area), centered within the boundary of the NPO of a given animal, with the area of dispersion of CTB granules indicated by the hatched area (in A and C). Abbreviations: CTB, cholera toxin B-subunit; DTgP, dorsal tegmental nucleus, posterior part; IC, inferior colliculus; LDT, laterodorsal tegmental nucleus; LL, lateral lemnicus; LSO, lateral superior nucleus; Me5, mesencephalic trigeminal nucleus; ml, medial lemnicus; Mo5, motor trigeminal nucleus; NPO, nucleus pontis oralis; P, pyramid; PPT, pedunculopontine tegmental nucleus; scp, superior cerebellar peduncle; Tz, nucleus of the trapezoid body; tz, trapezoid body.
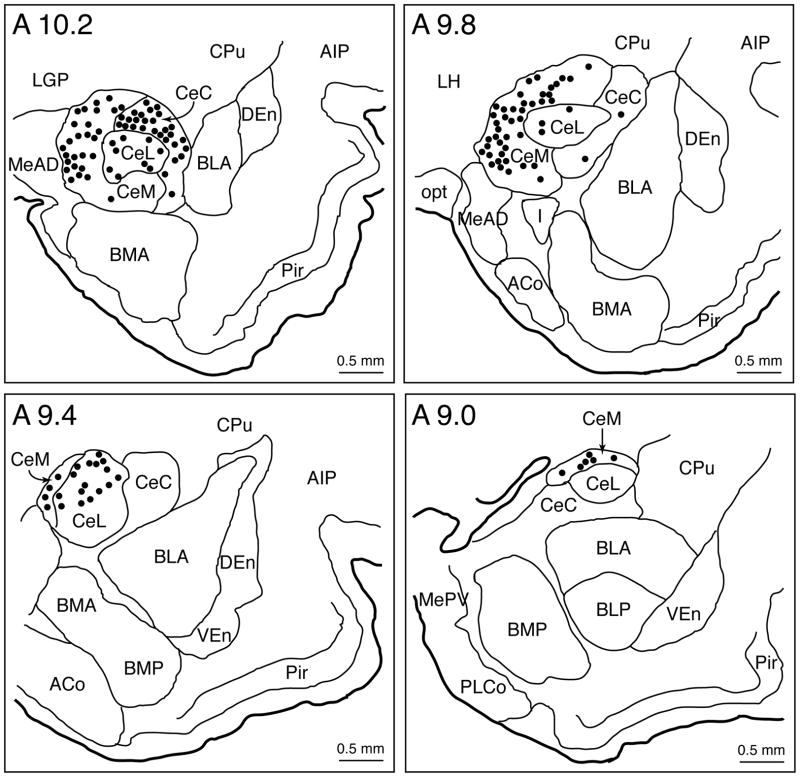
The distribution of retrogradely-labeled (CTB+) CNA neurons, in a representative animal, following CTB injections into the dorsolateral region of the NPO, is plotted in Figure 2. Large numbers of retrogradely-labeled CNA neurons were present ipsilaterally in the medial, lateral, and capsular divisions of the CNA; no contralateral labeling was observed. In addition, there were no labeled neurons in other (i.e., the basal, medial and lateral) nuclei of the amygdala (Figure 3). Plots of CTB+ neurons revealed that these labeled cells were distributed unevenly throughout the rostrocaudal extent of the CNA; the rostral portions of the CNA (Figure 2, A10.2-A9.8 stereotaxic planes) contained more CTB+ neurons than the caudal half of this nuclear group (Figure 2, A9.4-A9.0 stereotaxic planes).
Figure 2.
