Abstract
Mounting evidence suggests that Alzheimer disease (AD) is caused by the accumulation of the small peptide, Aβ, a proteolytic cleavage product of amyloid-β protein precursor (AβPP; or APP). Aβ is generated through a serial cleavage of APP by β- and γ-secretase. Aβ40 and Aβ42 are the two main components of amyloid plaques in AD brains, with Aβ42 being more prone to aggregation. APP can also be processed by α-secretase, which cleaves APP within the Aβ sequence, thereby preventing the generation of Aβ. Little is currently known regarding the effects of cell density on APP processing and Aβ generation. Here we assessed the effects of cell density on APP processing in neuronal and non-neuronal cell lines, as well as mouse primary cortical neurons. We found that decreased cell density significantly increases levels of Aβ40, Aβ42, total Aβ, and the ratio of Aβ42:Aβ40. These results also indicate that cell density is a significant modulator of APP processing. Overall, these findings carry profound implications for both previous and forthcoming studies aiming to assess the effects of various conditions and genetic/chemical factors, e.g. novel drugs on APP processing and Aβ generation in cell-based systems. Moreover, it is interesting to speculate whether cell density changes in vivo may also affect APP processing and Aβ levels in the AD brain.
Keywords: Alzheimer’s disease, amyloid-β, amyloid-β protein precursor, cell density
INTRODUCTION
AD is an insidious and progressive neurodegenerative disease and the primary cause of dementia in the elderly. It is a genetically complex and heterogenous disorder and based on the age of onset, it has two primary forms: early or late-onset of AD. More than 200 fully penetrant mutations in the amyloid-β protein precursor (APP), presenilin 1 (or PSEN1), and presenilin 2 (PSEN2) have been linked to early-onset familial AD (FAD) (<60 years old; 5–10% cases) [1–3], whereas 90–95% cases are late-onset AD (>60 years old) and a variant (ε4) of the gene encoding apolipoprotein E (APOE) has been associated with this disease type. Pathologically, AD is characterized by two hallmarks: amyloid plaques primarily composed of Aβ, and neurofibrillary tangles made up of the hyperphosphorylated tau. Multiple lines of evidence implicate excessive Aβ load in the brain as the primary cause of AD [1, 4, 5]. Aβ is a ~4 kDa peptide produced by a serial proteolytic cleavage of APP (Fig. 1) [6]. The initial cleavage of APP can be carried out by either α-, or β-secretase (or BACE1). α-Secretase cleavage produces sAPPα and the α-C-terminal fragment (α-CTF or APP-C83) while β-secretase cleavage produces sAPPβ and β-C-terminal fragment (β-CTF or APP-C99). C83 and C99 can be further cleaved by γ-secretase, which produces the P3 peptide and Aβ, respectively [1, 2, 5, 7]. Aβ can be 37-43 amino acids long depending on the γ-secretase cleavage site. While Aβ42 and Aβ40 are the two primary Aβ species, Aβ42 is more prevalent than Aβ40 in amyloid plaques. Most early-onset FAD mutations in APP, PSEN1, and PSEN2 increase the ratio of Aβ42:Aβ40, which drives the aggregation of Aβ into neurotoxic oligomeric assemblies [1, 2]. All three secretases are transmembrane proteins. α-Secretase activity can be carried out by at least 3 transmembrane proteins, ADAM9, 10 and 17 belonging to the adamalysin (or a disintegrin and metalloproteinase, ADAM) protein family[6]. Generally, these proteases play roles in cell adhesion, proteolytic processing, and ectodomain shedding of cell surface proteins. BACE1 (also named as Asp2, or memapsin 2), a type 1 transmembrane protein, has been identified as the β-secretase protein, which belongs to the pepsin family of aspartyl proteases [8]. γ-Secretase is a heterogeneous protein complex, which has been shown to contain at least four transmembrane proteins: presenilin (PS1), presenilin enhancer 2 (PEN2), nicastrin, and anterior pharynx-defective 1(APH-1) [1, 9, 10]. APP processing by α-, β- and γ-secretases is highly regulated, and the misregulation of APP processing is believed to underlie the pathogenesis of AD, particularly by increasing total Aβ levels or the Aβ42:Aβ40 ratio [1, 5]. AD brain is characterized by tremendous neuronal death and significantly decreased cell density, particularly in late stage of AD. However, currently, little is known regarding how Aβ levels and APP metabolism are altered by cell density. Thus, in this report, we set out to determine whether Aβ generation and APP metabolism are altered by cell density. We utilized both neuronal and non-neuronal cell models, as well as mouse primary cortical neurons in this study. We demonstrated that Aβ40, Aβ42, Aβ(40+42) levels, and the ratio of Aβ42:Aβ40 are all significantly increased with decreases in cell density. Thus, these findings carry profound implications for previous and forthcoming studies assessing the effects of various conditions and genetic/chemical factors on AD pathogenesis, e.g. novel drugs on APP processing and Aβ generation in cell-based systems.
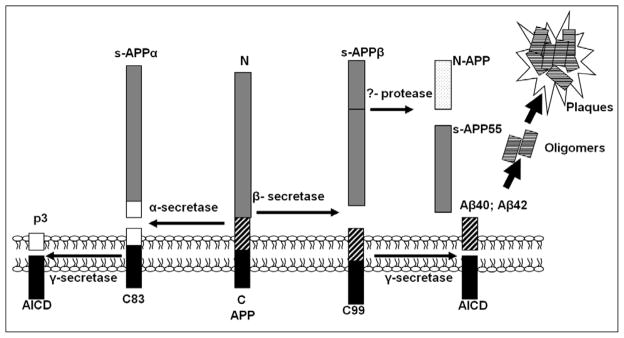
Figure 1. Summary of the proteolytic processing of APP.
