Abstract
Opioid addiction and HIV disease frequently co-occur. Adverse drug interactions have been reported between methadone and some HIV medications, but less is known about interactions between buprenorphine, an opioid partial agonist used to treat opioid dependence, and HIV therapeutics. This study examined drug interactions between buprenorphine and the protease inhibitors atazanavir and atazanavir/ritonavir. Opioid-dependent, buprenorphine/naloxone-maintained, HIV-negative volunteers (n=10 per protease inhibitor) participated in two 24-hour sessions to determine pharmacokinetics of (1) buprenorphine and (2) buprenorphine and atazanavir (400 mg daily) or atazanavir/ritonavir (300/100 mg daily) following administration for 5 days. Objective opiate withdrawal scale scores and Mini-Mental State Examination were determined prior to and following antiretroviral administration to examine pharmacodynamic effects. Pharmacokinetics of atazanavir and atazanavir/ritonavir were compared in subjects and matched, healthy controls (n=10 per protease inhibitor) to determine effects of buprenorphine. With atazanavir and atazanavir/ritonavir, respectively concentrations of buprenorphine (p<0.001, p<0.001), norbuprenorphine (p=0.026, p=0.006), buprenorphine glucuronide (p=0.002, p<0.001), and norbuprenorphine glucuronide (NS, p=0.037) increased. Buprenorphine treatment did not significantly alter atazanavir or ritonavir concentrations. Three buprenorphine/naloxone-maintained participants reported increased sedation with atazanavir/ritonavir. Atazanavir or atazanavir/ritonavir may increase buprenorphine and buprenorphine metabolite concentrations and might require a decreased buprenorphine dose.
Keywords: buprenorphine/naloxone, atazanavir, ritonavir, drug interactions
1. Introduction
Injection drug use continues to be a significant risk factor for HIV disease (Deany, 2000). The majority of injection drug users (IDUs) with HIV disease are opioid dependent and in need of treatment for both HIV disease and substance dependence. Adherence to medical regimens among IDUs is often poor (Arnsten at al., 2002; Metha at al., 1997). As a result, highly active antiretroviral therapy (HAART) is frequently underutilized in this population because of concerns regarding effective viral suppression (Celentano et al., 2001; Lucas et al., 2001; Strathdee at al., 1998). Treatment for opioid dependence that includes opioid-assisted therapy can promote adherence to HIV disease treatment regimens by stabilizing the chaotic lifestyle of the opioid-addicted individual. Studies have shown that the course of HIV disease in drug users receiving substance abuse treatment is similar to other groups with HIV infection (Cohn, 2002) and the rate of HIV progression can be slowed in IDUs who receive medical intervention (Cohn, 2002; Des Jarlais and Hubbard, 1999).
Methadone has been the most widely used opioid pharmacotherapy for the treatment of opioid dependence. However, its use has been associated with several adverse drug interactions with HIV therapeutics that can produce either elevated methadone concentrations with toxicity, or decreased methadone levels with withdrawal. Both effects may diminish adherence if uncorrected (Altice et al., 1999; McCance-Katz et al, 2002; McCance-Katz et al, 2003; McCance-Katz et al., 2004; McCance-Katz, 2005). Buprenorphine (BUP) has been shown to be equivalent to methadone in the treatment of opioid-dependent patients (Strain et al., 1996). Buprenorphine/naloxone (BUP/NLX) in a 4:1 ratio is the usual formulation used in the treatment of opioid dependence in the United States [McCance-Katz, 2004]. Naloxone, an opioid antagonist active only when administered parenterally, was added to BUP in a combination tablet to diminish diversion and abuse of the drug by injection (McCance-Katz, 2004). Further, the poor sublingual absorption of naloxone prevents its alteration of BUP opioid agonist effects. To date, BUP has not been shown to produce adverse drug interactions with delavirdine, efavirenz, nelfinavir, ritonavir (RTV) or lopinavir/ritonavir (McCance-Katz et al., 2006a; McCance-Katz et al., 2006 b).
We now report on the interaction between BUP and a newer protease inhibitor (PI), atazanavir (ATV). Because in clinical practice many PIs are now administered in combination with RTV as a means of boosting PI plasma concentrations and simplifying HAART, a second study in which the interaction of BUP with atazanavir/ritonavir (ATV/r) was determined is also reported.
2. Methods
2.1 Clinical Protocol
Forty individuals completed the protocol. Ten BUP/NLX-maintained individuals and 10 non-opioid-maintained participated in each of the ATV and ATV/r studies.
The study was open label and comprised of both (1) a within-subjects component which examined the effect of ATV or ATV/r administration on BUP disposition and (2) a between-subjects component that examined the effect of BUP on the disposition of ATV or ATV/r. Information about the study was available in local mental health centers and substance abuse treatment clinics in Richmond, Virginia, and potential subjects could self-refer. Other participants were recruited from the Richmond community at large through newspaper advertisement and word-of-mouth. Study investigators screened potentially eligible subjects who provided written, voluntary, informed consent following Virginia Commonwealth University Institutional Review Board-approved protocols. Opioid-dependent participants received BUP treatment of their opioid addiction at no charge and were offered monetary compensation for participation in the study protocol. Control participants were offered monetary compensation for their time and effort in the study protocol.
Participants admitted to this study were (1) opioid-dependent individuals treated and stable for at least 2 weeks on standard clinical doses of BUP/NLX (sublingual) daily and (2) a comparison group of age, gender, race and weight-matched, healthy, non-opioid dependent volunteers. Men and women were enrolled in the study if they were HIV-seronegative by enzyme-linked immunosorbent assay; were 18 years of age or older; were not being treated with medications that might alter hepatic function, and were without clinically significant medical conditions, as determined by medical history, physical examination, ECG, complete blood count, liver function tests (≥ 3 times the upper limit of normal was exclusionary), glucose, urea nitrogen, creatinine, pregnancy testing (for women) and urinalysis. Urine was also tested for recent use of cocaine, marijuana, opiates, amphetamines, and benzodiazepines; toxicology was repeated prior to conducting drug interaction/drug disposition studies. Participants were tested for HIV viral load in the week before study participation to exclude anyone with recent HIV infection.
Study procedures for opioid-dependent participants included standardized and validated measures of opioid withdrawal by clinician rating (Objective Opioid Withdrawal Scale [OOWS], scores ≥ 3 indicate moderate withdrawal symptoms) (Handlesman et al., 1997) and of cognitive impairment (Mini-Mental State Examination [MMSE] maximum score=30; scores of <27 indicate cognitive impairment) (Folstein et al., 1975). Adverse symptoms were recorded for all participants using an Adverse Symptoms Checklist that queried for a wide range of adverse experiences including changes in energy, gastrointestinal symptoms, central nervous system effects, genitourinary symptoms, and other somatic complaints scored for severity on an ordinal scale (0−3, with 0= not present, 1=mild, 2=moderate, and 3=severe, maximum possible score=87). These ratings were administered at baseline, following stabilization on BUP/NLX (prior to antiretroviral administration), and at completion of the PI dosing period, and for control subjects, prior to and at completion of PI administration.