Distribution of retrogradely (CTB) labeled neurons throughout the rostrocaudal extent of the CNA in a representative animal. Each distribution plot includes a single coronal section along the longitudinal axis of the CNA, i.e., from A 10.2 to A 9.0 (stereotaxic planes of the brain atlas of Rapisarda and Bacchelli, 1977). Each dot represents a single neuron containing punctate black granules of CTB immunoreactivity within its cytoplasm (see Figures 3–4). Note that CNA neurons that project to the NPO are located ipsilaterally in the 3 subnuclei of the CNA (medial, lateral, and capsular). Abbreviations: ACo, anterior cortical nucleus of the amygdala; AIP, agranular insular cortex, posterior part; BLA, basolateral nucleus of the amygdala, anterior part; BLP, basolateral nucleus of the amygdala, posterior part; BMA, basomedial nucleus of the amygdala, anterior part; BMP, basomedial nucleus of the amygdala, posterior part; CeC, central nucleus of the amygdala, capsular division; CeL, central nucleus of the amygdala, lateral division; CeM, central nucleus of the amygdala, medial division; CNA, central nucleus of the amygdala; CPu, caudate putamen; CTB, cholera toxin B-subunit; DEn, dorsal endopiriform nucleus; I, intercalated nucleus of the amygdala; LH, lateral hypothalamic area; LGP, lateral globus pallidus; MeAD, medial nucleus of the amygdala, anterodorsal part; MePV, medial nucleus of the amygdala, posteroventral part; NPO, nucleus pontis oralis; opt, optic tract; Pir, piriform cortex; PLCo, posterolateral cortical nucleus of the amygdala; VEn, ventral endopiriform nucleus.
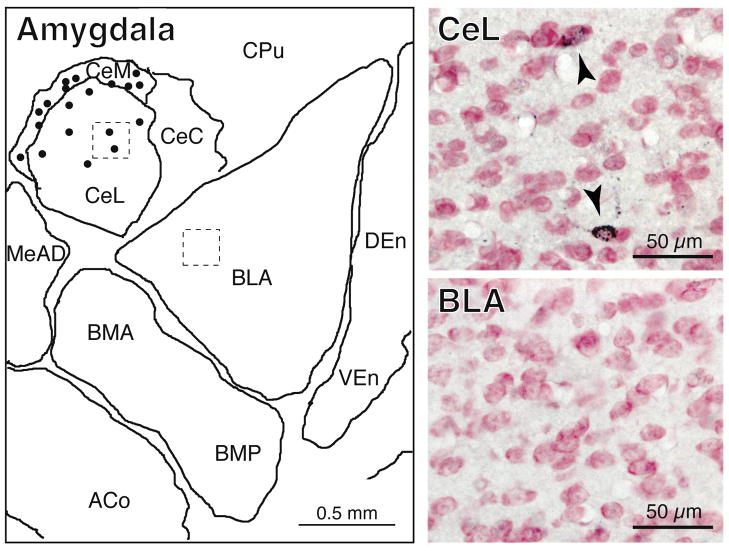
Figure 3.
Schematic and photomicrographs of the amygdala. In the schematic, retrogradely-labeled (CTB+) neurons (dots, left panel) are present exclusively in the central but not the basal, medial or lateral nuclei of the amygdala (left panel). The top and bottom photomicrographs (right panels) correspond to the dashed areas indicated in the central and lateral nuclei of the amygdala, respectively. The arrowheads in the top right panel point to CTB+ neurons. In the bottom photomicrograph, there are no CTB+ neurons. Abbreviations: ACo, anterior cortical nucleus of the amygdala; BLA, basolateral nucleus of the amygdala, anterior part; BMA, basomedial nucleus of the amygdala, anterior part; BMP, basomedial nucleus of the amygdala, posterior part; CeC, central nucleus of the amygdala, capsular division; CeL, central nucleus of the amygdala, lateral division; CeM, central nucleus of the amygdala, medial division; CPu, caudate putamen; CTB, cholera toxin B-subunit; DEn, dorsal endopiriform nucleus; MeAD, medial nucleus of the amygdala, anterodorsal part; VEn, ventral endopiriform nucleus.