The early-onset familiar AD gene APP encodes amyloid β-protein precursor, which generates Aβ through the serial proteolytic cleavage by β- and γ-secretase. β-secretase cleavage produces the secreted, ~90kDa protein, sAPPβ, and the β-carboxy-terminal fragment, β-CTF (or C99). sAPPβ is the substrate of an unidentified secretase, which produces N-APP (containing the N-terminal 286 amino acids of APP; ~35kDa) and s-APP55 (~55kDa). C99 can be cleaved by γ-secretase and gives rise to Aβ and AICD (APP intracellular domain). In contrast to this amyloidogenic process by β- and γ-secretase, APP undergoes an alternative cleavage pathway which precludes Aβ generation. This pathway is initiated by α-secretase and produces sAPPα and the carboxy-terminal fragment, α-CTF (or C83). C83 can be further cleaved by γ-secretase to produce P3 and AICD.
MATERIALS AND METHODS
Cell culture and mouse primary cortical neuron culture
Human neuroglioma H4 cells that stably over-express human APP751 (H4-APP751) cells have been reported previously [11–14]. Chinese hamster ovary (CHO) cells that stably over-express human APP751 (CHO-APP751) have been reported elsewhere [14, 15]. These cell lines were cultured on regular tissue culture plates in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, 100μg/ml streptomycin, and 200μg/ml G418. Mouse primary cortical neurons were obtained from Brainbits (E18) and were cultured on poly-D-lysine/laminin-coated plates in B27/Neurobasal medium supplemented with 1X GlutaMAX (Invitrogen) [13].
Chemicals and antibodies
The APP C-terminal antibody (targeting the last 19 amino acids of APP751, APP750 or APP695; 1:1000) was a polyclonal antibody, was purchased from Sigma (catalog #: A8717), and can detect full-length APP, and APP-CTFs (APP-C83 and APP-C99). β-Actin antibody (1:10,000) was purchased from Sigma. The HRP-conjugated secondary antibodies (anti-mouse and anti-rabbit) (1:100,000) were purchased from Thermofisher.
Cell lysis and protein amount quantification
Cells were lysed in M-PER (Mammalian Protein Extraction Reagent) (Thermoscientific) with 1X Halt protease and phosphatase inhibitor cocktail (Thermoscientific). The lysates were collected and centrifuged at 13,000 rpm (Eppendorf centrifuge 5417) for 10 minutes. Pellets were discarded and supernatants were transferred into a new micorcentrifuge tube [16]. Total protein was quantified using the bicinchoninic acid (BCA) protein assay kit (Pierce) [13].
Western blotting analysis
Western blotting analysis was carried out by the method described previously [13, 16–18]. Briefly, after centrifugation and protein concentration measurement, an equal amount of each protein sample was applied to electrophoresis followed by membrane transfer, antibody incubation, and signal development. The VersaDoc imaging system (Bio-Rad) was used to develop the blots and the Quantity One software (Bio-Rad) was used to quantify the proteins of interest by subtracting the background, following the protocols described previously [13, 16, 17]. Signals from full-length APP (APP-FL), APP-C83, APP-C99, and β-actin were assessed and quantified. β-Actin was used as an internal loading control.
Aβ measurement
Aβ measurement was performed following the manufacturer’s suggested protocols and was described previously [13, 17]. In brief, Aβ40 and Aβ42 levels were quantified using a sandwich enzyme-linked immunosorbent assay (ELISA), and were normalized to the protein concentration from the same cell lysates. The media from H4-APP751 cells were analyzed using the Wako Human kit to detect the levels of Aβ40 (catalog #:292-62301) and Aβ42 (catalog #:298-62401). The Aβ40 kit from Signet was also utilized to validate the Aβ40 results from Wako. The media from CHO cells and mouse primary cortical neurons were analyzed using the Wako Human/Rat kit to detect the levels of Aβ40 (catalog #:294-62501) and Aβ42 (catalog #: 290-62601). Briefly, the Wako ELISA kit used an assay based on colorimetric changes. First, 100μl standard and treatment samples were incubated at 4°C with plate seal overnight, and then the solution was gently washed off and 100μl of the HRP-conjugated antibody solution was added into each well and incubated for 2 hours. The solution was then washed off and the TMB solution was added with 30 minutes incubation. Lastly, 100μl Stop solution was added into each well and the absorbance was immediately read at 450nm with a microplate reader.
α- and β-Secretase activity assay
We used the fluorometric reaction-based kits that detect the APP cleavage activity level of α- and β-secretase. The assays were performed according to the manufacturer’s protocols (R&D Systems) and were described elsewhere [19].
Data analysis
Aβ40 and Aβ42 levels from each sample, as well as fluorometric readouts from the secretase activity experiment were normalized to the protein concentrations from the same cell lysates [11, 13, 18]. To account for any differences in loading, the levels of full-length APP and its CTFs (C83 and C99) were normalized to the β-actin levels from the same lane. The ratios of C83 and C99 to full-length APP were calculated by dividing C83 or C99 by APP-FL levels from the same lane. The ratios of total Aβ to C99 were calculated by dividing total Aβ levels by C99 levels from the same samples. The observations from higher cell densities were normalized to lowest cell densities, unless otherwise specified, and were demonstrated as mean ± S.E. from at least three independent experiments [11, 13, 18]. We used a two-tailed t test to compare the differences between two experimental groups. The Bonferroni correction analysis was used to correct for multiple comparisons within a single experiment. P values 0.05 were considered statistically significant.