Subject characteristics are summarized in Table 1. BUP/NLX-maintained subjects met DSM-IV-TR criteria (DSM-IV, 2000) for opioid dependence and were enrolled in the Virginia Commonwealth University Medical Center Buprenorphine Treatment Research program. Other substance use disorders and mental disorders were screened for by clinical assessment and administration of the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998).
Table 1.
Sample Characteristics
| Atazanavir Buprenorphine N=10 | Atazanavir Control N = 10 | Atazanavir/Ritonavir Buprenorphine N = 10 | Atazanavir/Ritonavir Control N = 10 | |
|---|---|---|---|---|
| Age (yrs) |
38 (2.7)* |
42 (3.1) |
37 (2.7) |
40 (3.1) |
| Weight (kg) |
83.4 (4.5) |
76.0 (4.4) |
87.6 (6.1) |
83.7 (4.9) |
| Buprenorphine/Naloxone Dose (mg/day) |
15.2 (0.8)/3.8 (0.2) |
N/A |
16.0 (0.0)/4.0 (0.0) |
N/A |
| Female |
3 |
4 |
4 |
3 |
| Race: | ||||
| African-American | 10 | 8 | 9 | 7 |
| Caucasian |
- |
2 |
1 |
3 |
| Substance Use Disorders: | ||||
| Opioid Dependence | 10 | - | 10 | - |
| Cocaine Abuse | 8 | 4 | 7 | 3 |
| Cannabis Abuse | 1 | 1 | 2 | 1 |
| Alcohol Abuse |
- |
1 |
1 |
1 |
| IDU |
1 |
0 |
2 |
0 |
| Nicotine Use (packs/day) |
0.6 (0.1) |
0.5 (0.2) |
0.7 (0.2) |
0.6 (0.2) |
| Hepatitis C Positive | 0 | 0 | 1 | 0 |
Mean (SE)
2.2 Pharmacokinetic study design
Study participants received study medications as outpatients where they reported to the Buprenorphine Treatment Research Program and were administered study medications daily. One day prior to the pharmacokinetic studies, they were admitted to an inpatient research unit (General Clinical Research Center) where the pharmacokinetics study protocol was completed. Prior to participation in a pharmacokinetics study, participants did not consume food or water after midnight of the previous evening. A urine toxicology screen was again obtained to document whether illicit substances had been recently ingested. A participant would be terminated from the study session if toxicology showed recent use of opiates. A breathalyzer test for blood alcohol level was required to be negative for the study to proceed.
2.3 BUP/NLX-maintained participants
BUP/NLX-maintained subjects then received their usual daily dose administered sublingually. Pharmacokinetic studies were initiated at the subject's usual dosing time. Five milliliter blood samples were collected immediately before BUP/NLX dosing (trough BUP concentration) and at pre-determined subsequent time points. BUP and metabolite plasma concentrations were obtained at 0.5, 1, 1.5, 2, 4, 6, 8, 12, and 24 hours after dosing. Participants were fed a standardized high-fat (36 grams) breakfast 30 minutes before administration of study drugs. Following completion of the initial pharmacokinetic study, a blood sample was collected for HIV viral load. Upon determination of a negative test result, participants were scheduled to begin PI administration. After administration of the daily dose of BUP, the first dose of ATV 400 mg or ATV/r (300/100 mg) was then administered (female subjects had another urine pregnancy test completed before the antiretroviral dose) and participants were monitored for 1 hour for the occurrence of immediate adverse events or side effects. Participants returned to the Buprenorphine Treatment Research program daily as outpatients to receive BUP/NLX dosing and HIV medication. PI medications were administered daily by research staff. Ingestion of all PI and BUP/NLX doses were witnessed by study staff. For these studies, PIs were administered over a 5-day period approximating the amount of time necessary to reach steady-state. Upon completion of the PI medication administration, a second pharmacokinetics study was completed in which blood samples were collected for determination of both BUP and PI plasma concentrations. At completion of the second pharmacokinetics study, participants underwent a physical examination, and a brief clinical assessment, were queried for adverse events, and had a blood sample collected for liver function tests. Participants were then discharged to their usual activities and to continue in the Buprenorphine Treatment Research program.
The OOWS, Adverse Symptoms Checklist, Mini-Mental State Examination, and ECG were administered at baseline, following stabilization on BUP/NLX (at the time of trough BUP plasma concentration) and prior to PI administration, and 30 minutes prior to initiation of the second pharmacokinetic study which occurred at the end of the PI dosing period.
2.4 Control (non-opioid-maintained) participants
A group of non-opioid-dependent controls were recruited for administration of PIs for the same duration as that for BUP/NLX-treated patients. Controls were generally matched to BUP/NLX-treated participants by age, weight, race, and gender. These participants underwent the same procedures as for BUP/NLX-treated participants except that they were administered only ATV or ATV/r for 5 days and underwent a single pharmacokinetics study to determine PI plasma concentrations.
2.5 Drug sampling
On pharmacokinetic study days, blood samples were collected by venipuncture into Vacutainer tubes immediately before dosing and at pre-determined time points (above). Blood samples to be assayed for BUP, ATV or RTV were collected in heparinized tubes from which plasma was collected. All blood samples were centrifuged at 3000 × g for 10 minutes. Plasma was then transferred into pre-labeled tubes and stored at −70°C until the time of analysis.
2.6 Biochemical assays
2.6.1 Buprenorphine assay
Buprenorphine and metabolite concentrations were determined using a recently described liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (Huang et al., 2006). Briefly, buprenorphine-d4 and norbuprenorphine-d3 were added to samples as the internal standards. The pH of the matrix was adjusted to 9.3 with ammonium carbonate buffer and samples were extracted using C18 solid-phase extraction columns. The eluate was evaporated and reconstituted with 0.1% formic acid in water and analyzed by LC-MS/MS using electrospray ionization. During validation inter-run precision was within 10.7, 5.2 and 8.2% at 0.25, 25 and 40 ng/mL, respectively.
2.6.2 Atazanavir and ritonavir assays
RTV was quantified using a previously published simultaneous high-performance liquid chromatography (HPLC) assay with slight modification (Keil et al., 2003; Keil et al., in press). ATV was added to this method at a later date following approval. Briefly, the method involved a liquid-liquid extraction of the plasma sample, followed by separation on a C8 Symmetry™ column (Waters Associates, Milford, MA) and detection at 248 and 215 nm for ATV and RTV, respectively, using a Model 996 photodiode array detector (Waters Associates, Milford, MA) to assure specificity. The coefficient of variation for the analytes was ≤6.6% at all quality control levels. Quality control concentrations were 300, 600, 3000, and 12,800 ng/mL for ATV and 600, 3000 and 12,800 ng/mL for RTV. The lower limit of quantitation (LLOQ) values for ATV and RTV were 100 and 200 ng/mL, respectively. During the course of assay application; inter-assay variation for ATV and RTV was ≤15%. Verification of accuracy for analyte concentrations was performed in conjunction with the ACTG and International Proficiency Testing Programs (Aarnoutse et al., 2002; Droste et al., 2003; Holland et al., 2004; Holland et al., 2006).