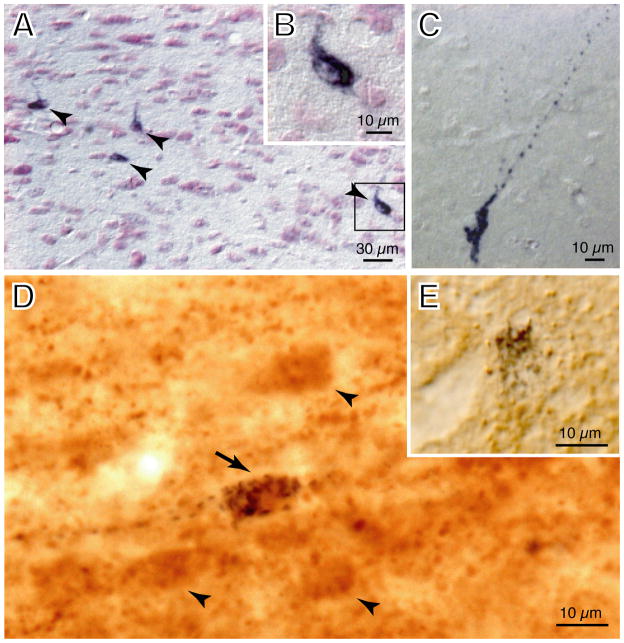
Under high-power magnification (Figure 4B), dense aggregates of punctate, black granules of CTB immunoreactivity were observed within the cytoplasm and the cell processes of individual retrogradely-labeled neurons (Figure 4A, arrowheads; Figure 4C). The somata of the CTB+ neurons were mostly fusiform in shape and their longitudinal axis measured approximately 15 μm in length.
Figure 4.
Photomicrographs of labeled CNA neurons. (A) Retrogradely-labeled (CTB+) neurons containing black CTB granules following single immunostaining for the presence of the retrograde tracer in the projection neurons (arrowheads). The CTB+ neuron in the bracket of A is further illustrated in the inset (B), at higher magnification, to demonstrate the presence of dense aggregates of black CTB granules within its cytoplasm. (C) Another example is presented of a CTB+ neuron with single labeling showing clear punctate black granules that are present not only within the soma, but which also fill the cell processes of this CNA neuron that projects to the NPO. (D) Double-labeling studies showing a glutamatergic neuron that projects to the NPO which contains black CTB granules with brown (VGLUT2)-immunostained cytoplasm (arrow). Brown-stained neurons, that were single-labeled for VGLUT2 (arrowheads), were present near this double-labeled neuron (arrow). The inset (E) depicts another type of single-labeled neuron (from a separate section) that contains black CTB granules without brown-stained VGLUT2 immunoreactivity in its cytoplasm. Brain sections of A to C were counter-stained with Pyronin-Y. Abbreviations: CNA, central nucleus of the amygdala; CTB, cholera toxin B-subunit; NPO, nucleus pontis oralis; VGLUT2, vesicular glutamate transporter 2.
Utilizing double immunostaining techniques with antibodies specifically directed against CTB and VGLUT2, the glutamatergic phenotype of CTB+ neurons was identified. Under high magnification, double-labeled (CTB+/VGLUT2+) neurons exhibited punctate black CTB granules plus brown-stained VGLUT2 immunoreactivity within their cytoplasm (e.g., Figure 4D, arrow). Two other types of single-labeled neurons were also present within the boundary of the CNA. Single-labeled VGLUT2+ neurons had homogenous, brown-stained cytoplasm (Figure 4D, arrowheads). On the other hand, non-glutamatergic projection neurons contained only black (CTB) granules (e.g., Figure 4E); they were similar in appearance to those neurons that were detected in the single-immunolabeling studies, as shown in Figure 4A (arrowheads) and Figure 4B–C (see DISCUSSION). For VGLUT2 immunolabeling controls, the omission of the primary antibody resulted in the absence of positive staining of glutamatergic neurons, confirming the specificity of the VGLUT2 antibody used in this study.
