Abstract
Alzheimer's disease (AD) is a progressive and irreversible neurodegenerative disorder in which the aggregation and deposition of amyloid-β (Aβ) peptides in the brain are central to its pathogenesis. In healthy brains, Aβ is effectively metabolized with little accumulation. Cellular uptake and subsequent degradation of Aβ is one of the major pathways for its clearance in the brain. Increasing evidence has demonstrated significant roles for the low-density lipoprotein receptor-related protein 1 (LRP1) in the metabolism of Aβ in neurons, glia cells, and along the brain vasculatures. Heparan sulfate proteoglycan (HSPG) has also been implicated in several pathogenic features of AD, including its colocalization with amyloid plaques. Here, we demonstrate that HSPG and LRP1 cooperatively mediate cellular Aβ uptake. Fluorescence-activated cell sorter and confocal microscopy revealed that knockdown of LRP1 suppresses Aβ uptake, whereas overexpression of LRP1 enhances this process in neuronal cells. Heparin, which antagonizes HSPG, significantly inhibited cellular Aβ uptake. Importantly, treatment with heparin or heparinase blocked LRP1-mediated cellular uptake of Aβ. We further showed that HSPG is more important for the binding of Aβ to the cell surface than LRP1. The critical roles of HSPG in cellular Aβ binding and uptake were confirmed in Chinese hamster ovary cells genetically deficient in HSPG. We also showed that heparin and a neutralizing antibody to LRP1 suppressed Aβ uptake in primary neurons. Our findings demonstrate that LRP1 and HSPG function in a cooperative manner to mediate cellular Aβ uptake and define a major pathway through which Aβ gains entry to neuronal cells.
Introduction
Alzheimer's disease (AD) is the most common form of dementia with amyloid plaques and neurofibrillary tangles as its pathological hallmarks (Selkoe, 2001, 2002). Increasing evidence supports the hypothesis that accumulation, aggregation, and deposition of amyloid-β (Aβ) peptides in the brain are critical steps in its pathogenesis (Selkoe, 2001; Shankar and Walsh, 2009). Aβ peptide is cleaved from amyloid precursor protein (APP) and secreted into the extracellular space (Bredesen, 2009). It is effectively cleared in healthy brains (Bu, 2009). In vivo microdialysis analysis demonstrated that Aβ in brain interstitial fluid of young mice is cleared within a relatively short half-life (∼2 h) (Cirrito et al., 2003). There are several pathways through which Aβ is cleared from the brain. These include cellular uptake and degradation, clearance along the interstitial fluid drainage pathway, through the blood–brain barrier, and through proteolytic degradation by Aβ-degrading enzymes (Bu, 2009). Members of the low-density lipoprotein (LDL) receptor family are expressed in different cell types in these pathways and play important roles in Aβ clearance. In particular, the LDL receptor-related protein 1 (LRP1) is shown to mediate the metabolism of Aβ in neurons (Qiu et al., 1999), glia cells (Wyss-Coray et al., 2003), and brain vessels (Urmoneit et al., 1997; Kanekiyo and Bu, 2009). LRP1 is a large endocytic receptor that recognizes an array of ligands, including APP, apolipoprotein E (apoE), α2-macroglobulin, and receptor-associated protein (RAP), which are involved in Aβ production and clearance (Herz and Strickland, 2001; Bu et al., 2006; Kanekiyo and Bu, 2009). Among these LRP1 ligands, an isoform of apoE (apoE4) is a strong genetic risk factor for AD (Bu, 2009).
Heparan sulfate proteoglycans (HSPGs) are abundant cell surface receptors that interact with a variety of ligands through electrostatic interactions (Poon and Gariépy, 2007). Several HSPGs colocalize with senile plaques and cerebral amyloid angiopathy (van Horssen et al., 2003). HSPGs found on the surface of almost all mammalian cells are members of the glycosaminoglycan family of polysaccharides and are involved in a large number of biological processes, including development, embryogenesis, cell growth and division, homeostasis, and coagulation (Turnbull et al., 2001). Heparan sulfate (HS) binds to Aβ (Brunden et al., 1993) and heparin attenuates neurotoxicity and inflammatory activity of Aβ, suggesting a potentially important role for HSPG in cellular metabolism of Aβ (Bergamaschini et al., 2002). In addition, LRP1 and HSPG are part of an immunoprecipitable complex at the cell surface to mediate lipid metabolism (Wilsie and Orlando, 2003). Internalization of apoE/lipoprotein particles is partially dependent on the HSPG and LRP1 complex (Mahley and Ji, 1999), suggesting a cooperative function for these apoE receptors at the cell surface.
Although Aβ clearance by its degrading enzymes has been studied extensively, less is known about receptor-mediated endocytic pathways for cellular Aβ uptake. In this study, we focused on both individual and potentially cooperative roles of LRP1 and HSPG in cellular Aβ uptake. We demonstrated that LRP1 and HSPG play critical, cooperative roles in neuronal Aβ uptake.
Materials and Methods
Reagents.
5(6)-Carboxyfluorescein (FAM)–Aβ40 and FAM–Aβ42 were purchased from AnaSpec. Aβ peptides were dissolved in dimethylsulfoxide at 200 μm and kept at −80°C before use. Recombinant RAP was purified as described previously (Warshawsky et al., 1993) and labeled using the Alexa Fluor 488 Protein Labeling kit (Invitrogen). Anti-LRP1 antibody was produced in-house using rabbit polyclonal antibodies (Liu et al., 2007). Heparin was purchased from Elkins-Sinn.
Cell culture.
Mouse hypothalamic neuronal GT1-7 cells and mouse embryonic fibroblast (MEF) cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and maintained at 37°C in humidified air containing 5% CO2. Mouse neuroblastoma N2a cells were stably transfected with empty vector plasmid (pcDNA3) or mLRP4 plasmid and maintained in DMEM/OPTIMEM (1:1) containing 5% FBS with the addition of 200 μg/ml G418. Chinese hamster ovary (CHO) cells were grown in Ham's F-12 medium containing 10% FBS, 100 U/ml penicillin G, and 100 μg/ml streptomycin sulfate. Primary cortical neurons were obtained from 17-d-old embryos of wild-type mice and grown in Neurobasal medium (Invitrogen) supplemented with 0.5 mm Glutamax (Invitrogen), 25 μm glutamate (Invitrogen), and B27 (Invitrogen) (Fuentealba et al., 2009).
LRP1 knockdown by small interfering RNA.
Single-stranded, mouse LRP1-specific sense (5′-GGA GUC ACU UAC AUC AAU AUU-3′) and antisense (5′-UAU UGA UGU AAG UGA CUC CUU-3′) RNA oligonucleotides were synthesized by Dharmacon. Double-stranded RNA molecules were generated according to the instructions of the manufacturer. Cells were transfected with small interfering RNA (siRNA) (50 nm) using Lipofectamine2000 (Invitrogen), according to the specifications of the manufacturer, and analyzed 48 h after transfection. In some experiments, pcDNA3 vector or mLRP4 plasmid was cotransfected with LRP1–siRNA using Lipofectamine2000 according to the specifications of the manufacturer.