2.7 Pharmacokinetics analysis
The pharmacokinetic parameters of BUP, norbuprenorphine (NBUP), buprenorphine-3-glucuronide (BUP-G), norbuprenorphine-3-glucuronide (NBUP-G), ATV, and RTV were evaluated as appropriate for each subject. BUP pharmacokinetics were calculated following sublingual administration of the BUP/NLX only, and again following ingestion of ATV 400 mg daily or ATV/r (300/100 mg daily) for 5 days. ATV or ATV/r pharmacokinetics parameters were evaluated after medications had been administered as described for BUP/NLX-maintained individuals. Standard non-compartmental methods were used to estimate pharmacokinetic parameters (WinNonlin, 2005). These parameters included the area under the concentration-time curve (AUC0−24,), maximum plasma concentration (Cmax), time of Cmax (Tmax), and oral clearance (CL/F), where F is the fraction of administered drug absorbed into the systemic circulation. For BUP metabolites, F represents the fraction of the original buprenorphine dose that was converted to circulating metabolite. All pharmacokinetic parameters were summarized and displayed by treatment period.
2.8 Statistical analysis
Based on our previous study of the interaction of methadone and lopinavir/ritonavir (McCance-Katz et al., 2003), power analysis showed a sample size of 10 was needed to detect a 40 percent change in either antiretroviral drug AUC or oral clearance between PI alone in the control group and in combination with BUP with a power of 0.8. Student's paired t-test was used to test the significance of the differences in pharmacokinetic parameters for BUP vs. BUP and ATV or ATV/r in combination (within-subjects analyses). The unpaired t-test was used to compare differences in pharmacokinetic parameters for ATV or RTV in the control groups vs. the BUP-treated groups. The Wilcoxon test was used for the within-subject comparison of the values of the discontinuous variable, Tmax, and the Mann-Whitney test was used for the between subject comparison of this parameter. A difference was considered statistically significant if the p value was ≤ 0.05 (two-tailed). Comparisons of subject characteristics were made by single factor ANOVA.
3.0 Results
3.1 Study participants
A total of 40 volunteers participated in the two PI protocols undertaken in this study. For ATV and ATV/r, 10 opioid-dependent participants who were receiving a stable, daily, sublingual, dose of BUP/NLX and who were otherwise physically healthy and without current mental disorders other than substance use disorders completed the study. Twenty (10 per PI) control participants who were generally matched by age, gender, and weight to opioid-dependent volunteers completed pharmacokinetic studies for ATV and ATV/r. Demographic characteristics for study participants are listed in Table 1. There were no significant differences in age, race, or gender. Several study participants in the BUP/NLX and control groups reported cocaine abuse, cannabis abuse, or alcohol abuse (no participants met diagnostic criteria for alcohol dependence), confirmed as substance use disorders by clinician interview with the MINI. Cigarette smoking was common in study participants, with daily use reported by all subjects of less than one pack per day (range: 0.5−0.7 packs/day). Injection drug use ranged from 10−20%, with nasal insufflation being the preferred route of administration for the remaining opioid-dependent participants. Serological evidence of Hepatitis C was present only in one opioid-dependent participant and in no controls (Table 1).
3.2 Interaction between buprenorphine and atazanavir or atazanavir/ritonavir
3.2.1 Effect of atazanavir or atazanavir/ritonavir on buprenorphine
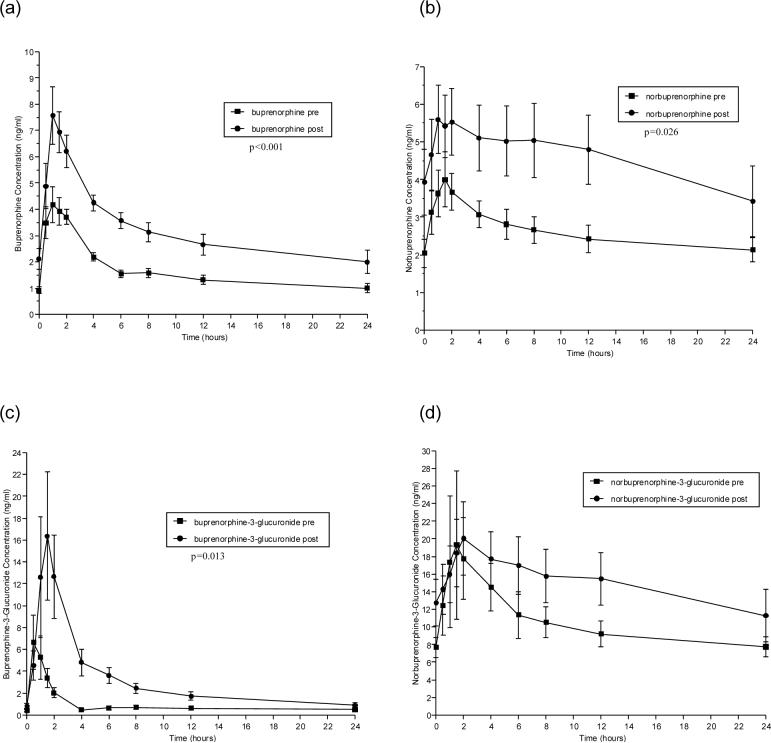
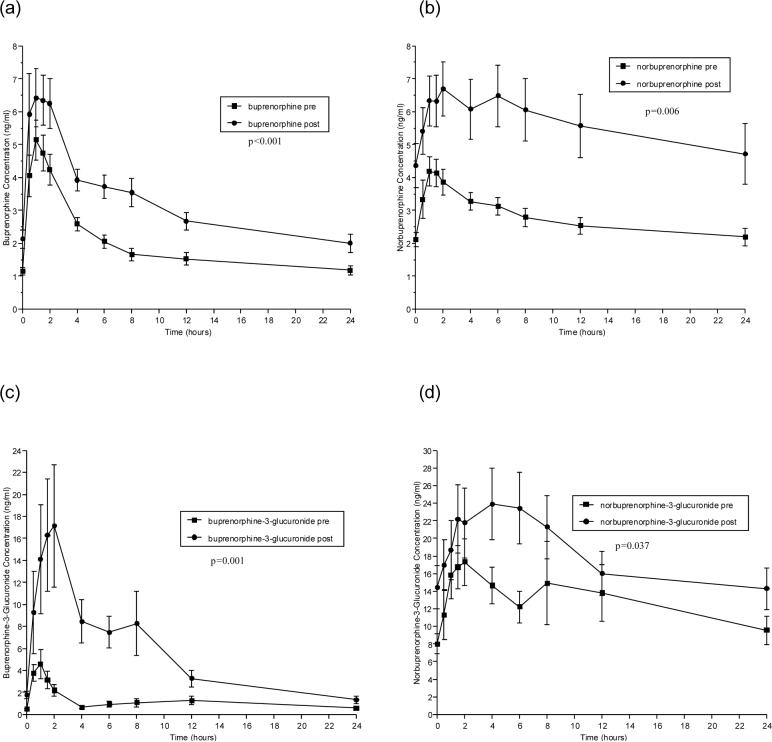
Pharmacokinetic parameters for BUP before and after ATV or ATV/r administration are shown in Tables 2 and 3, respectively. Figures 1 and 2 (a-d) show concentrations of BUP (a), the active metabolite, NBUP (b), and the inactive, conjugated metabolites BUP-G (c) and NBUP-G (d) over a 24-hour dosing interval for BUP alone administration versus BUP administered concomitantly with ATV or ATV/r, respectively.
