Abstract
OBJECTIVE
To estimate the probability of successful sterilization after hysteroscopic or laparoscopic sterilization procedure.
METHODS
An evidence-based clinical decision analysis using a Markov model was performed to estimate the probability of a successful sterilization procedure using laparoscopic sterilization, hysteroscopic sterilization in the operating room, and hysteroscopic sterilization in the office. Procedure and follow-up testing probabilities for the model were estimated from published sources.
RESULTS
In the base case analysis, the proportion of women having a successful sterilization procedure on first attempt is 99% for laparoscopic, 88% for hysteroscopic in the operating room and 87% for hysteroscopic in the office. The probability of having a successful sterilization procedure within one year is 99% with laparoscopic, 95% for hysteroscopic in the operating room, and 94% for hysteroscopic in the office. These estimates for hysteroscopic success include approximately 6% of women who attempt hysteroscopically but are ultimately sterilized laparoscopically. Approximately 5% of women who have a failed hysteroscopic attempt decline further sterilization attempts.
CONCLUSIONS
Women choosing laparoscopic sterilization are more likely than those choosing hysteroscopic sterilization to have a successful sterilization procedure within one year. However, the risk of failed sterilization and subsequent pregnancy must be considered when choosing a method of sterilization.
INTRODUCTION
Female sterilization is one of the most commonly used methods of contraception. Of the 38.2 million U.S. women using a contraceptive from 2006–2008, 27% utilized female sterilization (1). While this proportion has been stable since 1988, the methods employed to achieve sterilization have changed (2).
Hysteroscopic sterilization (HS) has been commercially available since 2002. Advantages of HS include that it is a non-incisional method, avoids abdominal entry (which may be especially important in women with adhesions or co-morbidities), can be performed as an office procedure, and avoids general anesthesia. A 6-year review of sterilization trends at a U.S. academic medical center from 2002–2006, showed a 50% decline in both laparoscopic sterilization (LS) and postpartum sterilizations and a corresponding 50% increase in HS (3). According to the manufacturer of Essure® (Mountain View, CA), the most popular HS system, approximately 310,000 devices have been placed as of 2010 (4).
However, unlike LS which conveys immediate reliability, HS is a multi-step process in which the HS procedure is followed by a confirmatory hysterosalpingogram (HSG) performed at least three months after the initial procedure to prove bilateral tubal occlusion before women can rely on this method of contraception (5). For women without occlusion but with devices present, an additional HSG may be indicated 3 months later. Alternative contraception must be used until occlusion is proven. Each step of this process introduces a chance of finding that the procedure failed, of non-compliance with use of alternative contraception, or loss to follow-up. Failed attempts at HS can subject women to multiple procedures, a delay in achieving sterilization, and increase the risk of unintended pregnancy.
Current published assessments of HS success do not adequately address these complex issues. Reported success rates often exclude women who failed initial microinsert placement or did not return for HSG, thereby falsely elevating the percentages of successful sterilization. Accordingly, we performed an evidence-based decision analysis that includes these complexities in order to better estimate the likelihood of a successful sterilization procedure after HS or LS.