Confocal microscopy.
Cells were cultured on eight-well slides (Lab-Tek II Chamber SlideTM System; Nalge Nunc International) at 37°C for at least 24 h before experiments. After incubation with FAM–Aβ40 or FAM–Aβ42 (500 nm) at 37°C for 4 or 24 h in the presence or absence of heparin (15 U/ml), fluorescence associated with Aβ was observed by confocal laser-scanning fluorescence microscopy (model LSM 510 invert; Carl Zeiss). In some experiments, non-immune IgG or anti-LRP1 IgG (75 μg/ml) was added 1 h before Aβ treatment. LysoTracker (50 nm; Invitrogen) was added 30 min before cell fixation and confocal imaging.
Fluorescence-activated cell sorter-based internalization and binding assays.
Cells were plated onto 12-well dishes and allowed to grow to 90% confluency. Cells were incubated with FAM–Aβ40 (500 nm), FAM–Aβ42 (500 nm), or Alexa Fluor 488–RAP (20 nm) in the presence or absence of heparin at 37°C for 24 h in DMEM with 10% FBS for internalization assay. In some experiments, cells were incubated with 5 Sigma U/ml Heparinase I (product number H2519, lot number 087K3790; Sigma) (Ji et al., 1998; Wilsie and Orlando, 2003) for 2 h at 37°C in DMEM. Cells were removed from the plate using Cell Dissociation Solution (Sigma) and incubated with Pronase (0.5 mg/ml; Roche Diagnostics) at 4°C for 20 min. Cells were then washed and resuspended in PBS containing 1.5% FBS, 1% sodium azide, and 1% paraformaldehyde. Cells (1 × 104) from each sample were analyzed for fluorescence on a BD FACS Calibur machine (BD Biosciences). Unstained cells without any exposure to fluorescence were used as a control for background fluorescence. Mouse primary cortical neurons were incubated with FAM–Aβ42 (500 nm) in the presence or absence of heparin at 37°C for 1, 4, 8, or 24 h. Non-immune IgG or anti-LRP1 IgG (75 μg/ml) was added 1 h before Aβ treatment. Cell-surface Aβ was removed using 0.25% trypsin/EDTA (Invitrogen). For binding assays, cells were incubated with FAM–Aβ42 (2 μm) or Alexa Fluor 488–RAP (100 nm) at 4°C for 2 h in PBS with 1.5% FBS after suspension by cell dissociation solution, and subjected to fluorescence-activated cell sorting (FACS) analysis. Only trace amount of SDS-stable Aβ42 oligomers and fibrils were detected in the media after incubation under these conditions (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Kinetic analysis of endocytosis.
Carrier-free [125I]Na was purchased from PerkinElmer Life and Analytical Science. RAP and Aβ42 (AnaSpec) were iodinated using the IODO-GEN (Thermo Fisher Scientific) and the Bolton-Hunter reagents (Thermo Fisher Scientific) according to the instructions of the manufacturer. N2a cells were plated onto 12-well dishes and allowed to grow to 90% confluency. Cells were incubated with 10 nm [125I]RAP or 100 nm [125I]Aβ42 in binding buffer (DMEM containing 0.6% bovine serum albumin) at 4°C for 60 min. Cells were then incubated at 37°C in binding buffer to initiate internalization. After each time interval, cells were placed on ice, and the binding buffer was replaced with ice-cold strip/stop solution (0.2 m acetic acid and 0.1 m NaCl, pH 2.6). Ligand remaining on the cell surface was stripped by incubation with stop/strip solution for 20 min and counted. Cells were then solubilized with low-SDS lysis buffer (62.5 mm Tris-HCl, pH 6.8, containing 0.2% SDS) and counted. The sum of radioactivity of ligands that were internalized plus that remaining on the cell surface after each assay was used as the maximum potential internalization. The fraction of internalized ligand at each time point was calculated and plotted.
Western blotting for Aβ.
Samples containing Aβ were electrophoretically separated on a 16% polyacrylamide Tris–tricine gels and transferred to polyvinylidene difluoride membrane. Immunoblotting with 6E10 antibody against Aβ (0.5 μg/ml) was performed overnight at 4°C, followed by the incubation with fluorescent anti-mouse IgG (1:10,000 dilution) for 1 h at room temperature. Fluorescence signals were detected with an Odyssey imaging scanner (LI-COR/Westburg).
Detection of cell-bound Aβ by ELISA.
Cells were incubated with Aβ42 (50 nm) for 3 h at 4°C in PBS with 1.5% FBS after suspension by Cell Dissociation Solution. After washing three times with PBS, cells were dissolved in 5 m guanidine in 50 mm Tris-HCl, pH 8.0. Samples were diluted 10-fold in DMEM and analyzed by sandwich ELISA for human Aβ42 (antibody 21F12), detected with biotin-conjugated antibody 3D6.
Statistical analysis.
All quantified data represent an average of triplicate samples. Error bars represent SD. Statistical significance was determined by Student's t test, and p < 0.05 was considered significant.
Results
Both LRP1 and HSPG play roles in Aβ uptake in GT1-7 neuronal cells
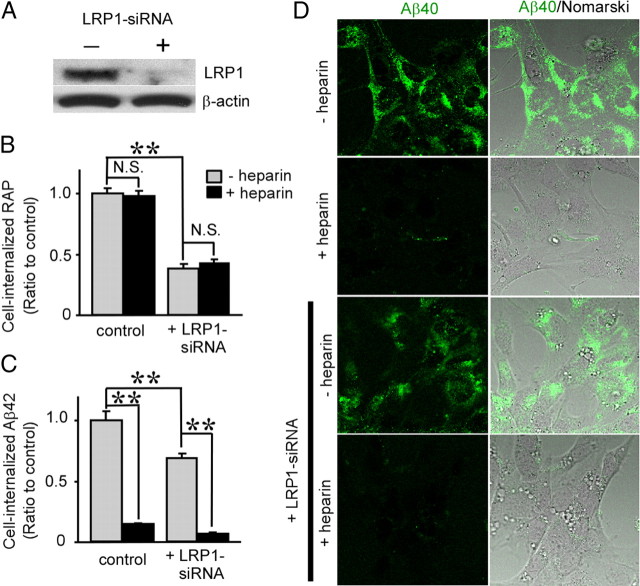
To investigate whether LRP1 and HSPG mediate cellular uptake of Aβ in neuronal cells, we used a mouse hypothalamic neuronal cell line, GT1-7 cells (Liposits et al., 1991). Cells were transfected with vehicle control or LRP1 siRNA and used for analysis 48 h after transfection. Expression of LRP1 was significantly suppressed by siRNA as confirmed by Western blotting (Fig. 1A). Control or LRP1-suppressed GT1-7 cells were incubated with 20 nm Alexa Fluor 488-labeled RAP for 4 h at 37°C, followed by treatment with Pronase to remove cell-surface RAP. Cell-internalized RAP was assessed by FACS. RAP is a chaperone for LDL receptor family members and tightly binds to LRP1 (Bu, 2001). Knockdown of LRP1 suppressed RAP internalization to 38% of the internalization for GT1-7 cells treated with vehicle control (Fig. 1B). When LRP1-suppressed GT1-7 cells were incubated with 500 nm FAM-labeled Aβ42 for 24 h at 37°C, Aβ42 internalization in LRP1-suppressed cells was reduced to 69% of the internalization for control cells (Fig. 1C). Although heparin did not affect RAP internalization (Fig. 1B), it did inhibit Aβ42 internalization to 14% of that for control cells (Fig. 1C). Importantly, heparin further inhibited Aβ42 internalization in LRP1-suppressd GT1-7 cells (Fig. 1C), indicating that the role of HSPG in Aβ uptake might be partially independent of LRP1. To confirm the effects of LRP1 knockdown and heparin on Aβ internalization, control and LRP1-suppressed GT1-7 cells were cultured with FAM–Aβ40 for 24 h at 37°C in the presence or absence of heparin and analyzed by confocal microscopy. Although cell-associated Aβ40 in LRP1-suppressed GT1-7 cells was slightly decreased, heparin nearly eliminated Aβ uptake in both control and LRP1-suppressed GT1-7 cells (Fig. 1D).