Table 2.
Effect of atazanavir on the plasma pharmacokinetic parameters of buprenorphine and buprenorphine metabolites
| Pharmacokinetic Parameter | Pre- Atazanavir | Post- Atazanavir | p value |
|---|---|---|---|
| Buprenorphine | |||
| AUC0-24 (ng*h/ml) | 39.5 (3.6) | 76.3 (8.6) | <0.001 |
| CI/F (L/h) | 408 (39) | 223 (29) | <0.001 |
| Cmax (ng/ml) | 4.87 (0.67) | 8.00 (1.02) | 0.001 |
| Tmax (h) | 1.25 (0.5-2) | 1.00 (0.5-1.5) | n.s. |
| Cmin (ng/ml) | 0.84 (0.14) | 1.67 (0.37) | 0.03 |
| Norbuprenorphine | |||
| AUC0-24 (ng*h/ml) | 62.4 (8.5) | 110 (20.7) | 0.026 |
| CI/F (L/h) | 282 (37) | 187 (38) | 0.01 |
| Cmax (ng/ml) | 4.51 (0.71) | 6.14 (0.97) | 0.08 |
| Tmax (h) | 1.75 (0.5-4) | 3.0 (1-8) | n.s. |
| Cmin (ng/ml) | 1.82 (0.30) | 2.98 (0.85) | 0.16 |
| Buprenorphine-3-Glucuronide | |||
| AUC0-24 (ng*h/ml) | 22.9 (5.3) | 76.2 (16.3) | 0.002 |
| CI/F (L/h) | 1132 (307) | 374 (123) | 0.012 |
| Cmax (ng/ml) | 7.53 (2.34) | 18.96 (5.69) | 0.04 |
| Tmax (h) | 0.5 (0.5-2) | 1.5 (1-6) | 0.04 |
| Cmin (ng/ml) | 0.35 (0.09) | 0.76 (0.22) | 0.04 |
| Norbuprenorphine-3-Glucuronide | |||
| AUC0-24 (ng*h/ml) | 252 (439) | 360 (72) | 0.13 |
| CI/F (L/h) | 0.07 (0.01) | 0.16 (0.47) | 0.99 |
| Cmax (ng/ml) | 81 (17) | 81 (30) | 0.98 |
| Tmax (h) | 1.75 (1-4) | 2.0 (0-12) | 0.05 |
| Cmin (ng/ml) | 6.72 (1.10) | 9.83 (2.51) | 0.23 |
Note: Values are the mean (standard error of the mean) for 10 subjects who participated in both sessions, except that the discontinuous variable, Tmax, is given as median (range). Student's paired t-test was used to determine p-values for all parameters except Tmax, where the Wilcoxon test was used.
Table 3.
Effect of atazanavir/ritonavir on the plasma pharmacokinetic parameters of buprenorphine and buprenorphine metabolites
| Pharmacokinetic Parameter | Pre- ATV/r | Post- ATV/r | p value |
|---|---|---|---|
| Buprenorphine | |||
| AUC0-24 (ng*h/ml) | 46.2 (4.3) | 77.0 (6.9) | <0.001 |
| CI/F (L/h) | 390 (55) | 226 (23) | 0.002 |
| Cmax (ng/ml) | 5.79 (0.59) | 7.95 (1.06) | 0.002 |
| Tmax (h) | 1.00 (0.5-2) | 1.25 (0.5-2) | n.s. |
| Cmin (ng/ml) | 1.09 (0.11) | 1.84 (0.22) | 0.002 |
| Norbuprenorphine | |||
| AUC0-24 (ng*h/ml) | 65.8 (6.3) | 135 (21.6) | 0.006 |
| CI/F (L/h) | 267 (28) | 155 (29) | <0.001 |
| Cmax (ng/ml) | 4.60 (0.52) | 7.42 (0.88) | 0.01 |
| Tmax (h) | 1.5 (0.5-4) | 2.0 (0.5-8) | n.s. |
| Cmin (ng/ml) | 1.97 (0.23) | 3.95 (0.66) | 0.01 |
| Buprenorphine-3-Glucuronide | |||
| AUC0-24 (ng*h/ml) | 28.1 (5.9) | 132 (25.5) | 0.001 |
| CI/F (L/h) | 1242 (425) | 184 (41) | 0.025 |
| Cmax (ng/ml) | 4.97 (1.28) | 25.6 (6.22) | 0.007 |
| Tmax (h) | 1.0 (0.5-1.5) | 1.75 (0.5-8) | 0.05 |
| Cmin (ng/ml) | 0.36 (0.08) | 1.28 (0.33) | 0.014 |
| Norbuprenorphine-3-Glucuronide | |||
| AUC0-24 (ng*h/ml) | 312 (54) | 432 (66) | 0.037 |
| CI/F (L/h) | 72 (15) | 56 (16) | 0.14 |
| Cmax (ng/ml) | 23.6 (4.6) | 31.5 (6.8) | 0.19 |
| Tmax (h) | 1.75 (1-12) | 5.0 (1.5-8) | n.s. |
| Cmin (ng/ml) | 7.62 (1.20) | 12.0 (1.92) | 0.018 |
Note: Values are the mean (standard error of the mean) for 10 subjects who participated in both sessions, except that the discontinuous variable, Tmax, is given as median (range). Student's paired t-test was used to determine p-values for all parameters except Tmax, where the Wilcoxon test was used.
Figure 1.
Effect of atazanavir on the time versus concentration curves of buprenorphine and its metabolites. Shown are mean concentrations at each time point in study participants taking sublingual buprenorphine/ naloxone (16/4 mg daily) alone (■) and study participants taking the same dose of buprenorphine/naloxone in combination with 400 mg of atazanavir daily (●). The panels show the mean concentrations of (a) buprenorphine, (b) norbuprenorphine, (c) buprenorphine-3-glucuronide and (d) norbuprenorphine-3-glucuronide.
Figure 2.
Effect of atazanavir/ritonavir on the time versus concentration curves of buprenorphine and its metabolites. Shown are mean concentrations at each time point in study participants taking sublingual buprenorphine/ naloxone (16/4 mg daily) alone (■) and study participants taking the same dose of buprenorphine/naloxone in combination with 300 mg atazanavir/100 mg ritonavir daily (●). The panels show the mean concentrations of (a) buprenorphine, (b) norbuprenorphine, (c) buprenorphine-3-glucuronide and (d) norbuprenorphine-3-glucuronide.