The number of the glutamatergic neurons in the CNA that project to the NPO was determined in 3 animals following double-immunolabeling procedures; in these animals, there were 21 CTB+/VGLUT2+ and 68 CTB+ neurons. Therefore, glutamatergic (CTB+/VGLUT2+) neurons accounted for 23.6% of all CNA neurons that projected to the NPO.
DISCUSSION
Previous findings have established that CNA neurons project to the NPO (see Introduction); however, no details are available regarding the distributional patterns of these CNA projection neurons or their phenotype. In the present study, we determined that amygdalofugal neurons that project directly to the NPO originate in the CeM, CeL, and the CeC subnuclei of the CNA. In addition, these neurons constitute a glutamatergic projection system that innervate cells in the NPO.
Previous studies have established that neurotransmitter phenotypes within the amygdala are heterogeneous, consisting of neurons that contain inhibitory as well as excitatory neurotransmitters; in addition, several neuropeptides are co-localized within individual CNA neurons (McDonald et al., 1989; Day et al., 1999; Poulin et al., 2008). Using a monoclonal Glu-1 antibody, early immunolabeling studies suggested that glutamatergic neurons were present in the CNA of rats (McDonald et al., 1989); these data have been recently confirmed by in situ hybridization studies that demonstrated that CNA neurons express VGLUT1 mRNA (Poulin et al., 2008). In the present study, we utilized a well-characterized antibody against the VGLUT2 antigen (see Materials and Methods for details; Hrabovsky et al., 2006a, b) to demonstrate the presence of neurons that were immunoreactive to VGLUT2 in the CNA. Both VGLUT1 and VGLUT2 label glutamatergic neurons, including those that are located in the amygdala (Herzog et al., 2001). Furthermore, CNA neurons contain abundant mRNA for VGLUT2 (Fremeau et al., 2001). Our findings of VGLUT2+ neurons in the CNA are therefore in accord with previous reports (ibid).
The presence of an excitatory amygdalofugal pathway to the NPO supports our preliminary study wherein we described non-GABAergic CTB+ neurons in the CNA (Chase et al., 2009). Previously, another CTB retrograde labeling study also demonstrated that CNA neurons that project to the sublaterodorsal nucleus (i.e., dorsal region of the NPO), albeit of unspecified phenotypes, are non-GABAergic (Boissard et al., 2003). Physiologically, our anatomical finding of a glutamatergic amygdalofugal pathway that innervates the NPO is supported by electrophysiological studies from our (Xi et al., 2009, 2010) and other (Koch and Ebert, 1993) laboratories. Other preliminary findings indicate that electrical stimulation of the CNA produces orthodromic discharges in NPO neurons as well as excitatory postsynaptic potentials in these cells (Xi et al., 2009, 2010). NPO neurons were activated in the absence of cholinergic excitation of LDT/PPT origin (ibid.). Hence, these findings are consistent with our hypothesis that the amygdala participates in the control of AS via the activation of AS-Generator neurons in the NPO, and that this control does not depend upon cholinergic projections from the LDT/PPT.
We propose that glutamatergic CNA neurons play a direct role in the control of AS via activation of the AS-Executive neurons in the NPO. This hypothesis is supported by previous findings that carbachol microinjection to the CNA (Calvo et al., 1996) as well as electrical stimulation of the CNA (Smith and Miskiman, 1975) promote AS and that pharmacological inactivation of the CNA (employing muscimol or tetrodotoxin) results in a reduction in AS (Sanford et al., 2002; Tang et al., 2005).