MATERIALS AND METHODS
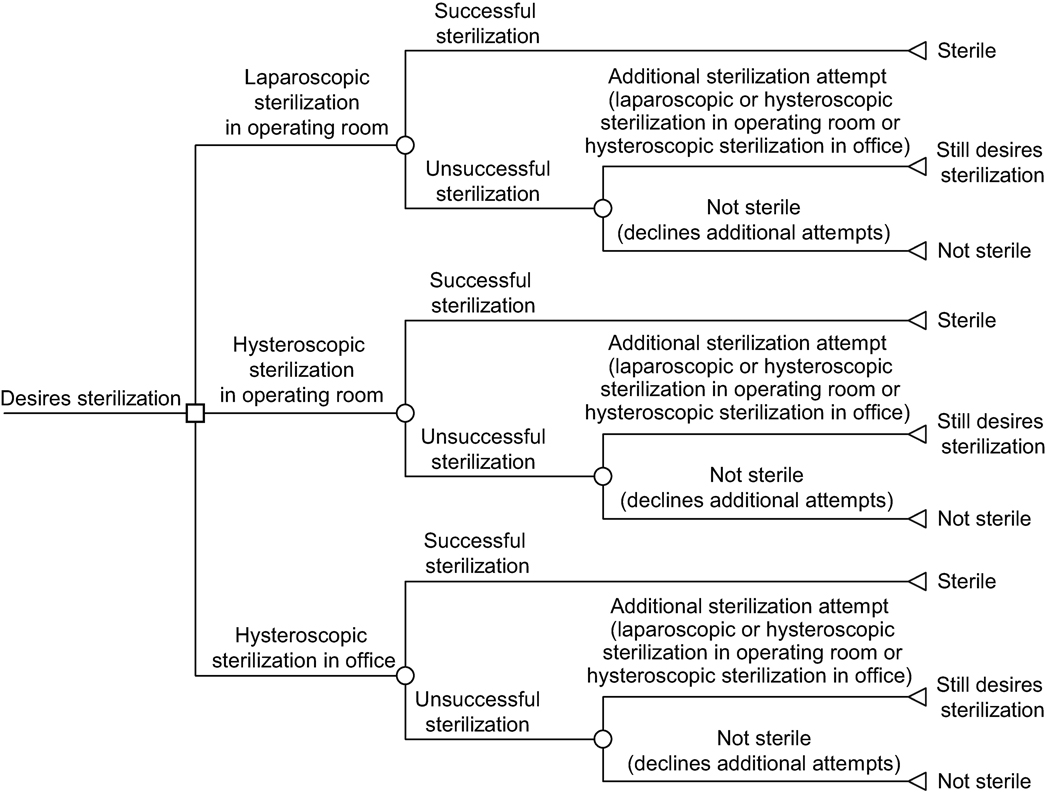
We developed an evidence-based Markov decision model (Fig. 1) to compare the probability of a successful sterilization procedure via 3 strategies: LS, HS in the operating room (OR), and HS in the office setting. The Markov model, using monthly cycles, contains health states and transition probabilities between those states corresponding to differing paths that could occur with each strategy, including the probabilities of successful sterilization, of follow-up procedures (and their outcomes), and of proceeding with alternative procedures (and their success) if prior procedures were unsuccessful.
Figure 1.
The Markov model for probability of successful sterilization procedure using the laparoscopic compared with the hysteroscopic approach.
The primary objective of the model was to estimate the probability of successful sterilization after HS or LS based on available data. HS and LS success were defined in accordance with standard clinical practice. A successful HS procedure was defined as having bilateral blockage of fallopian tubes on follow-up HSG evaluation. A successful LS procedure was defined as physical obstruction of the fallopian tubes at the time of surgery.
For the model, women were maintained in groups based on the original attempted procedure. Thus, women in HS strategies who ultimately received LS were counted as an HS success, biasing against the LS strategy. Cohorts were followed for 1 year. Standard decision-analysis software (TreeAge Pro Suite 2009) was used.
Procedure and follow-up testing probabilities were estimated from published sources (Table 1). This model used data pertaining to Essure® HS only, due to its dominance of the market. The major sources for base case values (and the ranges of lowest and highest reasonable values) were identified through a comprehensive literature search of all pertinent studies in English in PubMed and Ovid (last searched April 13, 2011), and by reviewing the bibliographies of identified references. All published studies that reported more than 50 subjects were included. However, some studies did not provide complete information for every outcome in the model. The base case values and ranges used in the model, as well as the studies referenced to provide this information, are described in Table 1. The values themselves are a mathematical average of the results from the referenced studies. When data was missing from published literature, we used data from our own practice’s active database, which was initiated in July 2003. Outcome data from studies that did not evaluate the success of HS using HSG, as required by the U.S. Food and Drug Administration (FDA), were not included in this analysis.
Table 1.