The co-administration of ATV with BUP for 5 days significantly increased BUP and BUP metabolite plasma concentrations (Table 2). AUC0−24 , Cmax and Cmin values were increased for BUP, NBUP and BUP-G with corresponding significant decreases in Cl/F for BUP and its metabolites. Differences in Tmax were statistically significant only for BUP-G, but the observed changes were clinically inconsequential. NBUP-G pharmacokinetics were not significantly altered by ATV administration, but observed values showed some increase in AUC0−24, Cmax and Cmin (Figure 1d).
ATV/r administration administered over a 5 day dosing interval produced similar or larger effects on BUP and BUP metabolite pharmacokinetics than did ATV alone administration (Table 3, Figure 2). Significant increases in AUC 0−24, Cmax, and Cmin were observed for BUP, NBUP, and BUP-G. Unlike ATV, the effect of ATV/r reached statistical significance for NBUP-G AUC 0−24 (Table 3).
BUP/NLX-maintained participants showed no evidence of cognitive impairment by repeated MMSE or opioid withdrawal symptoms by OOWS with either ATV or ATV/r co-administration (Table 4). Non-significant decreases in adverse symptoms were observed with ATV and ATV/r administration. The item “Drowsy/sleepy” was the only adverse symptom that increased following ATV/r administration in several BUP-maintained participants resulting in a trend for increased sedation when ATV/r was administered concomitantly with BUP (p=0.096) (Table 4).
Table 4.
Physiological, cognitive, and subjective responses in buprenorphine-maintained and control participants to atazanavir (400 mg daily) or atazanavir/ritonavir (300/100 mg daily)
| Atazanavir Buprenorphine N=10 | Atazanavir Control N = 10 | Atazanavir/Ritonavir Buprenorphine N = 10 | Atazanavir/Ritonavir Control N = 10 | |
|---|---|---|---|---|
| AST Pre/Post (U/L) | 23.2 (1.5)/25.5 (4.6) | 19.1 (1.2)/18.8 (2.5) | 20.4 (2.0)/19.1 (1.4) | 22.2 (1.3)/17.0 (1.3) |
| |
|
|
|
p=.012 |
| ALT Pre/Post (U/L) |
24.0 (5.4)/23.0 (6.5) |
18.4 (3.9)/18.3 (4.1) |
19.4 (4.3)/18.2 (3.8) |
22.4 (4.0)/18.3 (3.2) |
| Total Bilirubin Pre/Post (mg/dL) | 0.5 (0.1)/1.1 (0.2) | 0.6 (0.1)/1.7 (0.2) | 0.4 (0.1)/1.8 (0.4) | 0.6 (0.1)/2.9 (0.3) |
| |
p=.003 |
p=.001 |
p=.005 |
p<.001 |
| Direct Bilirubin Pre/Post (mg/dL) | 0.1 (0.01)/0.2 (0.02) | 0.1 (0.03)/0.2 (0.03) | 0.1 (0.01)/0.3 (0.05) | 0.1 (0.01)/0.4 (0.04) |
| |
p=.001 |
p=.005 |
p=.006 |
p<.0001 |
| PR Interval Pre/Post (ms) | 159.8 (6.3)/168.4 (8.0) | 163.0 (5.3)/176.0 (4.5) | 177.0 (10.4)/182.2 (13.0) | 156.2 (6.1)/166.2 (6.3) |
| |
|
p=.020 |
|
p=.014 |
| QTc Interval Pre/Post (ms) | 409.0 (5.4)/410.3 (3.8) | 417.0 (6.7)/405.2 (10.3) | 406.8 (3.5)/408.4 (4.9) | 419.4 (5.2)/407.8 (3.4) |
| |
|
|
|
p=.028 |
| OOWS Pre/Post |
0.1 (0.1)/0.0 (0.0) |
0.0 (0.0)/0.0 (0.0) |
0.0 (0.0)/0.0 (0.0) |
0.0 (0.0)/0.0 (0.0) |
| MMSE Pre/Post | 29.3 (0.3)/29.8 (0.1) | 29.6 (0.2)/29.4 (0.2) | 29.5 (0.2)/29.5 (0.2) | 29.5 (0.2)/29.4 (0.3) |
| |
p=.052 |
|
|
|
| Adverse Symptoms Total Score Pre/Post |
9.9 (3.1)/6.9 (1.6) |
1.3 (0.8)/0.7 (0.5) |
7.5 (2.6)/6.5 (1.7) |
0.0 (0.0)/1.1 (0.7) |
| Drowsy/Sleepy Score Pre/Post | 0.7 (0.25)/0.5(0.25) | 0.2(0.13)/0.1(0.09) | 0.1(0.09)/0.6(0.25) | 0.0(0.0)/0.2(0.19) |
* Mean (SE)
p values are shown for comparisons where significant differences were observed
3.2.2 Side effects of atazanavir or atazanavir/ritonavir
ATV administration at standard clinical doses produced significant increases in total bilirubin in BUP-maintained participants and in control participants (Table 4). ATV/r administration was associated with greater increases in total bilirubin than ATV alone in both BUP-maintained participants and control participants (Table 4). A comparison of total bilirubin following ATV or ATV/r administration showed significantly greater increases in control participants (Post ATV Total Bilirubin BUP-maintained: 1.1 mg/dL, Controls: 1.7, p=0.042, Post ATV/r BUP-maintained 1.8 mg/dL, Controls: 2.9 mg/dL p=0.042). Although statistically significant changes in direct (conjugated) bilirubin were also observed after PI administration in all groups, these changes accounted for only a small part of the increase in total bilirubin. Direct bilirubin concentrations also remained within normal limits for both BUP and control participants after ATV or ATV/r administration, indicating that the changes were of no clinical significance. Bilirubin increases were not associated with clinical symptoms and reverted to the normal range within one week of discontinuing ATV or ATV/r. The cardiac PR interval was increased for all groups, significantly for ATV control, and ATV/r control participants (Table 4) without adverse events or symptoms. Participants reported few adverse symptoms following ATV or ATV/r administration (Table 4). Three of ten BUP/NLX-maintained participants endorsed increases in “Drowsy/sleepy” on the Adverse Symptoms Checklist following ATV/r administration for 5 days, however this did not reach statistical significance.