RESULTS
Aβ40 and Aβ42 levels vary depending on cell density
We initially employed a neuronal cell line to assess the effects of varying cell densities on Aβ levels and APP processing. The stable human neuroglioma H4-APP751 cell line were plated in 6-well cell culture plates with different numbers (50K or 50 thousand, 100K, 150K, 200K, 250K, 300K and 400K) [13, 17, 18]. The surface area of one well in a 6-well plate is 9.6cm2 and the seeding densities were approximately 5, 10, 15, 20, 25, 30, and 40 X 103 cells/cm2, respectively. Cells were harvested after 48 hours. There was no observed cell death, but differences in morphology were observed at different densities. As expected, cells plated at lower cell densities exhibited less cell-cell contact and occupied less area based on our subjective observation. In addition, individual cells plated at lower densities possessed a larger cell size than individual cells at higher densities. Concordantly, cells at higher densities exhibited more cell-cell contact and occupied greater area, and individual cell revealed more circular shape and smaller size at higher densities.
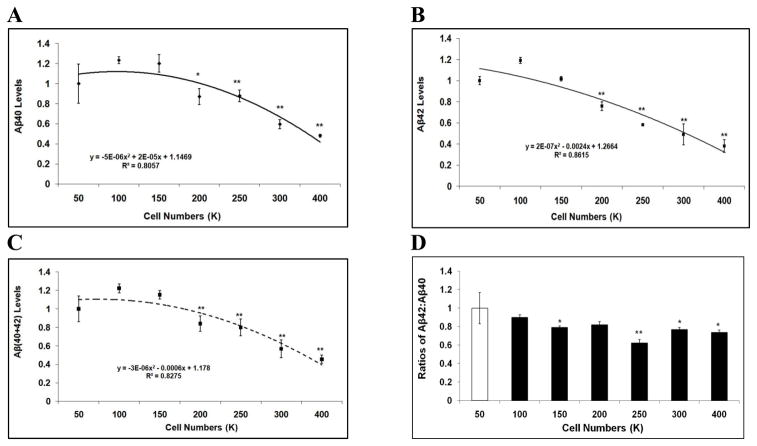
Conditioned media were harvested and subjected to ELISA to measure Aβ levels. For each sample, both Aβ40 and Aβ42 levels were measured and normalized to cell lysate protein concentration. At the plating of 100K, the levels of Aβ40 and Aβ42 exhibited a trend of, but not significant increase versus 50K (Fig. 2A-2B). At plating numbers from 200K to 400K, both Aβ40 and Aβ42 levels were significantly lower versus 100K (p<0.05; Fig. 2A-2B). For example, plating at 400K, Aβ40 and Aβ42 levels decreased 61.0%, and 68.0%, respectively, compared to plating at 100K (p<0.01; Fig. 2A-2B). The regression expression of Aβ40 is represented as a polynomial function: y = −5E-06x2 + 2E-05x + 1.1469 (R2 = 0.8057) (a fitting curve with highest R2 value was chosen) (Fig. 2A). The regression expression of Aβ42 is represented as a polynomial function: y = 2E-07x2 − 0.0024x + 1.2664 (R2 = 0.8615) (Fig. 2B). Next, we assessed Aβ(40+42) levels and the Aβ42:Aβ40 ratios at varying cell densities. Here we used the levels of Aβ(40+42) to represent total Aβ since these two Aβ species constitute the primary Aβ components. Plating at 100K revealed a trend to, but not significant increase in the level of total Aβ versus 50K (Fig. 2C). At plating of 200K to 400K, total Aβ levels markedly declined with increases in cell density versus 100K (p<0.05; Fig. 2C). Levels of Aβ(40+42) could be represented using a polynomial function: y = −3E-06x2 − 0.0006x + 1.178 (R2 = 0.8275). Similarly, the ratio of Aβ42:Aβ40 was decreased with increasing seeding densities, and presented as a polynomial function: y = 4E-06x2 − 0.0027x + 1.1257 (R2 = 0.7922) (Fig. 2D). For example, the Aβ42:Aβ40 ratio was 30.9% lower at 250K versus 100K (p<0.01).
Figure 2. Aβ40 and Aβ42 levels vary depending on cell density in H4-APP751 cells.
Cells of different numbers were seeded on 6-well plates. Conditioned media and cell lysates were collected after 48h. Cell lysates were applied to BCA assay to calculate protein concentration. Conditioned media were applied to ELISA to calculate Aβ40 and Aβ42 levels, which were then normalized to protein concentration. The Aβ40 and Aβ42 values represented in the figure were normalized values. The values of Aβ40, Aβ42, Aβ(40+42), and the Aβ42:Aβ40 ratio from higher cell densities were compared to those from 100K. A-C At higher seeding densities (200K-400K), the levels of Aβ40 (A), Aβ42 (B), and Aβ(40+42) (C) significantly decreased versus 100K. D. The ratio of Aβ42:Aβ40 was also reduced with increasing seeding densities (n=3 for each treatment group; mean ± S.E.; *p<0.05; **p<0.01).