Figure 1.
LRP1 knockdown and heparin inhibit Aβ uptake in GT1-7 cells. A, Western blotting using an anti-LRP1 antibody showed that LRP1 expression was effectively suppressed by siRNA transfection. B, C, GT1-7 cells with or without LRP1 knockdown by siRNA were incubated with Alexa Fluor 488-RAP (20 nm; B) or FAM–Aβ42 (500 nm; C) in the presence or absence of heparin (15 U/ml = ∼100 μg/ml). Internalization of RAP (B) or Aβ42 (C) was analyzed by FACS. Error bars indicate SD from three independent experiments. N.S., Not significant. **p < 0.01. D, The internalization of FAM–Aβ40 (500 nm) was analyzed by confocal microscopy. Left and right columns indicate FAM–Aβ40 and merged images with Nomarski images, respectively.
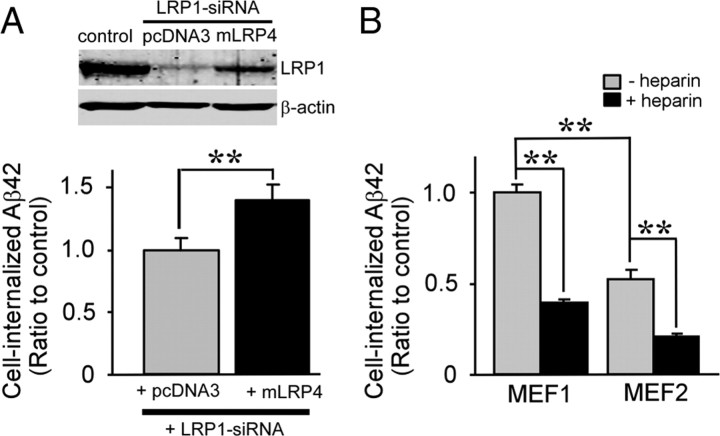
To confirm the effect of LRP1 knockdown on Aβ uptake in GT1-7 cells, we restored LRP1 function by cotransfecting an LRP1 minireceptor of domain IV, mLRP4, which exhibits similar functions to full-length LRP1 (Obermoeller-McCormick et al., 2001). This mLRP4 does not contain an LRP1–siRNA targeting sequence and is therefore resistant to knockdown by LRP1–siRNA. FACS analysis showed that mLRP4 expression restored FAM–Aβ42 uptake in GT1-7 cells (Fig. 2A). No significant differences in cell viability were detected after knockdown of LRP1 by siRNA and mLRP4 transfection (transfection efficiency, 52%) under these conditions (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). To rule out cell-type-specific effects, we performed similar FAM–Aβ42 uptake experiments in MEF cells from both wild-type (MEF1) and LRP1 knock-out mice (MEF2) (Fig. 2B). A decrease in the internalization of FAM–Aβ42 was observed in MEF2 cells compared with MEF1. In addition, heparin further inhibited Aβ42 internalization in MEF2 cells, indicating that LRP1 and HSPG are both involved in cellular Aβ uptake in neuronal and non-neuronal cells.
Figure 2.
LRP1-mediated cellular uptake of Aβ in GT1-7 and MEF cells. A, LRP1 minireceptor mLRP4 or empty vector pcDNA3 was cotransfected with LRP1–siRNA into GT1-7 cells, and cellular uptake of FAM–Aβ42 (500 nm) after 24 h incubation was analyzed by FACS. LRP1 knockdown and mLRP4 overexpression were analyzed by Western blotting. B, MEF1 (wild-type) and MEF2 (LRP1-deficient) cells were treated with 500 nm FAM–Aβ42 in the presence or absence of heparin (15 U/ml = ∼100 μg/ml) for 24 h; intracellular Aβ was then determined by FACS. Error bars indicate SD from three independent experiments. **p < 0.01.
Increased Aβ uptake in N2a cells overexpressing LRP1 depends on HSPG
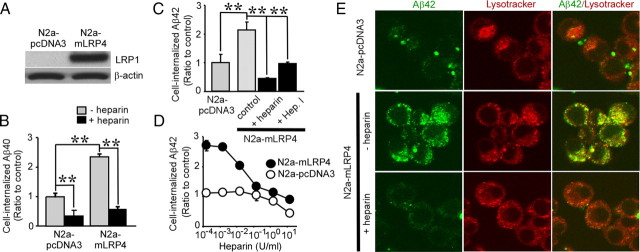
We next investigated whether LRP1 overexpression enhances Aβ uptake. Because mouse neuroblastoma N2a cells express low levels of endogenous LRP1 (Cuitino et al., 2005), they were stably transfected with the LRP1 minireceptor mLRP4. Western blotting revealed that mLRP4 was more effectively expressed in N2a–mLRP4 cells compared with vector pcDNA3-transfected cells (N2a–pcDNA3) (Fig. 3A). These cells were incubated with FAM–Aβ40 (500 nm) or FAM–Aβ42 (500 nm) in the presence or absence of heparin for 24 h at 37°C. FACS analysis showed that internalization of Aβ40 and Aβ42 in N2a–mLRP4 cells increased to 2.4-fold and 2.1-fold, respectively, compared with N2a–pcDNA3 cells (Fig. 3B,C). Interestingly, LRP1-mediated enhancement of Aβ uptake was inhibited by heparin (Fig. 3B,C) in a concentration-dependent manner (Fig. 3D). Heparinase treatment also decreased LRP1-mediated enhancement of cellular Aβ42 uptake (Fig. 3C). These results suggest that LRP1-mediated uptake of Aβ requires HSPG on the cell surface of N2a cells. To confirm the ability of LRP1 to promote Aβ uptake, N2a–pcDNA3 and N2a–mLRP4 cells were cultured with FAM–Aβ42, and analyzed by confocal microscopy. When cells were incubated with 500 nm FAM–Aβ42 for 24 h at 37°C, Aβ42 internalization was significantly higher in N2a–mLRP4 cells than in N2a–pcDNA3 cells. Internalized Aβ42 was colocalized with LysoTracker. Consistent with the results from FACS analysis, internalization of Aβ42 was almost eliminated by heparin (Fig. 3E). To determine whether internalized Aβ is degraded, N2a–pcDNA3 and N2a–mLRP4 cells were incubated with 500 nm FAM–Aβ42 in the presence or absence of heparin for 24 h at 37°C. The Aβ-containing media were then replaced with DMEM containing 10% FBS, and the cells were incubated for an additional 24 h before analysis of intracellular Aβ42 by FACS (supplemental Fig. 3A, available at www.jneurosci.org as supplemental material). Importantly, internalized Aβ42 was cleared after 24 h of incubation in both N2a–pcDNA3 and N2a–mLRP4 cells. When Aβ42 clearance was calculated under each condition, we found that it was enhanced in N2a–mLRP4 cells compared with N2a–pcDNA3 cells. In addition, heparin inhibited this cellular clearance of Aβ42 (supplemental Fig. 3B, available at www.jneurosci.org as supplemental material).