Following injections of CTB into the dorsolateral region of the NPO (Figure 1), large numbers of retrogradely labeled (CTB+) neurons were present within the ipsilateral CNA (Figure 2), which supports previous anatomical studies (Hopkins and Holstege, 1978; Krettek and Price, 1978; Price and Amaral, 1981; Shammah-Lagnado et al., 1987; Boissard et al., 2003). A similar innervation of the NPO by the CNA has been shown in autoradiographic tract-tracing studies (Hopkins and Holstege, 1978; Krettek and Price, 1978; Price and Amaral, 1981; Takeuch et al., 1988; Yasui et al., 2004). For example, anterograde labeling studies have shown that the CNA sends fibers to the central tegmental field (i.e., the dorsal region of the NPO at rostral levels) in cats and rats (Krettek and Price, 1978) as well as to the lateral tegmental field (i.e., the lateral region of the NPO at caudal levels) in monkeys (Price and Amaral, 1981) and cats (Hopkins and Holstege, 1978). Similarly, when anterograde tracers of wheat germ agglutinin conjugated with horseradish peroxidase (Takeuch et al., 1988) or biotinylated dextran amine (Yasui et al., 2004) were injected to the CNA in the rat, labeled amygdalofugal fibers were present in the ipsilateral lateral tegmental field of the pons.
Previous retrograde studies using horseradish peroxidase or its conjugates as tracers have revealed that neurons that project to the NPO arise mainly from the medial subdivsion of the CNA (Hopkins and Holstege, 1978; Takeuchi et al., 1988). In addition to this area, we also determined that retrogradely-labeled neurons were located within other (lateral and capsular) subdivisions of the CNA (see Figure 2). This finding likely reflects the superior neuronal labeling by the CTB tracer compared with other retrograde labeling techniques (Luppi et al., 1990). In support to our results, two previous retrograde-labeling studies have shown that structures other than the CNA, from the forebrain to medulla, also innervate the NPO (Shammah-Lagnado et al., 1987; Boissard et al., 2003).
Based upon the present study as well as previous data from our laboratory and that of others, we hypothesize that the CNA sends projections that directly induce AS by exciting AS-on neurons of the AS-Generator. We proposed that these projections also interact, in the production of AS, with cholinergic projections to the AS-Generator from the LDT/PPT. This hypothesis is supported by the pivotal role played by the amygdala in emotional and cerebral circuits (LeDoux, 2000) and related in pathological conditions, such as depression, wherein amygdaloid dysfunction together with disturbances of AS has been shown to occur (Maquet and Franck, 1997). Recently, we demonstrated that recurrent apnea in guinea pigs (which models the obstructive sleep apnea syndrome in humans) (Fung et al., 2007; 2009) produces neurodegeneration/apoptosis in CNA neurons (Zhang et al., 2009). This finding suggests that dysfunction of the excitatory CNA pathway to the NPO may contribute to AS disturbances as well as to the generation of comorbidities that occur due to pathologies that involve the amygdala and others that also occur in conjunction with Obstructive Sleep Apnea.
In conclusion, the present study demonstrates that glutamatergic neurons, which are present within the CNA, project directly to the NPO. These data support our hypothesis that NPO neurons that are involved in the control of active sleep can be activated by glutamatergic projections from the amygdala.
Acknowledgments
We are grateful to Vincent Lim, Nichole Stevens and Daniel Bronson for their excellent technical assistance. This research was supported by NIH grant NS 60917.