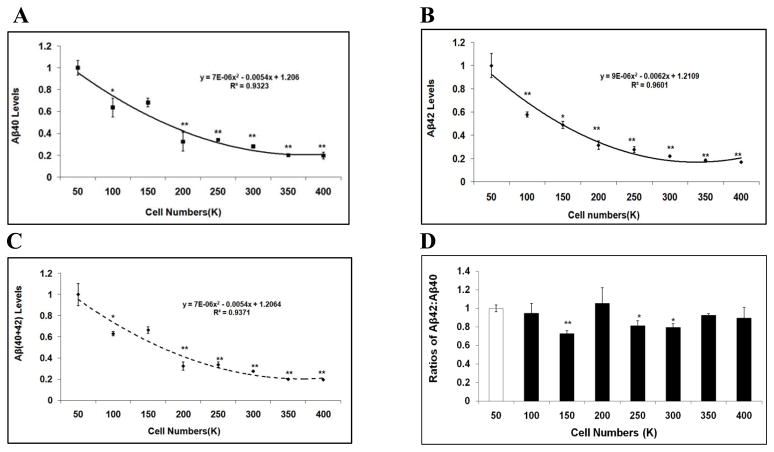
Cell type-specific differential effects in APP processing have been previously observed [16, 20, 21]. Therefore, we next validated the effect of cell density on Aβ levels utilizing different cell models. First, we utilized a non-neuronal cell model, chinese hamster ovary cells stably over-expressing human APP751 (CHO-APP751). CHO-APP751 cells of different numbers (50K, 100K, 150K, 200K, 250K, 300K, 350K and 400K) were initially seeded and incubated on 6-well cell culture plates and allowed to grow for 48 hours. Conditioned media were collected and applied to ELISA to measure Aβ levels. As a result, similar to what was observed in H4-APP751 cells, there was no cell death at each density, but there were differences in morphology at different densities. We showed that both Aβ40 and Aβ42 levels significantly decreased with increases in cell density (p<0.05; versus 50K) (Fig. 3A-3B). For example, at plating of 400K, Aβ40 and Aβ42 levels decreased by 80.2% and 83.1%, respectively, versus 50K (p<0.01; Fig. 3A-3B). In addition, total Aβ levels significantly declined with increases in cell density compared to those at 50K (p<0.05; Fig. 3C). For example, plating at 400K, total Aβ level decreased by 80.4% versus 50K (p<0.01; Fig. 3C). Similar to what was observed in H4-APP751 cells, the Aβ42:Aβ40 ratio was reduced with increasing cell densities (Fig. 3C). For example, the Aβ42:Aβ40 ratio decreased by 27.5% at plating of 150K versus 50K (p<0.01; Fig. 3C).
Figure 3. Aβ40 and Aβ42 levels vary depending on cell density in CHO-APP751 cells.
Cells of different numbers were seeded on 6-well plates and harvested after 48h. Cell lysates were applied to BCA assay to calculate protein concentration. Conditioned media were applied to ELISA to calculate Aβ40 and Aβ42 levels, which were then normalized to protein concentration. The values of Aβ40, Aβ42, Aβ(40+42), and the Aβ42:Aβ40 ratios from higher cell densities were compared to the values at 50K. AC At higher seeding densities, the levels of Aβ40 (A), Aβ42 (B), and Aβ(40+42) (C) significantly decreased versus 50K. D. The ratio of Aβ42:Aβ40 was also reduced with increasing seeding densities (n=3 for each treatment group; mean ± S.E.; *p<0.05; **p<0.01).
Aβ40 and Aβ42 levels vary depending on cell density in mouse primary cortical neurons
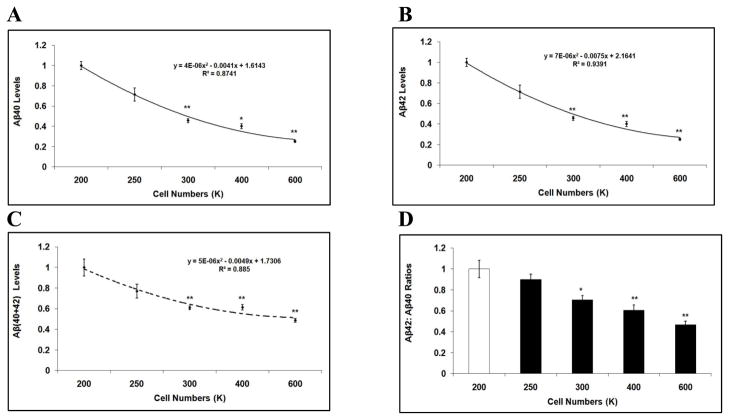
Next, we studied the effects of cell density on Aβ levels using mouse primary cortical neurons. The average size of a mouse primary neuron is approximately 25% of the size of an H4 or CHO cell. Therefore, in order to arrive at final cell densities for the mouse primary cortical neurons that would be similar to those of the H4-APP751 and CHO-APP751 cell lines, we used 24-well poly-D-lysine coated plates. Specifically, mouse primary cortical neurons were initially seeded at different densities (200K, 250K, 300K, 400K and 600K), and allowed to grow for 48 hours. Conditioned media were collected and subjected to ELISA to measure Aβ levels. (We had to start with 200K as the lowest seeding density in order to achieve reliable measurements of Aβ and control protein concentrations in the mouse primary neuronal cultures.) Our data showed that the levels of Aβ40, Aβ42, Aβ(40+42), and the ratio of Aβ42:Aβ40 markedly decreased with increases in cell density when compared to the lowest starting density of 200K (p<0.05; Fig. 4A-4D). For example, comparing the plating of 600K to 200K, the Aβ40 level decreased by 45.3% (p<0.01; Fig. 4A), the Aβ42 level declined by 74.9% (p<0.01; Fig. 4B), the total Aβ level dropped by 51.2% (p<0.01; Fig. 4C), and the ratio of Aβ42:Aβ40 decreased by 53.2% (p<0.01; Fig. 4D). Thus, studies of H4-APP751 cells, CHO-APP751 cells, and mouse primary cortical neurons consistently revealed that the levels of Aβ40, Aβ42, total Aβ, and Aβ42:Aβ40 ratios were inversely correlated with cell density.
Figure 4. Aβ40 and Aβ42 levels vary depending on cell density in mouse primary cortical neurons.