Figure 3.
Heparin inhibits LRP1-mediated cellular uptake of Aβ in N2a cells. A, N2a cells stably expressing a LRP1 minireceptor (N2a–mLRP4) or vector control (N2a–pcDNA3) were analyzed for LRP1 expression using an anti-LRP1 antibody. B, C, N2a–pcDNA3 or N2a–mLRP4 cells were incubated with FAM–Aβ40 (500 nm; B) or FAM–Aβ42 (500 nm; C), respectively, in the presence or absence of heparin (15 U/ml = ∼100 μg/ml) for 24 h at 37°C. Internalization of Aβ was analyzed by FACS. C, In some experiments, cells were treated with Heparinase I (Hep. I). D, Various concentrations of heparin (0.15 mU/ml to 15 U/ml) were incubated with N2a–pcDNA3 (open circles) and N2a–mLRP4 (filled circles) cells during their incubation with FAM–Aβ42 (500 nm). Error bars indicate SD from three independent experiments. **p < 0.01. E, Internalization of FAM–Aβ42 (500 nm) was analyzed by confocal microscopy. Left, middle, and right columns indicate FAM–Aβ42, Lysotracker, and merged images, respectively.
HSPGs, but not LRP1, mediate Aβ binding to neuronal cells
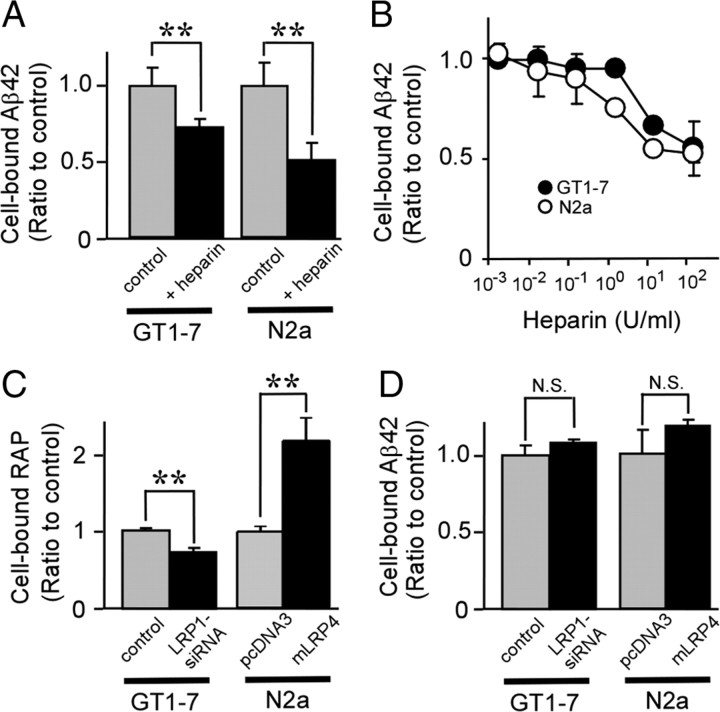
Next we investigated whether LRP1 and HSPG mediate binding of Aβ to neuronal cells. To analyze the role of HSPG in Aβ binding, GT1-7 and N2a cells were incubated with 2 μm FAM–Aβ42 for 2 h at 4°C in the presence or absence of heparin. Thereafter, Aβ42 binding was assessed by FACS (Fig. 4A). Heparin significantly inhibited the binding of Aβ42 in a concentration-dependent manner in both GT1-7 and N2a cells (Fig. 4A,B), suggesting that cell-surface HSPGs constitute major Aβ binding sites on neuronal cells. However, neither LRP1 knockdown nor LRP1 overexpression had any significant effect on Aβ42 binding in these neuronal cells (Fig. 4D). As a control, when LRP1-suppressed GT1-7 cells or LRP1-overexpressing N2a–mLRP4 cells were incubated with Alexa Fluor 488-labeled RAP for 2 h at 4°C, knockdown of LRP1 suppressed RAP binding to 38% of control cells, whereas LRP1 overexpression enhanced RAP binding to 2.2-fold of the N2a–pcDNA3 cells (Fig. 4C). Similar results were obtained when Aβ42 binding was analyzed by ELISA (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). Together, these results indicate that HSPGs, but not LRP1, play important roles in Aβ binding to neuronal cells.
Figure 4.
Heparin inhibits Aβ binding to neuronal cells. A, GT1-7 or N2a cells were incubated with FAM–Aβ42 (2 μm) in the presence or absence of heparin (15 U/ml = ∼100 μg/ml) for 2 h at 4°C. Binding of Aβ was analyzed by FACS. B, Various concentrations of heparin (1.5 mU/ml to 150 U/ml) were used with GT1-7 (filled circles) or N2a (open circle) cells during their incubation with FAM–Aβ42 (2 μm). C, D, GT1-7 cells without or with LRP1 knockdown, N2a-pcDNA3, or N2a–mLRP4 cells were incubated with Alexa Fluor 488–RAP (100 nm; C) or FAM–Aβ42 (2 μm; D) for 2 h at 4°C and analyzed by FACS. Error bars indicate SD from three independent experiments. N.S., Not significant. **p < 0.01.