Parameter values used in the model
| Probabilities of: | Baseline Value |
Range | Reference or Assumption |
|---|---|---|---|
| Laparoscopic Sterilization | |||
| Successful LS | 99–100 % | 99–100 % | 6, 7 |
| Choose HS in OR if LS failed | 20 % | 10–50 % | Expert opinion |
| Major complication | 1 % | 0.098–1.7 % | 7–11 |
| Minor complication | 0.5 % | 0.26–1 % | 10, 11 |
| Probability of death | 0 % | 7, 8, 12 | |
| HS in OR | |||
| Successful coil placement on first attempt | 90 % | 85–95 % | 12–19 |
| Major complication | 0.13 % | 0–0.4 % | 12, 14, 16 |
| Minor complication | 5 % | 4–7 % | 12–14, 16 |
| Choose second OR procedure | 70 % | 41–100 % | 13, 17–19 |
| Choose LS after one failed HS in OR | 83 % | 67–100 % | 13, 17–19 |
| Successful coil placement on second attempt | 84 % | 67–100 % | 12, 13 , 17, 19 |
| Probability of death | 0 % | 12 | |
| HS in Office | |||
| Successful coil placement on first HS attempt | 90 % | 76–96 % | 6, 15, 20–23 |
| Major complication | 0 % | 20, 24, 25 | |
| Minor complication | 5 % | 2–8 % | 20, 24, 25 |
| Choose second procedure after one failed | 70 % | 21–100 % | 6, 20, 24 |
| Choose HS | 33 % | 0–67 % | 20, 24 |
| Successful coil placement on second attempt | 80 % | 67–100 % | 6, 20, 22 |
| Probability of death | 0% | 15 | |
| HSG Outcomes | |||
| Returning for HSG at three months | 69 % | 13–94 % | 6, 13, 18, 21, 23, 24 |
| HSG: Coils present | 97 % | 95–99 % | 12, 14, 16, 24 |
| HSG: Blockage at three months | 96 % | 84–100 % | 6, 12–14, 16, 21, 23, 24 |
| Returning for HSG at six months | 69 % | 13–94 % | Assume same as for three months |
| HSG: Blockage at six months | 98 % | 93–100 % | 12–14, 24 |
| Assumed sterile if do not return for HSG | 96 % | 84–99 % | Assume same as for women who do return at three and six months |
| If HSG at three months shows non-occlusion | |||
| Initial procedure in OR | |||
| Choosing another procedure | 30 % | Practice database | |
| Choosing second HS in OR | 50 % | Practice database | |
| Occlusion with second HS in OR | 73 % | 45–100% | 16, Practice database |
| Initial procedure in office | |||
| Choosing another procedure | Assume same as OR | ||
| Choosing second HS in office | Assume same as OR | ||
| Occlusion with second HS in office | Assume same as OR | ||
| If two failed HS attempts | |||
| LS after two failed HS in OR | 87 % | 16, Practice database | |
| LS after two failed HS in office | 100% | 20 |
HS = Hysteroscopic Sterilizatinon
HSG = Hysterosalpingogram
LS = Laparoscopic Sterilization
OR = Operating Room
Follow-up rates of HSG at 3 months vary widely (Table 1), with the highest follow-up rates reported in the original HS clinical trials performed by the manufacturer. The base case values used in this analysis were limited to subsequent case series or cohort analyses in an effort to avoid bias. However, we did include the manufacturer’s follow-up rates of 98% to 100% in the sensitivity analyses (12, 14, 16).
In the absence of published data, the following assumptions were made for the model:
20% of women who failed LS would accept HS,
the probability of choosing another HS procedure after one failed HS (defined as a negative HSG) would be the same after HS performed in the OR or office,
the probability of choosing a repeat HS procedure (as opposed to choosing a LS procedure) after a failed HS in the office (defined as a negative HSG) would be the same as is reported for the OR,
a second HS had similar success regardless of operating room or office location,
the probability of returning for HSG at 6 months is similar to the probability at 3 months, and
women would not want a third HS attempt.
HS success was identical whether or not women completed follow-up testing.
A schematic diagram of the Markov model is shown in Figure 1. Sterilization via LS, HS in the operating room, and HS in the office setting were tested in identical hypothetical cohorts of women. Complications related to HS and LS are also included in the model, but are not shown in Figure 1.
Using published data, the model also calculates the number of women who pursue a second or third attempt after a failed sterilization and the number of women who stop pursuing sterilization after one failed attempt.
One way sensitivity analysis was performed for the reasonable range of values identified for all parameters.
RESULTS
In the base case analysis, the percentage of women able to rely on their method of sterilization at 3 months post-procedure (without having any other procedures done) is 99% for LS, 86% for HS in OR, and 85% for HS in office. The reliance rate at 6 months post-procedure (without having any other procedures done) is 99%, 88% and 87%, respectively.