Mouse primary cortical neurons were seeded on poly-D-lysine/laminin-coated 24-well plates and harvested after 48h. Cell lysates were applied to BCA assay to calculate protein concentration. Conditioned media were applied to ELISA to calculate Aβ40 and Aβ42 levels, which were then normalized to protein concentration. The values of Aβ40, Aβ42, total Aβ, and the Aβ42:Aβ40 ratios from high seeding densities were compared to those from 200K. A-D At higher seeding densities (300K-600K), the levels of Aβ40 (A), Aβ42 (B), Aβ(40+42) (C), and the ratios of Aβ42:Aβ40 (D) significantly decreased compared to those at 200K (n=3 for each treatment group; mean ± S.E.; *p<0.05; **p<0.01).
APP proteolytic processing varies depending on cell density
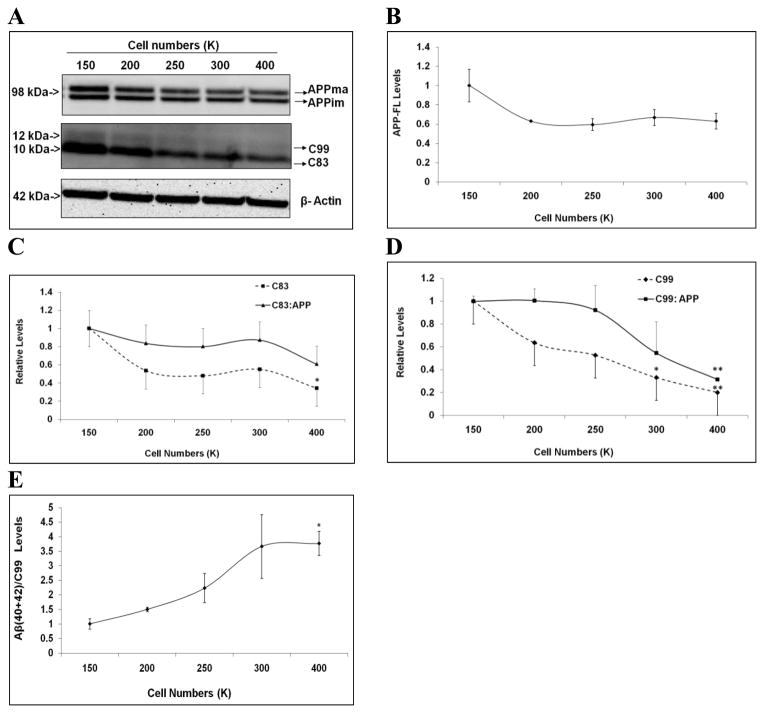
In the next set of experiments, we asked whether APP processing was altered with changes in cell density. H4-APP751 cells of different numbers were seeded on 6-well cell culture plates and allowed to grow for 48 hours. Cell lysates were collected and applied to Western blotting analysis. Samples of 50K and 100K densities were not applied due to their low protein concentrations for the Western blotting analysis. Equal amounts of protein were loaded onto the electrophoresis gel. We observed that full-length APP (or APP-FL) were represented with immature (N-glycosylated) and mature (N, O-glycosylated) forms of APP, which has been observed in previous reports [16, 17, 20, 22]. The levels of full-length APP and its CTFs (C83 and C99) were quantified and normalized to β-actin from the same samples [11, 16, 17] and compared to 150K versus the higher densities (200K to 400K). With increased cell density, full-length APP exhibited a trend toward decreased levels (Fig. 5A-5B). Next we measured the levels of APP proteolytic products APP-C83 and APP-C99, as well as the ratios of C83/APP and C99/APP. All of these measures varied inversely with cell density. For example, comparing the values at 400K to 150K, the APP-C83 level decreased by 65.6% (p<0.05), the C83/APP ratio declined by 39.3%, the APP-C99 level decreased by 80.0% (p<0.01), and the ratio of C99/APP decreased by 68.5% (p<0.01) (Fig. 5C-5D). Thus, these data suggested that the APP processing activities of α- and β-secretase were attenuated with increases in cell density.
Figure 5. APP proteolytic processing varies depending on cell density in H4-APP751 cells.
A-D. H4-APP751 cells were seeded on 6-well plates with different densities. Cell lysates were collected after 48h and applied to Western blotting analysis. The levels of full-length APP, APP-CTFs (C83 and C99), and their ratios to APP from higher seeding densities were compared to those at 150K. At higher cell densities, full-length APP levels had a trend to decrease versus 150K (A-B). The levels of C83 and C99, as well as their ratios to full-length APP decreased at higher seeding densities (C-D). E. The ratios of Aβ(40+42):APP-C99 were obtained by normalizing the levels of Aβ(40+42) to APP-C99 levels from the same samples, and the ratios from higher seeding densities were compared to those at 150K. The ratios of Aβ(40+42):APP-C99 increased with higher seeding densities (n=3 for each treatment group; mean ± S.E.; *p<0.05; **p<0.01).
We also used the ratio of Aβ(40+42):C99 to assess γ-secretase processing of APP-C99. The levels of Aβ(40+42) (normalized to cell lysate protein concentrations) were divided by the C99 levels from the same samples. The ratios of Aβ(40+42):C99 at higher seeding densities were compared to those at 150K. We found that the level of Aβ(40+42):C99 initially increased at higher plating densities and reached a plateau at 300K (Fig. 5E). For example, at initial seeding of 400K, the ratio of Aβ(40+42):C99 increased by 276.3% versus 150K (p<0.01; Fig. 5E). Thus, these data suggested that γ-secretase processing of APP is potentiated with increases in cell density, which is different from the changes in α- and β-secretase processing of APP.