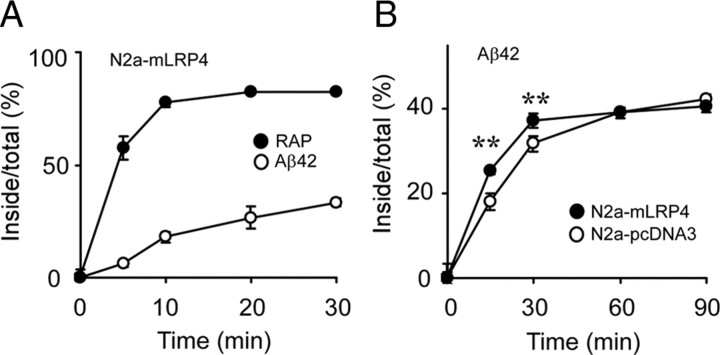
LRP1 mediates Aβ endocytosis in N2a cells
To compare the endocytosis rates of RAP and Aβ, N2a–mLRP4 cells were first incubated with 10 nm [125I]RAP or 100 nm [125I]Aβ42 at 4°C for 1 h to allow binding to occur. Cells were then warmed for different periods of time to allow ligand internalization and to determine endocytosis rates (Li et al., 2000). We found that [125I]Aβ42 had a slower rate of endocytosis than [125I]RAP (Fig. 5A). Next, N2a–pcDNA3 and N2a–mLRP4 cells were incubated with 100 nm [125I]Aβ42 at 4°C for 1 h and then warmed for different periods of time. Despite similar endocytosis capacities, the initial endocytosis rate of [125I]Aβ42 was faster in N2a–mLRP4 cells than in N2a–pcDNA3 cells (Fig. 5B). These results indicate that LRP1 plays an important role in mediating Aβ endocytosis in N2a cells; however, other mechanisms also contribute to this process.
Figure 5.
LRP1 regulates Aβ endocytosis in N2a cells. A, N2a–mLRP4 cells were incubated with 10 nm [125I]RAP (filled circles) or 100 nm [125I]Aβ42 (open circles) for 1 h at 4°C and then warmed to 37°C for the indicated times. B, N2a–pcDNA3 and N2a–mLRP4 cells were similarly treated with 100 nm [125I]Aβ42. The percentage of ligand internalized at each time interval is equal to the amount of ligand internalized divided by the total cell-associated ligand. Error bars indicate SD from three independent experiments. **p < 0.01.
HSPG deficiency significantly decreases cellular Aβ binding and uptake
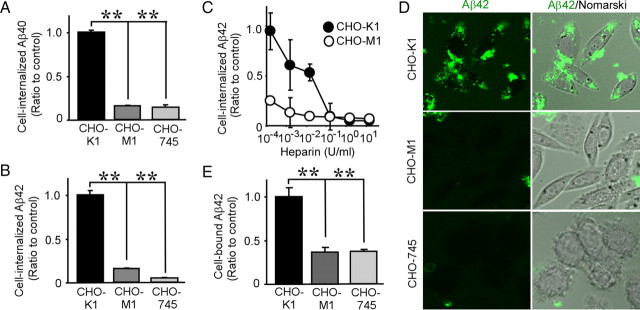
To further examine the role of HSPG in cellular Aβ uptake, we next used wild-type CHO-K1 cells, CHO-M1 cells deficient in N-acetylglucosaminyltransferase/glucuronyltransferase (required for HS biosynthesis) (Broekelmann et al., 2005), and CHO-745 cells deficient in xylosyltransferase [required for both HS and chondroitin sulfate (CS) biosynthesis] (Esko et al., 1985). These CHO cells were incubated with 500 nm FAM–Aβ40 or FAM–Aβ42 for 24 h at 37°C, and the uptake of Aβ was assessed by FACS. We found that internalization of both Aβ40 and Aβ42 in HS-deficient CHO-M1 and HS/CS-deficient CHO-745 cells was significantly lower than in wild-type CHO-K1 cells. Specifically, internalization of Aβ40 in CHO-M1 and CHO-745 was 16 and 14% of that in CHO-K1 cells, respectively (Fig. 6A). In the case of Aβ42, Aβ uptake in CHO-M1 and CHO-745 was 16 and 5% of that in CHO-K1 cells, respectively (Fig. 6B). The differences in Aβ internalization between CHO-M1 and CHO-745 cells were significantly less than between CHO-K1 and CHO-M1 cells, suggesting that HSPG is the predominant cell-surface proteoglycan that mediates Aβ uptake. Although heparin suppressed Aβ42 internalization in CHO-K1 cells in a concentration-dependent manner, it had little effect in CHO-M1 cells (Fig. 6C). To further confirm the role of HSPG in Aβ internalization, CHO cells were cultured with FAM–Aβ42 for 24 h at 37°C, and Aβ uptake was analyzed by confocal microscopy. Although cell-associated Aβ42 in CHO-K1 cells was abundantly detected, very little internalized Aβ42 was detected in CHO-M1 and CHO-745 cells (Fig. 6D). To analyze the roles of proteoglycans in Aβ binding in these CHO cells, they were incubated with 2 μm FAM–Aβ42 for 2 h at 4°C. FACS analysis revealed that binding of Aβ42 in CHO-M1 and CHO-745 cells was also significantly lower than in CHO-K1 cells (Fig. 6E). Collectively, these results clearly indicate that HSPG plays critical roles in both Aβ binding and uptake.
Figure 6.
HSPG mediates Aβ uptake and binding. CHO-K1 (wild-type), CHO-M1 (HS-deficient), or CHO-745 (HS/CS-deficient) cells were incubated with FAM–Aβ40 (500 nm; A) or FAM–Aβ42 (500 nm; B) for 24 h at 37°C. Internalization of Aβ was analyzed by FACS. C, Various concentrations of heparin (0.15 mU/ml to 15 U/ml) were used with CHO-K1 (filled circles) or CHO-M1 (open circles) cells during their incubation with FAM–Aβ42 (500 nm). D, Internalization of FAM–Aβ42 (500 nm) in CHO-K1, CHO-M1, or CHO-745 cells was analyzed by confocal microscopy. Left and right columns indicate FAM–Aβ42 and merged images with Nomarski images, respectively. E, Cells were incubated with Aβ42 (2 μm) for 3 h at 4°C, and binding of Aβ was analyzed by FACS. Error bars indicate SD from three independent experiments. **p < 0.01.