3.2.3 Effect of buprenorphine on atazanavir and ritonavir pharmacokinetics
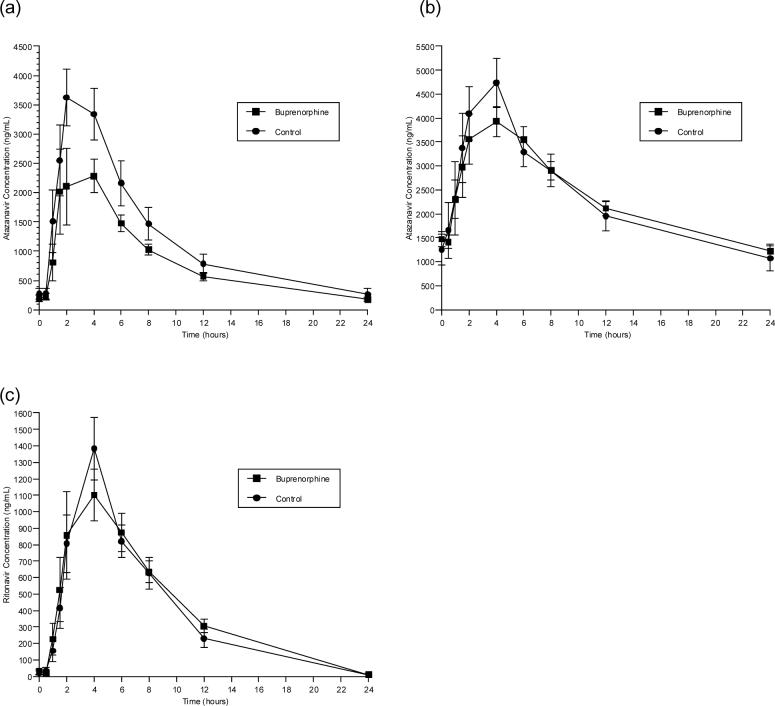
ATV concentrations were measured following ATV 400 mg daily alone or ATV/r 300/100 mg daily over a 24-hour dosing interval in BUP/NLX-maintained individuals. RTV concentrations were measured over a 24-hour dosing interval when administered in combination with ATV. BUP/NLX had no significant effect on ATV or RTV pharmacokinetics (Figure 3, Table 5). Concentrations of ATV and RTV remained in the therapeutic range during BUP/NLX treatment.
Figure 3.
Effect of buprenorphine on the time versus concentration curves of atazanavir and ritonavir. Shown are mean concentrations at each time point in study participants taking daily sublingual buprenorphine/ naloxone (16/4 mg daily) (■) and in control participants who were not taking buprenorphine/naloxone (●). The panels show the mean concentrations of (a) atazanavir in participants taking 400 mg of atazanavir daily; (b) atazanavir in participants taking 300 mg atazanavir/100 mg ritonavir daily; and (c) ritonavir in participants taking 300 mg atazanavir/100 mg ritonavir daily.
Table 5.
Effect of buprenorphine on the plasma pharmacokinetic parameters of atazanavir and ritonavir
| Atazanavir Parameter | ATV | ATV-BUP/NLX | p-value |
|---|---|---|---|
| AUC0-24 (h*ng/mL) | 29863 (4696) | 20649 (2206) | n.s. |
| CI/F (L/h) | 16.5 (2.32) | 21.64 (2.60) | n.s. |
| Cmax (ng/mL) | 4435 (410) | 3223 (494) | n.s. |
| Tmax (h) | 2.00 (1.45-4.00) | 3.00 (1.50-6.05) | n.s. |
| Cmin (ng/mL) |
259 (100) |
173 (41) |
n.s. |
|
Atazanavir/ritonavir Parameter |
ATV |
ATV-BUP/NLX |
|
| AUC0-24 (h*ng/mL) | 55980 (7653) | 56137 (3783) | n.s. |
| CI/F (L/h) | 5.78 (0.70) | 5.62 (0.47) | n.s. |
| Cmax (ng/mL) | 5359 (501) | 4597 (346) | n.s. |
| Tmax (h) | 3.00 (1.50-4.00) | 3.00 (1.50-6.00) | n.s. |
| Cmin (ng/mL) |
1072 (269) |
1223 (136) |
n.s. |
| RTV | RTV-BUP/NLX | ||
| AUC0-24 (h*ng/mL) | 9460 (1330) | 9730 (1310) | n.s. |
| CI/F (L/h) | 12.68 (1.88) | 12.77 (2.35) | n.s. |
| Cmax (ng/mL) | 1490 (170) | 1240 (180) | n.s. |
| Tmax (h) | 4.00 (2.00-4.00) | 4.00 (2.00-8.00) | n.s. |
| Cmin (ng/mL) | <200 | <200 | n.s. |
Note: Values are the mean (standard error of the mean) for 10 subjects who participated in both sessions, except that the discontinuous variable, Tmax, is given as median (range). Student's unpaired t-test was used to determine p-values for all parameters except Tmax, where the Wilcoxon test was used.
4.0 Discussion
The findings from this study indicate that administration of ATV or ATV/r in doses that are regularly used in clinical care of HIV disease is associated with significant increases in BUP and BUP metabolite exposure. These increases were associated with sedation in several (30%) BUP-NLX maintained study participants receiving ATV/r. BUP treatment had no significant effect on ATV or RTV concentrations. ATV and ATV/r administration were associated with significant, reversible increases in total bilirubin in BUP/NLX-maintained participants as well as healthy, control participants. These findings indicate that a reduction in dose could sometimes be indicated in BUP-maintained patients receiving ATV or ATV/r.
In the current study, BUP and BUP metabolite concentrations were significantly increased following five days of daily ATV or ATV/r. This pharmacokinetic interaction was associated with increased daytime sedation in three BUP/NLX-maintained participants administered ATV/r. However, the remaining BUP/NLX-maintained study participants suffered no ill effects of ATV or ATV/r administration. These findings may offer an explanation for observations in a recent clinical report of three BUP/NLX-maintained patients with HIV disease who required BUP dose reductions when treated with ATV/r (Bruce and Altice, 2006). In that report, patients developed somnolence and impaired cognition that required a decrease in BUP dose within 1−2 days of beginning a HAART regimen that included ATV/r (300/100 mg daily--the dose tested in this study as well). In the current study, 30% of participants receiving ATV/r reported mild to moderate sedation, but none required a BUP dose decrease. Participants noted few other adverse events, despite increases in BUP and BUP metabolite exposure in all BUP-treated participants receiving ATV or ATV/r. Similarly, no participants complained of cognitive difficulties. This observation, coupled with unchanged MMSE performance, confirmed a lack of acute cognitive deficits with ATV or ATV/r in BUP/NLX-maintained participants.
It is not clear why 3 subjects treated with ATV/r developed sedation. Concentrations of BUP and its metabolites were not higher in these subjects than in the other 7 subjects who did not develop sedation. No BUP-maintained participants in the ATV 400 mg/d protocol complained of sedation, despite similar increases in the concentrations of BUP and its metabolites. In a previous investigation, coadministration of delavirdine produced a much larger increase in BUP AUC, to a mean of 186±28 ng*h/mL, without producing sedation or other side effects (McCance-Katz et al., 2006 a). The ATV/r group had higher ATV concentrations than the ATV-only group (Fig. 3). Although sedation is not a typical side-effect of ATV, it is possible that there may be an idiosyncratic pharmacodynamic interaction between high BUP concentrations and high ATV concentrations that could explain our observations as well as those of the previous clinical report (Bruce and Altice, 2006).
BUP is principally metabolized by CYP 3A4 (Chang et al., 2006; Iribarne et al., 1997). Since ATV (Perloff et al., 2005) and RTV (King et al., 2004) are both in vitro inhibitors of CYP 3A4, it is likely that inhibition of metabolic clearance of BUP contributes to the higher BUP concentrations observed in both studies. However, if this were the only effect, one would expect to see increased concentrations of BUP and BUP-G, but decreased concentrations of the metabolites NBUP and NBUP-G, which result from dealkylation catalyzed by CYP 3A4.