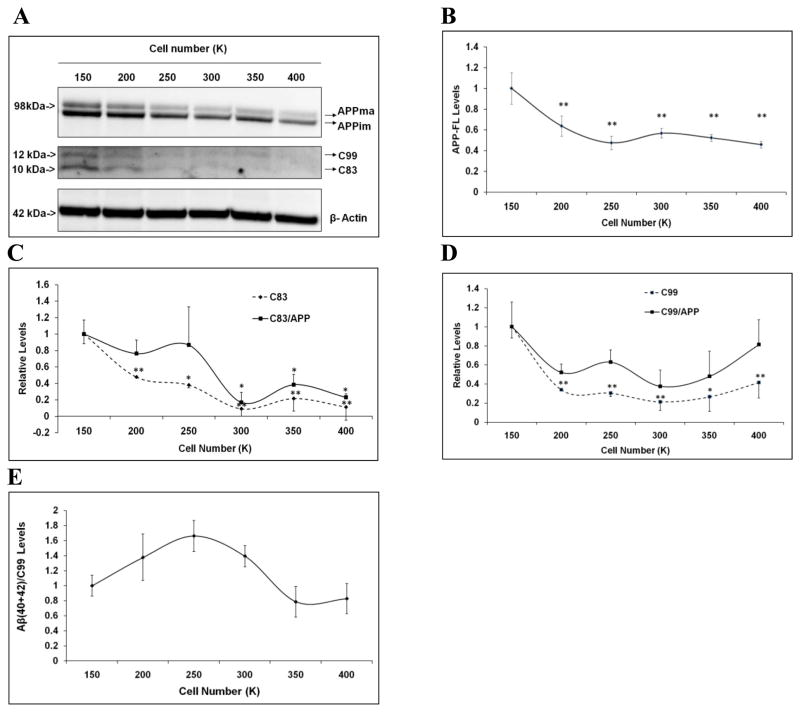
Next we utilized CHO-APP751 cells to validate our observation of cell density-dependent APP processing in H4 cells. CHO-APP751 cells of different numbers were seeded on 6-well cell culture plates and allowed to grow for 48 hours. Cell lysates were collected and applied to Western blotting analysis, excluding the samples from the densities of 50K and 100K due to low protein concentrations. Equal amount of protein from each sample was loaded onto the electrophoresis gel. Full-length APP, APP-C83 and APP-C99 levels were quantified and normalized to β-actin from the same samples. Full-length APP levels at higher densities were normalized to the values at 150K. Our data showed that at higher cell densities, full-length APP levels were markedly decreased versus those at 150K (Fig. 6A-6B). As an example, at the plating of 400K, full-length APP levels decreased by 54.2% compared to those at 150K (p<0.05; Fig. 6A-6B).
Figure 6. APP proteolytic processing varies depending on cell density in CHO-APP751 cells.
A-D. CHO-APP751 cells were seeded on 6-well plates with different densities. Cell lysates were collected after 48h and applied to Western blotting analysis. The levels of full-length APP, APP-CTFs (C83 and C99), and their ratios to APP from higher seeding densities were compared to those at 150K. At higher cell densities (200K-400K), full-length APP levels significantly decreased versus those at 150K (A-B). At higher seeding densities, the levels of C83 and C99, as well as their ratios to full-length APP decreased versus 150K (C-D). E. The ratios of Aβ(40+42):APP-C99 were obtained by normalizing the levels of Aβ(40+42) to APP-C99 levels from the same samples. The ratios of Aβ(40+42): APP-C99 initially increased with higher seeding densities (versus 150K), but then began to decrease with seeding densities > 250K (n=3 for each treatment group; mean ± S.E.; *p<0.05; **p<0.01).
We next measured levels of APP proteolytic fragments (C83 and C99), their ratios to full-length APP, as well as the ratio of Aβ(40+42) to C99 in response to increasing cell density. Our data showed that both C99 and C83 levels and their ratios to APP markedly decreased with the increasing cell density (Fig. 6C-6D). For example, at the plating of 400K, the ratio of C83:APP decreased by ~80%, and the ratio of C99:APP decreased by ~60%, respectively, when compared to those at 150K (p<0.05; Fig. 6C-6D). In contrast, at higher plating densities, the ratio of Aβ(40+42):C99 had a trend to initially increase from 150K to 250K, then start to decrease > 250K (Fig. 6E). Collectively, our data show that APP processing and Aβ generation are profoundly influenced by changes in cell density.
Validation of altered APP processing with changes in cell density
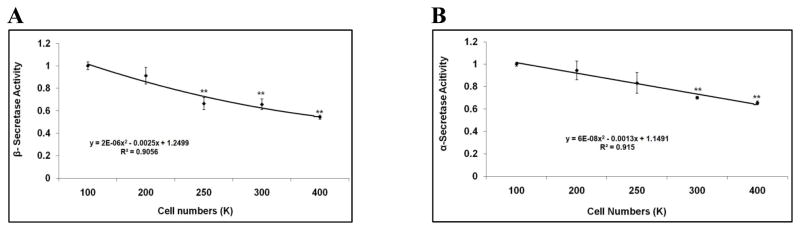
We next employed a fluorometric-based in-vitro assay for measuring α- and β-secretase activity levels to validate the effects of cell density on APP processing in H4-APP751 cells. We used 10cm-plates to obtain sufficient protein amounts for the assay, as recommended by the manufacturer. Based on the cell surface area, seeding densities of 0.5M (million), 1M, 1.5M, 2M and 3M in 10cm-plates are equivalent to those at 100K, 200K, 250K, 300K and 400K in 6-well plates, respectively. Cells were plated and harvested after 48 hours. Cell lysates were collected using the extraction buffer provided by the manufacturer and applied to the fluorometric assay. The fluorometric readouts were normalized to the cell lysate protein concentration of each sample. The normalized fluorometric levels at higher densities were compared to those at 100K. Our data showed that at higher seeding densities, both α-, and β-secretase activities were attenuated when compared to those at 100K (Fig. 7A-7B). For example, at plating of 400K, β-secretase activity decreased by 45.8% versus 100K (p<0.01; Fig. 7A), while α-secretase activity decreased by 34.5% versus 100K (p<0.01; Fig. 7B). These data suggest that the effects of cell density on APP processing and Aβ levels are modulated, at least in part by changes in the activity levels of α- and β-secretase.