The probability of having any successful sterilization procedure within 1 year is 99% for women starting with LS, 95% for women starting with HS in the operating room, and 94% for women starting with HS in the office. However, the method by which the woman was ultimately sterilized sometimes differs from the method initially chosen. In the base case analysis, 7.0% and 5.3% of women who undergo an initial HS procedure in the operating room or office, respectively, actually achieve sterilization via LS. Of the women who experienced one failed attempt at a HS procedure, approximately 5% will decline any further sterilization attempts.
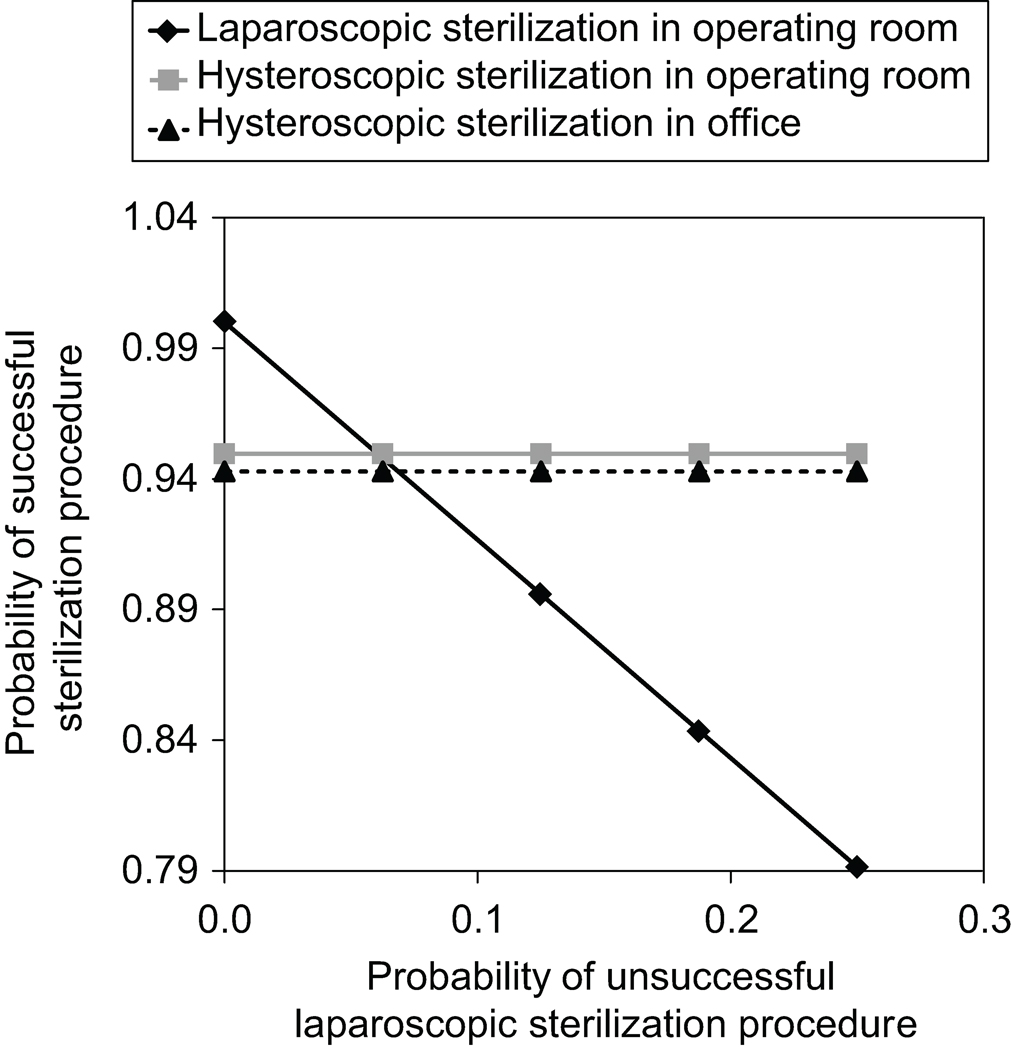
In sensitivity analyses, we found that the model is most strongly influenced by the high probability of success with LS (Figure 2). However, even if the model is biased against LS by using the highest probability of success for HS (97.9% for HS in operating room and 97.2% for HS in office) and the lowest probability of success for LS (98.3%), we found that LS would still outperform HS and 0.4% more women would have successful sterilization procedures if they initially chose LS. In contrast, if we use the highest probability of success for LS (99.6%) and the lowest for HS (89.5% for HS in Office), the difference in successful sterilization procedures within 1 year is as large as 10%.