Grant support: NIH grant NS 60917
References
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–261. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Ehrhardt S, Friauf E, Nothwang HG. Developmental pattern of three vesicular glutamate transporters in the rat superior olivary complex. Cell Tissue Res. 2005;320:33–50. doi: 10.1007/s00441-004-1054-8. [DOI] [PubMed] [Google Scholar]
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- Calvo JM, Simon-Arceo K, Fernandez-Mas R. Prolonged enhancement of REM sleep produced by carbachol microinjection in to the amygdala. NeuroReport. 1996;7:577–580. doi: 10.1097/00001756-199601310-00048. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR. Control of motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Philadelphia: W.B. Saunders Co; 2005. pp. 154–168. [Google Scholar]
- Chase MH, Torterolo P, Cabrera G, Lagos P, Sampogna S. Projections from the amygdala to the active (REM) sleep executive area of the nucleus pontis oralis in the guinea pig. Sleep. 2009;32(Supp):A21. [Google Scholar]
- Day HEW, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1β. J Comp Neur. 1999;413:113–128. [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Xi MC, Zhang JH, Sampogna S, Yamuy J, Morales FR, Chase MH. Apnea promotes glutamate-induced excitotoxicity in hippocampal neurons. Brain Res. 2007;1179:42–50. doi: 10.1016/j.brainres.2007.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Xi MC, Zhang JH, Yamuy J, Sampogna S, Tsai KL, Lim V, Morales FR, Chase MH. Eszopiclone prevents excitotoxicity and neurodegeneration in the hippocampus induced by experimental apnea. Sleep. 2009;32:1593–1601. doi: 10.1093/sleep/32.12.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Res. 2001;903:257–262. doi: 10.1016/s0006-8993(01)02318-6. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Csapo AK, Kallo I, Wilheim T, Turi GF, Liposits Z. Localization and osmotic regulation of vesicular glutamate transporter-2 in magnocellular neurons of the rat hypothalamus. Neurochem Int. 2006a;48:753–761. doi: 10.1016/j.neuint.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Turi GF, May K, Wittmann G, Fekete C, Liposits Z. Expression of vesicular glutamate transporter-2 in gonadotrope and thyrotrope cells of the rat pituitary. Regulation by estrogen and thyroid hormone status. Endocrinology. 2006b;147:3818–3825. doi: 10.1210/en.2005-1229. [DOI] [PubMed] [Google Scholar]
- Jha SK, Ross R, Morrison AR. Sleep-related neurons in the central nucleus of the amygdala of rats and their modulation by the dorsal raphe nucleus. Physiol Behav. 2005;86:415–426. doi: 10.1016/j.physbeh.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Jones BE. Paradoxical REM sleep promoting and permitting neuronal networks. Arch Ital Biol. 2004;142:379–396. [PubMed] [Google Scholar]
- Koch M, Ebert U. Enhancement of the acoustic startle response by stimulation of an excitatory pathway from the central amygdala/basal nucleus of Meynert to the pontine reticular formation. Exp Brain Res. 1993;93:231–241. doi: 10.1007/BF00228390. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherma D, Devor M, Saper C. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Fort P, Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 1990;534:209–224. doi: 10.1016/0006-8993(90)90131-t. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Gervasoni D, Verret L, Goutagny R, Peyron C, Salvert D, Leger L, Fort P. Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J Physiol Paris. 2006;100:271–283. doi: 10.1016/j.jphysparis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Maquet P, Franck G. REM sleep and amygdala. Mol Psychiatry. 1997;2:195–196. doi: 10.1038/sj.mp.4000239. [DOI] [PubMed] [Google Scholar]
- Maquet P, Peters J, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Beitz AJ, Larson AA, Kuriyama R, Sellitto Madl JE. Co-localization of glutamate and tubulin in putative excitatory neurons of the hippocampus and amygdala: an immunohistochemical study using monoclonal antibodies. Neurosci. 1989;30:405–421. doi: 10.1016/0306-4522(89)90261-3. [DOI] [PubMed] [Google Scholar]