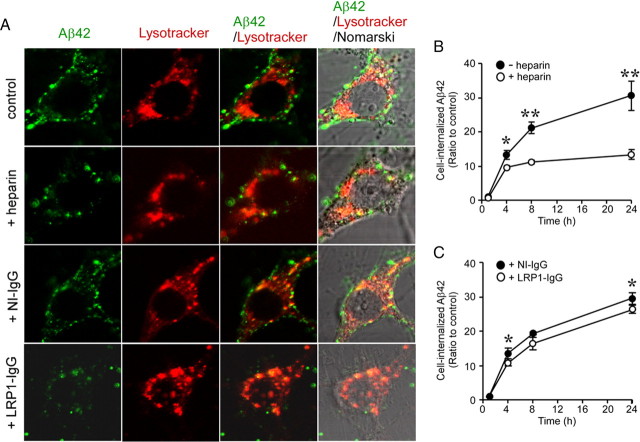
Heparin and LRP1 antibody inhibit Aβ uptake in primary neurons
To further determine the roles of HSPG and LRP1 in neuronal Aβ uptake, we analyzed the effects of heparin and an LRP1 blocking antibody on Aβ uptake in mouse primary cortical neurons. Neurons were incubated with FAM–Aβ42 (500 nm) in the presence of heparin, non-immune IgG, or anti-LRP1 IgG for 4 h at 37°C and analyzed by confocal microscopy (Fig. 7A). When neuronal cell bodies were observed by confocal microscopy, we found that Aβ42 internalization was suppressed by the treatment of heparin and anti-LRP1 IgG. In contrast, non-immune IgG had no effect on Aβ42 uptake. Internalized Aβ was partially colocalized with a lysosomal marker, LysoTracker. Next, neurons were incubated with 500 nm FAM–Aβ42 for 1, 4, 8, or 24 h at 37°C in the presence of heparin (Fig. 7B), non-immune IgG, or anti-LRP1 IgG (Fig. 7C), followed by treatment with trypsin to remove cell-surface Aβ42. Cell-internalized Aβ42 was assessed by FACS. Although neurons effectively internalized Aβ42 in a time-dependent manner, heparin significantly suppressed Aβ42 uptake (Fig. 7B), consistent with the results from confocal microscopy. In addition, anti-LRP1 antibody significantly inhibited Aβ42 internalization compared with non-immune IgG (Fig. 7C), albeit to a lesser degree compared with heparin. Together, these results indicate that both HSPG and LRP1 play critical roles in Aβ uptake in neurons.
Figure 7.
Heparin and anti-LRP1 antibody inhibit cellular Aβ uptake in primary neurons. A, Mouse primary cortical neurons were incubated with FAM–Aβ42 (500 nm) in the presence or absence of heparin (15 U/ml = ∼100 μg/ml), non-immune IgG, or anti-LRP1 IgG (75 μg/ml) for 4 h at 37°C and analyzed by confocal microscopy. Non-immune IgG or anti-LRP1 IgG was added 1 h before Aβ treatment. B, C, Internalization of Aβ was analyzed by FACS after incubation with FAM–Aβ42 (500 nm) for 1, 4, 8, and 24 h in the presence or absence of heparin (B) and in the presence of non-immune IgG or anti-LRP1 IgG (C). Error bars indicate SD from three independent experiments. *p < 0.05; **p < 0.01.
Discussion
Intracellular or extracellular accumulation, aggregation, and deposition of Aβ in the brain are pathogenic events of AD (Selkoe, 2001, 2002). One of the major pathways for Aβ clearance is its cellular uptake and degradation by neurons, glia, and cells along the brain vasculature (Bu, 2009). In this study, we focused on investigating individual and cooperative roles of LRP1 and HSPG in neuronal Aβ uptake. Using complementary approaches, we showed that neuronal cell-surface HSPGs constitute major Aβ binding sites and are critical for Aβ uptake. Although not required for Aβ binding, LRP1 cooperates with HSPG in neuronal Aβ uptake.
HSPG is composed of a core protein with long polysaccharide chains of repeated heparan sulfate. There are two groups of HSPGs: extracellular matrix HSPGs (e.g., perlecan, agrin, and collagen XVIII) and cell-surface HSPGs (syndecan and glypican) (van Horssen et al., 2003). Both extracellular matrix and cell-surface HSPGs were detected by immunohistochemistry in senile plaques, cerebral amyloid angiopathy, and neurofibrillary tangles (van Horssen et al., 2003). These evidences strongly suggest that HSPGs may interact with Aβ and play some roles in AD pathogenesis. Consistent with this notion, we demonstrated that heparin binds to Aβ (supplemental Fig. 5A, available at www.jneurosci.org as supplemental material) and significantly inhibits neuronal Aβ binding and uptake. Heparin binds to several classes of extracellular proteins, including growth factors, matrix proteins, and apolipoproteins (Bergamaschini et al., 2009). Heparan sulfate was shown to bind to the amino acid 13–16 region (HHQK) of Aβ (Brunden et al., 1993) and to antagonize the binding of Aβ to HSPG in vitro (Castillo et al., 1997). To block heparin binding proteins and/or displace HSPG binding proteins at the cell surface, GT1-7 cells were pretreated with heparin and then incubated with Aβ42 after washing. No significant effect of heparin pretreatment on Aβ42 uptake was detected (supplemental Fig. 5B, available at www.jneurosci.org as supplemental material), suggesting that heparin antagonizes the interaction of Aβ to HSPG through its binding to Aβ. In addition, HSPG deficiency in CHO cells significantly decreased Aβ binding and uptake. Therefore, our findings indicate that Aβ binding to neuronal cell-surface HSPG may be the first and critical step for its internalization, degradation, accumulation, and/or toxicity. Aβ binding to cell-surface and/or extracellular matrix HSPG may also be important for its aggregation and eventual formation of amyloid plaques.
We showed that heparin suppresses Aβ binding and internalization in neuronal cell lines and mouse primary neurons. Similar findings have been demonstrated in brain vascular smooth muscle cell (Kanekiyo and Bu, 2009) and in microglia (Giulian et al., 1998). However, heparin and heparinase treatment did not affect Aβ uptake in brain capillary endothelial cells (Yamada et al., 2008). Because heparan sulfate biosynthesis differs depending on tissue/cell types (Kreuger et al., 2006), the discrepancies in cellular Aβ uptake may result from differences in tissue/cell-specific HSPG structure. Several studies have demonstrated that heparin and heparan sulfate suppress Aβ cellular toxicity (Woods et al., 1995; Bergamaschini et al., 2002), likely by inhibiting cellular Aβ binding and/or uptake. Cells internalize and degrade Aβ through the lysosomal pathway (Hu et al., 2009). We demonstrated that LRP1 and HSPG mediate uptake of Aβ to lysosomes and its subsequent cellular clearance in N2a cells. However, when the capacity is overwhelmed through continuous exposure to high concentrations of extracellular Aβ, intracellular accumulation and aggregation of Aβ may be induced, leading to cellular toxicity. We recently showed that enhancement of Aβ uptake through LRP1 leads to eventual lysosomal accumulation and slightly decreased cell viability when cells were exposed to high Aβ concentrations and long periods of incubation (Fuentealba et al., 2010). Furthermore, the remnants of these aggregates after cell death may induce extracellular amyloid plaque formation through its seeding effect (Hu et al., 2009). In this view, heparin and heparin-like compounds may be promising candidates for AD therapy because of their competitive function against HSPGs. Bergamaschini et al. (2004) demonstrated that the effect of long-term, peripheral treatment with a low-molecular-weight heparin significantly decreased Aβ concentration and deposition in the brain of APP transgenic mice (Bergamaschini et al., 2004).