Figure 7. Validation of altered APP processing with changes in cell density.
A-B, H4-APP751 cells were seeded on 10cm-plates with different densities (0.5M, 1M, 1.5M, 2M, and 3M; equivalent to the densities of 100K, 200K, 250K, 300K, and 400K in 6-well plates, respectively). Cell lysates were collected after 48h and were applied to the BCA assay to determine protein concentration and the fluorometric assay to measure α- or β-secretase activity. The fluorometric readout was normalized to the protein concentration for each sample. Normalized fluorometric values at higher cell densities were compared to those at 100K. At higher seeding densities, the APP processing activity of α- and β-secretase decreased when compared to those at 100K (n=3 for each treatment group; mean ± S.E.; *p<0.05; **p<0.01).
DISCUSSION
AD pathogenesis is believed to be primarily triggered by Aβ accumulation in the brain [1–5]. Thus, identifying factors that influence APP processing and Aβ generation may help elucidate the etiology and pathogenesis of AD. Here, we tested cell density as a putative effector of APP processing using multiple cell models. We found that Aβ levels and APP processing are strongly influenced by cell density. These findings have profound implications for thousands of previously published studies assessing APP processing and Aβ generation in vitro. In addition, the data described here may be relevant to changes in APP processing and Aβ generation in the AD brains as cell densities are altered with disease progression. First, we showed that both Aβ40 and Aβ42 levels vary inversely with cell density. Second, we found that the ratios of Aβ42:Aβ40 are also reduced with increasing cell density. Importantly, the increase of Aβ42:Aβ40 ratio has been identified in more than 200 FAD mutations in APP, PSEN1, and PSEN2, which drive the aggregation of Aβ into neurotoxic oligomeric assemblies [1, 2]. Thus, in previous countless studies assessing the effects of various conditions and factors on Aβ levels and the Aβ42:Aβ40 ratio, study-to-study variations in cell density may have had profound and heretofore-unnoticed effects on the reported experimental conclusions.
Along with these findings, we show that Aβ levels and APP processing by proteolytic secretases are altered at different cell densities. Particularly, both α- and β-secretase processing of APP are potentiated, whereas γ-secretase processing appear to be attenuated with decreasing cell density overall (based on the ratio of Aβ/C99). In the neuronal cell model (H4-APP751), γ-secretase processing of APP reaches a maximum level at high densities (300K-400K), and becomes attenuated at lower densities (150K-250K). In the non-neuronal model (CHO-APP751), γ-secretase processing of APP achieves a maximum level at medium cell densities (250K), and is attenuated at both lower densities (150–250K) and higher densities (250K-400K).
PS1/γ-secretase can process several other substrates apart from APP, e.g. molecules regulating cell-cell contact and signal transduction [23–26]. The cadherin molecules, primarily E(epithelial)- and N(neuronal)-cadherin, form cadherin-based adherens junctions (CAJs) at sites of cell-cell interaction and modulate fundamental intercellular functions, e.g. cell-cell interaction and development [26, 27]. When cell densities are high, PS1 is concentrated at the intercellular sites on the cell surface in CAJs and promotes CAJ assembly and cell-cell interaction [23]. However, when cells are exposed to stimuli, e.g. apoptosis and calcium influx, PS1/γ-secretase cleaves full-length E-cadherin and a transmembrane E-cadherin CTF, derived from a metalloproteinase cleavage [26]. This cleavage leads to the disassembly of the CAJs, and increases soluble β- and α-catenin. The cytosolic pool of β-catenin has been indicated to be a key regulator of the Wnt signaling pathway [28, 29], which is associated with not only APP processing and Aβ metabolism [30], but also the pathogenesis of AD and other neurological disorders [29]. Moreover, over-expressing PS1 leads to elevated cell–cell interaction and aggregation, while this function is lost with the artificial loss-of-function PSEN1 mutation, D257A [23]. Collectively, these multiple lines of evidence indicate that γ-secretase activity may be regulated by cell density and vice-versa, and support our current finding that γ-secretase processing of APP may also be dependent on cell density.
In summary, we show that Aβ40 and Aβ42 levels, total Aβ levels, and the ratios of Aβ42:Aβ40 vary inversely with cell density. Moreover, the activities of both α- and β-secretase were potentiated with decreases in cell density. Finally, based on the ratio of Aβ/C99, γ-secretase activity appears to be attenuated with decreases in cell density, although, additional studies will be necessary to confirm this. Overall, these findings carry profound implications for both previous and forthcoming studies investigating the effects of various conditions and genetic/chemical factors on APP processing and Aβ generation in cell-based systems. Moreover, it is interesting to speculate whether cell density changes in vivo may also affect APP processing and Aβ levels in the AD brain.
Acknowledgments
Sponsor’s role
The sponsors played no role in the study design, collection, analysis and interpretation of data and preparation and submission of the manuscript.
Funding sources
The research and salary support were from Cure Alzheimer Fund (http://www.curealzfund.org/), the Funds for Medical Discovery (FMD) from the Massachusetts General Hospital (http://www.massgeneral.org/give/mghfund/), a Ruth L. Kirschstein National Research Service Award (NRSA) (5T32AG000222-18) (http://grants.nih.gov/training/nrsa.htm), and the Neurodegenerative Disease Pilot Study Program from Harvard NeuroDiscovery Center and Massachusetts Alzheimer’s Disease Research Center (http://madrc.mgh.harvard.edu/).