- McGregor R, Damian A, Fabbiani G, Torterolo P, Pose I, Chase M, Morales FR. Direct hypothalamic innervation of the trigeminal motor nucleus: a retrograde tracer study. Neurosci. 2005;136:1073–1081. doi: 10.1016/j.neuroscience.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Morales FR, Sampogna S, Yamuy J, Chase MH. c-fos expression in brainstem premotor interneurons during cholinergically induced active sleep in the cat. J Neurosci. 1999;19:9508–9518. doi: 10.1523/JNEUROSCI.19-21-09508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. New York: Academic Press; 1998. [Google Scholar]
- Ponzio TA, Ni Y, Montana V, Parpura V, Hatton GI. Vesicular glutamate transporter expression in supraoptic neurons suggests a glutamatergic phenotype. J Neuroendocrinol. 2006;18:253–265. doi: 10.1111/j.1365-2826.2006.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pose I, Fung S, Sampogna S, Chase MH, Morales FR. Nitrergic innervation of trigeminal and hypoglossal motoneurons in the cat. Brain Res. 2005;1041:29–37. doi: 10.1016/j.brainres.2005.01.092. [DOI] [PubMed] [Google Scholar]
- Poulin J-F, Castonguay-Lebel Z, Laforest S, Drolet G. Enkephalin co-expression with classic neurotransmitters in the amygdaloid complex of the rat. J Comp Neur. 2008;506:943–959. doi: 10.1002/cne.21587. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapisarda C, Bacchelli B. The brain of the guinea pig in stereotaxic coordinates. Arch Sci Biol (Bologna) 1977;61:1–37. [PubMed] [Google Scholar]
- Roth MT, Fleegal MA, Lydic R, Baghdoyan HA. Pontine acetylcholine release is regulated by muscarinic autoreceptors. Neuroreport. 1996;7:3069–3072. doi: 10.1097/00001756-199611250-00055. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Parris B, Tang X. GABAergic regulation of the central nucleus of the amygdala: implications for sleep control. Brain Res. 2002;956:276–84. doi: 10.1016/s0006-8993(02)03552-7. [DOI] [PubMed] [Google Scholar]
- Siegel JM. REM sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Philadelphia: W.B. Saunders Co; 2005. pp. 120–35. [Google Scholar]
- Shammath-Lagnado SJ, Negrao N, Silva BA, Ricardo JA. Afferent connections of the nuclei reticularis pontis oralis and caudalis: a horseradish peroxidase study in the rat. Neuroscience. 1987;20:961–89. doi: 10.1016/0306-4522(87)90256-9. [DOI] [PubMed] [Google Scholar]
- Smith CT, Miskiman DE. Increases in paradoxical sleep as a result of amygdaloid stimulation. Physiol Behav. 1975;15:17–19. doi: 10.1016/0031-9384(75)90272-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Satoda T, Tashiro T, Matsushima R, Uemura-Sumi M. Amygdaloid pathway to the trigeminal motor nucleus via the pontine reticular formation in the rat. Brain Res Bull. 1988;21:829–833. doi: 10.1016/0361-9230(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Tang X, Yang L, Liu X, Sanford LD. Influence of tetrodotoxin inactivation of the central nucleus of the amygdala on sleep and arousal. Sleep. 2005;28:923–930. doi: 10.1093/sleep/28.8.923. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Sampogna S, Chase MH. MCHergic projections to the nucleus pontis oralis participate in the control of active (REM) sleep. Brain Res. 2009;1268:76–87. doi: 10.1016/j.brainres.2009.02.055. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Xi MC, Fung SJ, Chase MH. Projections from the central nucleus of the amygdala to neurons of the nucleus pontis oralis: an intracellular study. Sleep. 2010;33(Supp):A55. [Google Scholar]
- Xi MC, Fung SJ, Zhang JH, Sampogna S, Morales FR, Chase MH. Projections from the central nucleus of the amygdala excite neurons of the active (REM) sleep-generator in the nucleus pontis oralis. Soc Neurosci Abstr Program no. 375.26 2009 [Google Scholar]
- Xi MC, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–2019. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Tsumori T, Oka T, Yokota S. Amygdaloid axon terminals are in contact with trigeminal premotor neurons in the parvicellular reticular formation of the rat medulla oblongata. Brain Res. 2004;1016:129–134. doi: 10.1016/j.brainres.2004.04.080. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Fung SJ, Xi MC, Sampogna S, Chase MH. Recurrent apnea induces neuronal apoptosis in the guinea pig forebrain. Exp Neur. 2009;216:290–294. doi: 10.1016/j.expneurol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Xi MC, Fung SJ, Sampogna S, Lim V, Chase MH. Glutamatergic neurons in the central nucleus of the amygdala project directly to the NPO. Sleep. 2010;33(Supp):A55–A56. [Google Scholar]