LRP1 plays critical roles in cellular Aβ uptake in the brain. Harris-White et al. (2004) showed that TGFβ2-mediated intraneuronal accumulation of brain-injected Aβ depends on LRP1 function (Harris-White et al., 2004). We showed that the internalization of Aβ was also decreased in LRP1-deficient neuronal cells and increased in LRP1-overexpressing neuronal cells. LRP1 expression restored Aβ uptake in LRP1 knocked down GT1-7 cells. We also demonstrated that treatment with a neutralizing antibody to LRP1 decreased Aβ uptake in mouse primary cortical neuron. In addition, LRP1 enhanced the Aβ endocytosis rate in N2a cells. Importantly, LRP1-mediated Aβ uptake was also inhibited by heparin, indicating that the function of LRP1 in cellular Aβ uptake depends on HSPG. In contrast to HSPG, we showed that neither LRP1 knockdown nor overexpression affects Aβ binding. These results can be explained by two possibilities. First, HSPG may function as a coreceptor for LRP1. Previous work has shown that LRP1 forms a complex with HSPG (Wilsie and Orlando, 2003). Aβ may initially bind to the HSPG sites on the surface of the complex and then may undergo endocytosis via LRP1, in a process analogous to another LRP1 ligand, coagulation factor VIII (Sarafanov et al., 2001). We have shown that inhibition of LRP1 endocytosis decreases cellular Aβ uptake in N2a cells (Fuentealba et al., 2010). LRP1 is a large endocytotic receptor that recognizes >30 ligands, including lipoproteins, proteinases, proteinase inhibitor complexes, extracellular matrix proteins, bacterial toxins, viruses, and various intracellular proteins (Herz and Strickland, 2001; Bu, 2009). Although the ligand-binding sites on LRP1 localize primarily on domains II and IV (Herz and Strickland, 2001), some LRP1 ligands seem to require coreceptors for their interaction (Nykjaer et al., 1992; Sarafanov et al., 2001). Aβ was shown to bind directly to domains II and IV of LRP1 (Sagare et al., 2007); however, another study did not support direct Aβ binding to LRP1 (Yamada et al., 2008). Our results suggest that HSPG may be one of the candidate coreceptors of LRP1 for Aβ binding and uptake.
We showed that Aβ had a slower rate of endocytosis than RAP in N2a–mLRP4 cells, indicating that LRP1 mediates Aβ endocytosis through different mechanisms from its other ligands. Therefore, the other possibility is that HSPG, which is abundantly expressed in neurons, may capture Aβ on the cell surface and subsequently internalize Aβ through an LRP1-regulated signaling pathway. Increasing evidence also shows that LRP1 functions as a signal-transducing receptor (Herz and Strickland, 2001) and that LRP1 plays a critical role in neuronal viability by regulating key survival signaling pathways (Fuentealba et al., 2009). In addition, LRP1 regulates cell migration (Song et al., 2009) and the integrity of the blood–brain barrier (Yepes et al., 2003). Aβ binding to HSPG may activate LRP1 signaling pathways, resulting in an enhancement of cellular Aβ uptake. Consistent with an LRP1-mediated signaling function, actin polymerization, which is critical for macropinocytosis and Aβ uptake (Mandrekar et al., 2009), is impaired in LRP1-deficient cells (Zhou et al., 2009). This evidence suggests that LRP1 may mediate Aβ cellular uptake by regulating macropinocytosis.
In summary, we have demonstrated that HSPG and LRP1 function cooperatively in neuronal Aβ binding and uptake. We show that HSPG mediates Aβ binding on the cell surface and that both HSPG and LRP1 play important roles in Aβ uptake. Our findings provide novel insights into the molecular mechanisms of AD pathogenesis. Additional studies of the HSPG/LRP1-mediated Aβ pathways may lead to novel therapeutic strategies to treat AD.
Footnotes
This work was supported by National Institutes of Health Grants R01AG027924 and P01AG030128 (G.B.), a Zenith Fellows Award from the Alzheimer's Association (G.B.), a research grant from the American Health Assistance Foundation (G.B.), and a postdoctoral fellowship from the American Heart Association (T.K.). We thank the Alafi Neuroimaging Laboratory of the Hope Center for Neurological Disorders for the use of imaging facilities, which are supported by National Institutes of Health Neuroscience Blueprint Center Core Grant P30NS057105 (to Washington University, St. Louis, MO). We also thank Albert Tzeng and Samantha Shasserre for technical assistance and careful reading of this manuscript.
References
- Bergamaschini L, Donarini C, Rossi E, De Luigi A, Vergani C, De Simoni MG. Heparin attenuates cytotoxic and inflammatory activity of Alzheimer amyloid-beta in vitro. Neurobiol Aging. 2002;23:531–536. doi: 10.1016/s0197-4580(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Bergamaschini L, Rossi E, Storini C, Pizzimenti S, Distaso M, Perego C, De Luigi A, Vergani C, De Simoni MG. Peripheral treatment with enoxaparin, a low molecular weight heparin, reduces plaques and β-amyloid accumulation in a mouse model of Alzheimer's disease. J Neurosci. 2004;24:4181–4186. doi: 10.1523/JNEUROSCI.0550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschini L, Rossi E, Vergani C, De Simoni MG. Alzheimer's disease: another target for heparin therapy. ScientificWorldJournal. 2009;9:891–908. doi: 10.1100/tsw.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen DE. Neurodegeneration in Alzheimer's disease: caspases and synaptic element interdependence. Mol Neurodegener. 2009;4:27. doi: 10.1186/1750-1326-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekelmann TJ, Kozel BA, Ishibashi H, Werneck CC, Keeley FW, Zhang L, Mecham RP. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J Biol Chem. 2005;280:40939–40947. doi: 10.1074/jbc.M507309200. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Richter-Cook NJ, Chaturvedi N, Frederickson RC. pH-dependent binding of synthetic beta-amyloid peptides to glycosaminoglycans. J Neurochem. 1993;61:2147–2154. doi: 10.1111/j.1471-4159.1993.tb07453.x. [DOI] [PubMed] [Google Scholar]
- Bu G. The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int Rev Cytol. 2001;209:79–116. doi: 10.1016/s0074-7696(01)09011-8. [DOI] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G, Cam J, Zerbinatti C. LRP in amyloid-beta production and metabolism. Ann N Y Acad Sci. 2006;1086:35–53. doi: 10.1196/annals.1377.005. [DOI] [PubMed] [Google Scholar]