Figure 2.
Sensitivity analysis of the probability of successful sterilization procedure using the laparoscopic compared with the hysteroscopic approach.
DISCUSSION
Women seeking permanent sterilization deserve an accurate assessment of the likelihood of success for both HS and LS. The time required to achieve sterilization before women can rely on it must also be included as part of the informed consent process. According to this model, 85% to 86% of women who chose HS will have a successful sterilization procedure by 3 months. This finding is consistent with the results from the Essure® Pivotal clinical trial (26).
By 12 months, 95% of women who chose HS in the operating room will have a successful sterilization procedure. However, the additional percentage gained by one year is mostly due to subsequent successful sterilization via LS. Seven percent of women choosing HS in the OR and 5% of women choosing HS in the office will ultimately require LS to be sterilized, and may only reach their goal of sterilization after multiple attempts at microinsert placement, HSGs, office visits, time missed from work, insurance co-pays, and need for other reliable interim contraception.
In the base case analysis, the large majority of women choosing HS in the office or operating room will have a successful sterilization procedure. However, women choosing HS are less likely to have a successful sterilization procedure than women choosing LS. In this analysis, this difference in success rates between the two approaches could be as large as 10%, although the true difference will vary by patient population.
In the U.S., 345,000 women undergo sterilization annually (1). If all female sterilizations in the U.S. were performed only by HS, this model predicts that approximately 31,050 women would not achieve actual sterilization within one year of their initial HS procedure. This estimate is comprised of the 5% of women who experience one failed attempt at HS and decline any further sterilization attempts and the 4% of women (99% minus 95%) that do not achieve sterilization by HS as compared to those choosing LS.
Failed attempts at sterilization can result in unintended pregnancies. In a recent analysis of outcomes after unfulfilled postpartum sterilization, the risk of pregnancy within one year was twice that of women not requesting sterilization (27).
Failed sterilizations resulting in unintended pregnancy can also occur after a successful procedure. Data on pregnancy after successful HS is reported to be zero by the manufacturer of Essure® and is currently limited to case reports. For LS, the CREST (Collaborative Review of Sterilization) study showed a cumulative pregnancy rate at one year of 0.68% (28). At one year, if we assume a pregnancy rate of zero after HS and 0.68% after LS, the likelihood of being able to rely on sterilization at one year is still lower with HS than LS.
The Adiana system, another multi-step method of hysteroscopic sterilization, was approved by the FDA in 2009. In the pivotal clinical trial for Adiana, the number of patients able to ultimately rely on Adiana at one year after placement was similar to that reported for Essure (29). Thus, the findings of this model are likely applicable to Adiana.
This model and its findings are limited by the uncertainty of the data it is based on. The biggest limitation of published HS data is that the majority comes from observational case series or cohort designs and the lack of any randomized trials directly comparing HS and LS (30). Published studies are also limited by low follow-up rates and possible conflicts of interest since most HS studies are performed by the manufacturer (12, 16). Also, we could not incorporate the results of 5 years of post-approval data collection that were presented by the manufacturer to the FDA in the Spring of 2010 as they have not been published (as of PubMed and Ovid search 4/13/2011).
Limitations with published LS data do not take into account women who were never offered LS due to co-morbid conditions that were relative contraindications for general anesthesia or trendelenberg positioning required for LS, or due to knowledge of significant adhesive disease. While the same argument could be applied to women who weren’t offered HS, the absolute and relative contraindications to HS are less frequently encountered than for LS.
However, contraindications to HS may be difficult to identify preoperatively. According to the package insert, Essure® should not be used for any patient, “for whom only one micro-insert can be placed (including patients with apparent contralateral proximal tubal occlusion and patients with a suspected unicornuate uterus)” (31). Uterine anomalies and tubal scarring are often asymptomatic.