ATV is also known to significantly inhibit bilirubin glucuronidation by UDP-glucuronosyltransferase 1A1 (UGT 1A1) (Bristol Myers Squibb, 2006; Zhang et al., 2005). BUP and N-BUP glucuronidation is mediated by UGT 1A1 (King et al., 1996), although apparently using a different active site than is used for bilirubin (Rios and Tephly, 2002). BUP and NBUP are also effectively glucuronidated by UGT 2B7 and 1A3, respectively (Y Chang and DE Moody, personal communication, Rios and Tephly, 2002). If glucuronidation of BUP and NBUP is inhibited by ATV, this would be expected to increase concentrations of BUP and NBUP while decreasing concentrations of BUP-G and NBUP-G.
If the only effects of ATV and ATV/r were inhibition of BUP metabolism by dealkylation and glucuronidation, one might expect so see increased exposure to BUP, and decreased exposure to all metabolites, with NBUP-G being most affected. In fact, increased levels of BUP and all of the metabolites were observed, suggesting that additional processes must be involved.
The most straightforward explanation for parallel increases in the AUC of a drug, as well as the AUC's of all of its metabolites, is an increase in the bioavailability of the drug. The short sublingual hold time and low overall bioavailability of ∼15−35 % for sublingual tablets (Chiang and Hawks, 2003) suggests that a substantial amount of the administered BUP may be swallowed. Under normal circumstances, BUP has poor gastrointestinal bioavailability.
RTV is well known for its ability to greatly increase bioavailability of other protease inhibitors by inhibition of both CYP3A4 and P-glycoprotein (King et al., 2004) and ATV also exhibits both inhibitory activities (Perloff et al., 2005). In the presence of ATV and/or RTV, the gastrointestinal bioavailability of swallowed BUP may go from poor to excellent, leading to a substantial increase in overall BUP bioavailability and plasma levels, with downstream increases in metabolite concentrations as well. An effect from increased bioavailability of BUP in the presence of ATV or ATV/r is a hypothesis that provides a plausible and parsimonious explanation for the simultaneous increases in buprenorphine and its metabolites. Inhibition of dealkylation and glucuronidation certainly contribute to the observed increases as well.
The increases in the AUC0−24 of BUP-G after PI treatment are larger than would be expected from a simple increase in bioavailability of BUP. The increase also differentiates the effect of ATV and ATV/r from the effect of RTV alone (McCance-Katz et al., 2006b). The disproportionate increase in BUP-G may reflect diversion of buprenorphine metabolism from dealkylation to glucuronidation via inhibition of CYP 3A4. Another possible explanation would be inhibition of secretion of glucuronides into the bile. The increases in plasma levels of direct bilirubin (primarily bilirubin mono- and diglucuronide) that were observed after initiation of ATV or ATV/r (Table 4) are consistent with this possibility. However, the failure to observe a parallel increase in NBUP-G relative to NBUP would require the inhibition of biliary glucuronide secretion to be highly selective.
ATV (Perloff et al., 2005) and RTV (King et al., 2004) may also act as inducers of CYP 3A4 and P-glycoprotein. Such induction could oppose their direct inhibitory effects. The 5-day administration period in this study would have allowed only partial development of inductive effects. It is possible that the increased concentrations of BUP and its metabolites might have been moderated with more prolonged administration of ATV or ATV/r.
It is also noteworthy that total bilirubin was significantly and reversibly increased with ATV and ATV/r administration, while direct (conjugated) bilirubin remained within normal limits. Increase in total bilirubin is a known adverse event associated with ATV and ATV/r treatment (Bristol Myers Squibb, 2006) and is probably related to ATV inhibition of UGT1A1 (Bristol Myers Squibb, 2006; Zhang et al., 2005) resulting in reduction of bilirubin conjugation. Increased total bilirubin was observed in both BUP-maintained and control study participants. This finding was not associated with icterus or other signs or symptoms of hepatic impairment, nor was any clinical intervention required. Furthermore, total bilirubin returned to within normal clinical limits within one week of termination of ATV or ATV/r administration (participants were tested 4−7 days following cessation of ATV or ATV/r administration). In clinical practice, all patients with HIV disease receiving ATV should be warned of the potential complication of hyperbilirubinemia.
Prolonged PR interval, including first degree atrioventricular (AV) block has been reported with ATV use and appears to be related to drug concentrations. Significant increases in cardiac PR interval were observed in BUP and control samples in the current study; although no cardiac adverse symptoms were observed in any participant. However, these findings indicate caution with ATV use in those with underlying cardiac disease, those receiving other medications known to alter cardiac electrical activity, and/or the concomitant use of medications that may alter (decrease) drug metabolism.
The findings for increased total bilirubin and cardiac PR interval appear to be associated with ATV administration, as increases were similar in BUP-maintained and control participants. This study did not demonstrate any additional increases associated with combined therapy with BUP and ATV or ATV/r.
In conclusion, the administration of ATV or ATV/r for treatment of HIV disease in opioid-dependent patients maintained on BUP/NLX is likely to produce a pharmacokinetic interaction characterized by increased buprenorphine and metabolite concentrations. Associated with these increases is some potential for increased sedation and, possibly, as has been reported by others (Bruce and Altice, 2006), impaired cognition. BUP/NLX treatment should not significantly alter ATV plasma concentrations when these medications are administered at standard clinical dosages. The interaction described in this report is the first drug interaction reported between BUP and HIV medications that has been associated with adverse events, albeit mild ones. Concomitant treatment with these medications warrants clinical monitoring and possibly dose reduction in BUP should unacceptable adverse events occur.
Acknowledgements
Sources of Support: This study was supported by NIDA/NIH grants: RO1 DA 13004 (EMK), KO2 DA00478 (EMK), RO1 DA 10100 (DEM), and the General Clinical Research Center at Virginia Commonwealth University (M01RR00065 NCRR/NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarnoutse RE, Verweij-van Wissen CP, van Ewijk-Beneken Kolmer EW, Wuis EW, Koopmans PP, Hekster YA, Burger DM. International interlaboratory quality control program for measurement of antiretroviral drugs in plasma. Antimicrob Agents and Chemother. 2002;46:884–886. doi: 10.1128/AAC.46.3.884-886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Friedland GH, Cooney EL. Nevirapine-induced opiate withdrawal among injection drug users with HIV infection receiving methadone. 1999;13:957–962. doi: 10.1097/00002030-199905280-00012. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol Myers Squibb Reyataz (atazanavir) Prescribing information. PDR. 2006. pp. 948–957.
- Bruce RD, Altice FL. Three case reports of a clinical pharmacokinetic interaction with buprenorphine and atazanavir plus ritonavir. AIDS. 2006;20:783–784. doi: 10.1097/01.aids.0000216384.22432.9a. [DOI] [PubMed] [Google Scholar]
- Celentano DD, Galai N, Sethi AK, Shah NG, Strathdee SA, Vlahov D, Gallant JE. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- Chang Y, Moody DE, McCance-Katz EF. Novel metabolites of buprenorphine detected in human liver microsomes and human urine. Drug Metab Dispos. 2006;34:440–448. doi: 10.1124/dmd.105.006148. [DOI] [PubMed] [Google Scholar]
- Chiang CN, Hawks RL. Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug Alcohol Depend. 2003;70(supp 2):S39–S47. doi: 10.1016/s0376-8716(03)00058-9. [DOI] [PubMed] [Google Scholar]
- Cohn JA. HIV-1 infection in injection drug users. Infect Dis Clin North Am. 2002;16:745–770. doi: 10.1016/s0891-5520(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Deany P. HIV and injecting drug use: A new challenge to sustainable human development. 2000 Available at: http://www.undp.org/hiv/publications/deany.htm. Accessed 30 May 2006.