- Castillo GM, Ngo C, Cummings J, Wight TN, Snow AD. Perlecan binds to the beta-amyloid proteins (A beta) of Alzheimer's disease, accelerates A beta fibril formation, and maintains A beta fibril stability. J Neurochem. 1997;69:2452–2465. doi: 10.1046/j.1471-4159.1997.69062452.x. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuitino L, Matute R, Retamal C, Bu G, Inestrosa NC, Marzolo MP. ApoER2 is endocytosed by a clathrin-mediated process involving the adaptor protein Dab2 independent of its Rafts' association. Traffic. 2005;6:820–838. doi: 10.1111/j.1600-0854.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- Esko JD, Stewart TE, Taylor WH. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba RA, Liu Q, Kanekiyo T, Zhang J, Bu G. Low density lipoprotein receptor-related protein 1 promotes anti-apoptotic signaling in neurons by activating Akt survival pathway. J Biol Chem. 2009;284:34045–34053. doi: 10.1074/jbc.M109.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba RA, Liu Q, Zhang J, Kanekiyo T, Hu X, Lee JM, LaDu MJ, Bu G. Low-density lipoprotein receptor-related protein 1 (LRP1) mediates neuronal Abeta42 uptake and lysosomal trafficking. PLoS One. 2010;5:e11884. doi: 10.1371/journal.pone.0011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Haverkamp LJ, Yu J, Karshin W, Tom D, Li J, Kazanskaia A, Kirkpatrick J, Roher AE. The HHQK domain of beta-amyloid provides a structural basis for the immunopathology of Alzheimer's disease. J Biol Chem. 1998;273:29719–29726. doi: 10.1074/jbc.273.45.29719. [DOI] [PubMed] [Google Scholar]
- Harris-White ME, Balverde Z, Lim GP, Kim P, Miller SA, Hammer H, Galasko D, Frautschy SA. Role of LRP in TGFbeta2-mediated neuronal uptake of Abeta and effects on memory. J Neurosci Res. 2004;77:217–228. doi: 10.1002/jnr.20149. [DOI] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Crick SL, Bu G, Frieden C, Pappu RV, Lee JM. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc Natl Acad Sci U S A. 2009;106:20324–20329. doi: 10.1073/pnas.0911281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji ZS, Pitas RE, Mahley RW. Differential cellular accumulation/retention of apolipoprotein E mediated by cell surface heparan sulfate proteoglycans. Apolipoproteins E3 and E2 greater than e4. J Biol Chem. 1998;273:13452–13460. doi: 10.1074/jbc.273.22.13452. [DOI] [PubMed] [Google Scholar]
- Kanekiyo T, Bu G. Receptor-associated protein interacts with amyloid-beta peptide and promotes its cellular uptake. J Biol Chem. 2009;284:33352–33359. doi: 10.1074/jbc.M109.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, Spillmann D, Li JP, Lindahl U. Interactions between heparan sulfate and proteins: the concept of specificity. J Cell Biol. 2006;174:323–327. doi: 10.1083/jcb.200604035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275:17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Merchenthaler I, Wetsel WC, Reid JJ, Mellon PL, Weiner RI, Negro-Vilar A. Morphological characterization of immortalized hypothalamic neurons synthesizing luteinizing hormone-releasing hormone. Endocrinology. 1991;129:1575–1583. doi: 10.1210/endo-129-3-1575. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, Herz J, Muglia L, Bu G. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Aβ through fluid phase macropinocytosis. J Neurosci. 2009;29:4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Petersen CM, Møller B, Jensen PH, Moestrup SK, Holtet TL, Etzerodt M, Thøgersen HC, Munch M, Andreasen PA, Gliemann J. Purified alpha 2-macroglobulin receptor/LDL receptor-related protein binds urokinase.plasminogen activator inhibitor type-1 complex. Evidence that the alpha 2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J Biol Chem. 1992;267:14543–14546. [PubMed] [Google Scholar]
- Obermoeller-McCormick LM, Li Y, Osaka H, FitzGerald DJ, Schwartz AL, Bu G. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J Cell Sci. 2001;114:899–908. doi: 10.1242/jcs.114.5.899. [DOI] [PubMed] [Google Scholar]
- Poon GM, Gariépy J. Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem Soc Trans. 2007;35:788–793. doi: 10.1042/BST0350788. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Strickland DK, Hyman BT, Rebeck GW. Alpha2-macroglobulin enhances the clearance of endogenous soluble beta-amyloid peptide via low-density lipoprotein receptor-related protein in cortical neurons. J Neurochem. 1999;73:1393–1398. doi: 10.1046/j.1471-4159.1999.0731393.x. [DOI] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafanov AG, Ananyeva NM, Shima M, Saenko EL. Cell surface heparan sulfate proteoglycans participate in factor VIII catabolism mediated by low density lipoprotein receptor-related protein. J Biol Chem. 2001;276:11970–11979. doi: 10.1074/jbc.M008046200. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. J Clin Invest. 2002;110:1375–1381. doi: 10.1172/JCI16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Walsh DM. Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Li Y, Lee J, Schwartz AL, Bu G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009;69:879–886. doi: 10.1158/0008-5472.CAN-08-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull J, Powell A, Guimond S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 2001;11:75–82. doi: 10.1016/s0962-8924(00)01897-3. [DOI] [PubMed] [Google Scholar]
- Urmoneit B, Prikulis I, Wihl G, D'Urso D, Frank R, Heeren J, Beisiegel U, Prior R. Cerebrovascular smooth muscle cells internalize Alzheimer amyloid beta protein via a lipoprotein pathway: implications for cerebral amyloid angiopathy. Lab Invest. 1997;77:157–166. [PubMed] [Google Scholar]
- van Horssen J, Wesseling P, van den Heuvel LP, de Waal RM, Verbeek MM. Heparan sulphate proteoglycans in Alzheimer's disease and amyloid-related disorders. Lancet Neurol. 2003;2:482–492. doi: 10.1016/s1474-4422(03)00484-8. [DOI] [PubMed] [Google Scholar]
- Warshawsky I, Bu G, Schwartz AL. 39-kD protein inhibits tissue-type plasminogen activator clearance in vivo. J Clin Invest. 1993;92:937–944. doi: 10.1172/JCI116669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsie LC, Orlando RA. The low density lipoprotein receptor-related protein complexes with cell surface heparan sulfate proteoglycans to regulate proteoglycan-mediated lipoprotein catabolism. J Biol Chem. 2003;278:15758–15764. doi: 10.1074/jbc.M208786200. [DOI] [PubMed] [Google Scholar]
- Woods AG, Cribbs DH, Whittemore ER, Cotman CW. Heparan sulfate and chondroitin sulfate glycosaminoglycan attenuate beta-amyloid(25-35) induced neurodegeneration in cultured hippocampal neurons. Brain Res. 1995;697:53–62. doi: 10.1016/0006-8993(95)00775-l. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Yamada K, Hashimoto T, Yabuki C, Nagae Y, Tachikawa M, Strickland DK, Liu Q, Bu G, Basak JM, Holtzman DM, Ohtsuki S, Terasaki T, Iwatsubo T. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. J Biol Chem. 2008;283:34554–34562. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Takayama Y, Boucher P, Tallquist MD, Herz J. LRP1 regulates architecture of the vascular wall by controlling PDGFRbeta-dependent phosphatidylinositol 3-kinase activation. PLoS One. 2009;4:e6922. doi: 10.1371/journal.pone.0006922. [DOI] [PMC free article] [PubMed] [Google Scholar]