Comparative trials are needed to assess true long-term efficacy and costs of HS. Reported success rates for HS that exclude patients that failed bilateral microinsert placement, failed to return for HSG, or were ultimately sterilized by LS are disingenuous. For physicians and patients, reporting of HS success rates must include the actual number of women who can truly rely on the method for its desired effect. We plan further analyses to evaluate cost and comparative pregnancy rates over time to provide additional data for women and their providers.
Acknowledgments
Funded by an Anonymous Foundation. Dr. Schwarz was funded by NICHD grant K23 HD051585.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented as a poster at the Society for Medical Decision Making, Toronto, Canada, Oct 25–27, 2010.
REFERENCES
- 1.Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat. 2010;23:1–44. [PubMed] [Google Scholar]
- 2.Piccinino LJ, Mosher WD. Trends in contraceptive use in the United States: 1982–1995. Fam Plann Perspect. 1998;30:4–10. 46. [PubMed] [Google Scholar]
- 3.Shavell VI, Abdallah ME, Shade GH, Jr, Diamond MP, Berman JM. Trends in sterilization since the introduction of Essure hysteroscopic sterilization. J Minim Invasive Gynecol. 2009;16:22–27. doi: 10.1016/j.jmig.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Conceptus I. [Retrieved December 12, 2010];What is Essure? Available at: http://www.essuremd.com/Home/AboutEssure/WhatisEssure/tabid/219/Default.aspx.
- 5.ACOG Committee Opinion No. 458: Hysterosalpingography after tubal sterilization. Obstet Gynecol. 2010;115:1343–1345. doi: 10.1097/AOG.0b013e3181e45ac2. [DOI] [PubMed] [Google Scholar]
- 6.Duffy S, Marsh F, Rogerson L, Hudson H, Cooper K, Jack S, et al. Female sterilisation: a cohort controlled comparative study of ESSURE versus laparoscopic sterilisation. BJOG. 2005;112:1522–1528. doi: 10.1111/j.1471-0528.2005.00726.x. [DOI] [PubMed] [Google Scholar]
- 7.Destefano F, Greenspan JR, Dicker RC, Peterson HB, Strauss LT, Rubin GL. Complications of interval laparoscopic tubal sterilization. Obstet Gynecol. 1983;61:153–158. [PubMed] [Google Scholar]
- 8.Jamieson DJ, Hillis SD, Duerr A, Marchbanks PA, Costello C, Peterson HB. Complications of interval laparoscopic tubal sterilization: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 2000;96:997–1002. doi: 10.1016/s0029-7844(00)01082-6. [DOI] [PubMed] [Google Scholar]
- 9.Franks AL, Kendrick JS, Peterson HB. Unintended laparotomy associated with laparoscopic tubal sterilization. Am J Obstet Gynecol. 1987;157:1102–1105. doi: 10.1016/s0002-9378(87)80269-7. [DOI] [PubMed] [Google Scholar]
- 10.Hendrix NW, Chauhan SP, Morrison JC. Sterilization and its consequences. Obstet Gynecol Surv. 1999;54:766–777. doi: 10.1097/00006254-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Huber AW, Mueller MD, Ghezzi F, Cromi A, Dreher E, Raio L. Tubal sterilization: complications of laparoscopy and minilaparotomy. Eur J Obstet Gynecol Reprod Biol. 2007;134:105–109. doi: 10.1016/j.ejogrb.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Kerin JF, Cooper JM, Price T, Herendael BJ, Cayuela-Font E, Cher D, et al. Hysteroscopic sterilization using a micro-insert device: results of a multicentre Phase II study. Hum Reprod. 2003;18:1223–1230. doi: 10.1093/humrep/deg256. [DOI] [PubMed] [Google Scholar]
- 13.Chen BA, Hayes JL, Reeves MF, Creinin MD. Outcomes of transcervical hysteroscopic sterilization in an urban academic medical center. Contraception. 2009;80:205. [Google Scholar]