- Des Jarlais DC, Hubbard R. Treatment for drug dependence. Proc Assoc Am Physicians. 1999;111:126–130. doi: 10.1046/j.1525-1381.1999.09248.x. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. (4th edition) 2000 doi: 10.1016/j.psychres.2011.06.006. American Psychiatric Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste JAH, Aarnoutse RE, Koopmans PP, Hekster YA, Burger DM. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. J Acquir Immune Defic Syndr. 2003;32:287–291. doi: 10.1097/00126334-200303010-00007. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Holland D, DiFrancesco R, Stone S, Hamzeh F, Connor JD, Morse GD. Quality assurance program for clinical measurement of antiretrovirals: AIDS clinical trials group proficiency testing program for pediatric and adult pharmacology laboratories. Antimicrob Agent Chemother. 2004;48:824–831. doi: 10.1128/AAC.48.3.824-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland DT, DiFrancesco R, Connor JD, Morse GD. Quality assurance program for pharmacokinetic assay of antiretrovirals: ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004: a requirement for therapeutic drug monitoring. Ther Drug Monit. 2006;28:367–374. doi: 10.1097/01.ftd.0000211817.58052.b8. [DOI] [PubMed] [Google Scholar]
- Huang W, Moody DE, McCance-Katz EF. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-eletrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28:245–251. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- Iribarne C, Picart D, Dreano Y, Bail JP, Berthou F. Involvement of cytochrome P450 3A4 in N-dealkylation of buprenorphine in human liver microsomes. Life Sci. 1997;60:1953–1964. [Google Scholar]
- Keil K, Frerichs VA, DiFrancesco R, Morse GD. Reverse phase high performance liquid chromatography method for the analysis of amprenavir, efavirenz, indinavir, lopinavir, nelfinavir and its active metabolite (M8), ritonavir, and saquinavir in heparinized human plasma. Ther Drug Monit. 2003;25:340–346. doi: 10.1097/00007691-200306000-00015. [DOI] [PubMed] [Google Scholar]
- Keil K, Hochreiter JS, DiFrancesco R, Zingman BS, Reichman RC, Fischl MA, Gripshover B, Morse GD. Integration of atazanavir into an existing liquid chromatography UV method for protease inhibitors: validation and application. Ther Drug Monit. 2006 doi: 10.1097/FTD.0b013e3180318ef3. (in press) [DOI] [PubMed] [Google Scholar]
- King CD, Green MD, Rios GR, Coffman BL, Owens IS, Bishop WP, Tephly TR. The glucuronidation of exogenous and endogenous compounds by stably expressed rat and human UDP-glucuronosyltransferase 1.1. Arch Biochem Biophys. 1996;332:92–100. doi: 10.1006/abbi.1996.0320. [DOI] [PubMed] [Google Scholar]
- King JR, Wynn H, Brundage R, Acosta EP. Pharmacokinetic enhancement of protease inhibitor therapy. Clin Pharmacokinet. 2004;43:291–310. doi: 10.2165/00003088-200443050-00003. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF. Office-based buprenorphine treatment for opioid-dependent patients. Harv Rev Psychiatry. 2004;12:321–338. doi: 10.1080/10673220490905688. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF. Treatment of opioid dependence and coinfection with HIV and hepatitis C virus in opioid-dependent patients: the importance of drug interactions between opioids and antiretroviral medications. Clin Infect Dis. 2005;41:S89–95. doi: 10.1086/429503. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Gourevitch MN, Arnsten J, Sarlo J, Rainey P, Jatlow P. Modified directly observed therapy for injection drug users with HIV disease. Am J Addict. 2002;11:271–278. doi: 10.1080/10550490290088072. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Moody DE, Morse G, Pade P, Friedland G, Baker J, Alvanzo A, Smith P, Abayomi O, Jatlow P, Rainey PM. Interactions between buprenorphine and antiretrovirals I: Non-nucleoside reverse transcriptase inhibitors efavirenz and delavirdine. Clin Infect Dis. 2006 a;43:S224–S234. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Moody DE, Morse G, Pade P, Friedland G, Baker J, Alvanzo A, Smith P, Jatlow P, Rainey PM. Interactions between buprenorphine and antiretrovirals II: Protease inhibitors, nelfinavir, lopinavir/ritonavir, or ritonavir. Clin Infect Dis. 2006 b;43:S235–S246. doi: 10.1086/508188. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey P, Friedland G, Jatlow P. The protease inhibitor lopinavir/ritonavir may produce opiate withdrawal in methadone-maintained patients. Clin Infect Dis. 2003;37:476–482. doi: 10.1086/376907. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Rainey P, Smith P, Morse G, Friedland G, Gourevitch M, Jatlow P. Drug interactions between opioid and antiretroviral medications: interaction between methadone, LAAM, and nelfinavir. Am J Addict. 2004;13:163–180. doi: 10.1080/10550490490436037. [DOI] [PubMed] [Google Scholar]
- Mehta S, Moore RD, Graham NM. Potential factors affecting adherence with HIV therapy. AIDS. 1997;11:1665–1670. doi: 10.1097/00002030-199714000-00002. [DOI] [PubMed] [Google Scholar]
- Perloff ES, Duan SX, Skolnik PR, Greeblatt DJ, von Moltke LL. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33:764–770. doi: 10.1124/dmd.104.002931. [DOI] [PubMed] [Google Scholar]
- Rios GR, Tephly TR. Inhibition and active sites of UDP-glucuronosyltransferases 2B7 and 1A1. Drug Metab. Dispos. 2002;30:1364–1367. doi: 10.1124/dmd.30.12.1364. [DOI] [PubMed] [Google Scholar]
- Rotger M, Taffe P, Bleiber G, Gunthard HF, Furrer H, Vernazza P, Drechsler H, Bernasconi E, Rickenbach M, Telenti A. Gilbert syndrome and the development of antiretroviral therapy-associated hyperbilirubinemia. J Infect Dis. 2005;192:1381–1386. doi: 10.1086/466531. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan HK, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;20(59 Suppl):22–33. [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependence: self-reports, urinalysis, and addiction severity index. J Clin Psychopharmacol. 1996;16:58–67. doi: 10.1097/00004714-199602000-00010. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PG, Yip B, O'Shaughnessy MV, Montaner JS, Schechter MT, Hogg RS. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- WinNonLin Professional, Version 5.0. Pharsight, Inc.; Mountain View, CA: 2005. [Google Scholar]
- Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33:1729–1739. doi: 10.1124/dmd.105.005447. [DOI] [PubMed] [Google Scholar]