References
- 1.Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 5.Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J Clin Invest. 2005;115:1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Saunders AJ. Therapeutic targeting of the alpha-secretase pathway to treat Alzheimer's disease. Discov Med. 2007;7:113–117. [PubMed] [Google Scholar]
- 7.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 8.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 9.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 10.Sisodia SS, St George-Hyslop PH. gamma-Secretase, Notch, Abeta and Alzheimer's disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z, Dong Y, Maeda U, Xia W, Tanzi RE. RNA interference silencing of the adaptor molecules ShcC and Fe65 differentially affect amyloid precursor protein processing and Abeta generation. J Biol Chem. 2007;282:4318–4325. doi: 10.1074/jbc.M609293200. [DOI] [PubMed] [Google Scholar]
- 12.Xie Z, Romano DM, Tanzi RE. RNA interference-mediated silencing of X11alpha and X11beta attenuates amyloid beta-protein levels via differential effects on beta-amyloid precursor protein processing. J Biol Chem. 2005;280:15413–15421. doi: 10.1074/jbc.M414353200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Browne A, Child D, Divito JR, Stevenson JA, Tanzi RE. Loss of function of ATXN1 increases amyloid beta-protein levels by potentiating beta-secretase processing of beta-amyloid precursor protein. J Biol Chem. 285:8515–8526. doi: 10.1074/jbc.M109.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Browne A, Child D, Tanzi RE. Curcumin decreases amyloid beta-peptide levels by attenuating the maturation of amyloid-beta precursor protein. J Biol Chem. doi: 10.1074/jbc.M110.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huttunen HJ, Guenette SY, Peach C, Greco C, Xia W, Kim DY, Barren C, Tanzi RE, Kovacs DM. HtrA2 regulates beta-amyloid precursor protein (APP) metabolism through endoplasmic reticulum-associated degradation. J Biol Chem. 2007;282:28285–28295. doi: 10.1074/jbc.M702951200. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Khandelwal PJ, Chakraborty R, Cuellar TL, Sarangi S, Patel SA, Cosentino CP, O'Connor M, Lee JC, Tanzi RE, Saunders AJ. An AICD-based Functional Screen to Identify APP Metabolism Regulators. Mol Neurodegener. 2007;2:15. doi: 10.1186/1750-1326-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiltunen M, Lu A, Thomas AV, Romano DM, Kim M, Jones PB, Xie Z, Kounnas MZ, Wagner SL, Berezovska O, Hyman BT, Tesco G, Bertram L, Tanzi RE. Ubiquilin 1 modulates amyloid precursor protein trafficking and Abeta secretion. J Biol Chem. 2006;281:32240–32253. doi: 10.1074/jbc.M603106200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Browne A, Kim DY, Tanzi RE. Familial Alzheimer's Disease Mutations in Presenilin 1 Do Not Alter Levels of the Secreted Amyloid-beta Protein Precursor Generated by beta-Secretase Cleavage. Curr Alzheimer Res. 2009 doi: 10.2174/156720510790274428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons RB, Price GC, Farrant JK, Subramaniam D, Adeagbo-Sheikh J, Austen BM. Statins inhibit the dimerization of beta-secretase via both isoprenoid- and cholesterol-mediated mechanisms. Biochem J. 2006;399:205–214. doi: 10.1042/BJ20060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia W, Zhang J, Kholodenko D, Citron M, Podlisny MB, Teplow DB, Haass C, Seubert P, Koo EH, Selkoe DJ. Enhanced production and oligomerization of the 42-residue amyloid beta-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem. 1997;272:7977–7982. doi: 10.1074/jbc.272.12.7977. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Kang DE, Xia W, Okochi M, Mori H, Selkoe DJ, Koo EH. Subcellular distribution and turnover of presenilins in transfected cells. J Biol Chem. 1998;273:12436–12442. doi: 10.1074/jbc.273.20.12436. [DOI] [PubMed] [Google Scholar]
- 22.Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe DJ. The Swedish mutation causes early-onset Alzheimer's disease by beta-secretase cleavage within the secretory pathway. Nat Med. 1995;1:1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- 23.Georgakopoulos A, Marambaud P, Efthimiopoulos S, Shioi J, Cui W, Li HC, Schutte M, Gordon R, Holstein GR, Martinelli G, Mehta P, Friedrich VL, Jr, Robakis NK. Presenilin-1 forms complexes with the cadherin/catenin cell-cell adhesion system and is recruited to intercellular and synaptic contacts. Mol Cell. 1999;4:893–902. doi: 10.1016/s1097-2765(00)80219-1. [DOI] [PubMed] [Google Scholar]
- 24.Kouchi Z, Barthet G, Serban G, Georgakopoulos A, Shioi J, Robakis NK. p120 catenin recruits cadherins to gamma-secretase and inhibits production of Abeta peptide. J Biol Chem. 2009;284:1954–1961. doi: 10.1074/jbc.M806250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serban G, Kouchi Z, Baki L, Georgakopoulos A, Litterst CM, Shioi J, Robakis NK. Cadherins mediate both the association between PS1 and beta-catenin and the effects of PS1 on beta-catenin stability. J Biol Chem. 2005;280:36007–36012. doi: 10.1074/jbc.M507503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J Cell Sci. 1997;110 ( Pt 17):2065–2077. doi: 10.1242/jcs.110.17.2065. [DOI] [PubMed] [Google Scholar]
- 28.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 29.De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- 30.Magdesian MH, Carvalho MM, Mendes FA, Saraiva LM, Juliano MA, Juliano L, Garcia-Abreu J, Ferreira ST. Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. J Biol Chem. 2008;283:9359–9368. doi: 10.1074/jbc.M707108200. [DOI] [PMC free article] [PubMed] [Google Scholar]