- 14.Kerin JF, Carignan CS, Cher D. The safety and effectiveness of a new hysteroscopic method for permanent birth control: results of the first Essure pbc clinical study. Aust N Z J Obstet Gynaecol. 2001;41:364–370. doi: 10.1111/j.1479-828x.2001.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 15.Nichols M, Carter JF, Fylstra DL, Childers M. A comparative study of hysteroscopic sterilization performed in-office versus a hospital operating room. J Minim Invasive Gynecol. 2006;13:447–450. doi: 10.1016/j.jmig.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59–67. doi: 10.1016/s0029-7844(03)00373-9. [DOI] [PubMed] [Google Scholar]
- 17.Ubeda A, Labastida R, Dexeus S. Essure: a new device for hysteroscopic tubal sterilization in an outpatient setting. Fertil Steril. 2004;82:196–199. doi: 10.1016/j.fertnstert.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 18.Shavell VI, Abdallah ME, Diamond MP, Kmak DC, Berman JM. Post-Essure hysterosalpingography compliance in a clinic population. J Minim Invasive Gynecol. 2008;15:431–434. doi: 10.1016/j.jmig.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Shavell VI, Abdallah ME, Diamond MP, Berman JM. Placement of a permanent birth control device at a university medical center. J Reprod Med. 2009;54:218–222. [PubMed] [Google Scholar]
- 20.Andersson S, Eriksson S, Mints M. Hysteroscopic female sterilization with Essure in an outpatient setting. Acta Obstet Gynecol Scand. 2009;88:743–746. doi: 10.1080/00016340902934704. [DOI] [PubMed] [Google Scholar]
- 21.Savage UK, Masters SJ, Smid MC, Hung YY, Jacobson GF. Hysteroscopic sterilization in a large group practice: experience and effectiveness. Obstet Gynecol. 2009;114:1227–1231. doi: 10.1097/AOG.0b013e3181c2a10d. [DOI] [PubMed] [Google Scholar]
- 22.Mino M, Arjona JE, Cordon J, Pelegrin B, Povedano B, Chacon E. Success rate and patient satisfaction with the Essure sterilisation in an outpatient setting: a prospective study of 857 women. BJOG. 2007;114:763–766. doi: 10.1111/j.1471-0528.2007.01354.x. [DOI] [PubMed] [Google Scholar]
- 23.Levie MD, Chudnoff SG. Prospective analysis of office-based hysteroscopic sterilization. J Minim Invasive Gynecol. 2006;13:98–101. doi: 10.1016/j.jmig.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Sinha D, Kalathy V, Gupta JK, Clark TJ. The feasibility, success and patient satisfaction associated with outpatient hysteroscopic sterilisation. BJOG. 2007;114:676–683. doi: 10.1111/j.1471-0528.2007.01351.x. [DOI] [PubMed] [Google Scholar]
- 25.Arjona JE, Mino M, Cordon J, Povedano B, Pelegrin B, Castelo-Branco C. Satisfaction and tolerance with office hysteroscopic tubal sterilization. Fertil Steril. 2008;90:1182–1186. doi: 10.1016/j.fertnstert.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 26. [Retrieved December 12, 2010];Frequently asked questions: The Essure clinical trial data; What is the reliance rate for Essure? Available at: http://www.essuremd.com/Home/AboutEssure/FAQs/tabid/285/Default.aspx.
- 27.Thurman AR, Janecek T. One-year follow-up of women with unfulfilled postpartum sterilization requests. Obstet Gynecol. 2010;116:1071–1077. doi: 10.1097/AOG.0b013e3181f73eaa. [DOI] [PubMed] [Google Scholar]
- 28.Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Am J Obstet Gynecol. 1996;174:1161–1168. doi: 10.1016/s0002-9378(96)70658-0. discussion 8-70. [DOI] [PubMed] [Google Scholar]
- 29.Vancaillie TG, Anderson TL, Johns DA. A 12-month prospective evaluation of transcervical sterilization using implantable polymer matrices. Obstet Gynecol. 2008;112:1270–1277. doi: 10.1097/AOG.0b013e31818d8bda. [DOI] [PubMed] [Google Scholar]
- 30.Hurskainen R, Hovi SL, Gissler M, Grahn R, Kukkonen-Harjula K, Nord-Saari M, et al. Hysteroscopic tubal sterilization: a systematic review of the Essure system. Fertil Steril. 2010;94:16–19. doi: 10.1016/j.fertnstert.2009.02.080. [DOI] [PubMed] [Google Scholar]
- 31.Conceptus I. [Retrieved February 9, 2011];Quick Links; Instructions for Use. Available from: http://www.essuremd.com/portals/essuremd/PDFs/TopDownloads/L3002%2009_09_09%20smaller.pdf.