Abstract
Background
Concurrent HPV testing and cervical cytology (co-testing) is an approved and promising alternative to cytology alone in women aged 30 and older. However, broad acceptance of co-testing is being hindered by a lack of evidence about its performance in routine clinical practice. We evaluated the safety of three-year screening intervals for women testing HPV-negative with normal cytology (Pap-negative) and assessed the ability of co-testing to identify women at high risk of CIN3+ or cervical cancer over five years.
Methods
We analyzed five-year cumulative incidence of cervical cancer and cervical intraepithelial neoplasia grade 3 or worse (CIN3+) for 331,818 women aged 30 and older who enrolled in co-testing at Kaiser Permanente Northern California starting 2003-2005 (and had adequate enrollment co-test results) and were followed through December 31, 2009.
Findings
Five-year cumulative incidence of cancer for all 315,061 HPV-negative women was extremely low (3.8 per 100,000 women per year), only slightly higher than for the 306,969 women who were both HPV-negative and Pap-negative (3.2 per 100,000 women per year), and half the cancer risk of all 319,177 women who were Pap-negative (7.5 per 100,000 women per year). Almost all (99.5%; 313,465) HPV-negative women had either normal cytology or minor abnormalities. Abnormal cytology greatly increased cumulative incidence of CIN3+ over five years for the 16,757 HPV-positive women (12% vs. 5.9%, p<0.0001). In contrast, although statistically significant, abnormal cytology did not increase 5-year CIN3+ risk for HPV-negative women to a substantial level (0.86% vs. 0.16%). 73% of HPV-positive women had no cytologic abnormality (12,208 women). HPV-positive women with no cytologic abnormality experienced 34% of the CIN3+, 29% of the cancers, and 63% of the adenocarcinomas.
Interpretation
For women aged 30 and older in routine clinical practice, a single negative HPV test sufficed to provide strong reassurance against cervical cancer over five years, demonstrating the safety of 3-year screening intervals for HPV-negative/Pap-negative women and suggesting that five-year intervals may also be safe. Concurrent HPV testing resulted in earlier identification of the women at high risk of cervical cancer, especially adenocarcinoma. HPV testing without adjunctive cytology may be sufficiently sensitive for primary cervical cancer screening.
Keywords: Human Papillomavirus (HPV), cancer prevention, cytology, cervical intraepithelial neoplasia (CIN), Hybrid Capture 2 (HC2)
Introduction
Overwhelming evidence from long-term prospective cohorts and randomized clinical trials demonstrates that HPV DNA testing is considerably more sensitive than cervical cytology for the detection of cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3) and cancer.1-9 Incorporation of HPV testing into cervical cancer screening programs could reduce cervical cancer incidence in women 30 and older4-5, 8, 10 (most markedly for adenocarcinoma, the precursors of which are often missed by cytologic methods11), and even cervical cancer mortality12. Cohorts and trials suggest that women’s risk of CIN3 or cancer (CIN3+) following a negative HPV test (HPV-negative) is very low for five years.3, 10, 13-14 This evidence was the basis for regulatory and clinical guideline approval15-16 of routine HPV testing in conjunction with cervical cytology (co-testing) for cervical cancer screening of women 30 and older. In particular, guidelines discourage screening of women testing HPV-negative with normal cytology (Pap-negative) before three years to avoid detection of new HPV infections. New HPV infections have extremely low cancer risk because they usually resolve themselves without requiring medical intervention.17
Although very promising and approved, co-testing has not been widely adopted in the US. In a recent survey, only 19% of US clinicians would recommend the 3-year screening interval for women testing HPV-negative with normal cytology18, suggesting concern about the cancer risk accrued over three years. Studies in routine clinical practice are needed to estimate the feasibility and safety of co-testing guidelines19. Although clinical trials and research cohorts in specially selected populations can show efficacy in specific, tightly-controlled, idealized, circumstances, the final proof of the value of medical interventions is their effectiveness in general clinical practice, with all its attendant complexity, such as non-standard protocols, potential non-adherence by clinicians and patients to protocols, or screening tests conducted in non-ideal circumstances. Furthermore, very large samples are required to determine actual cancer risks for each possible cytologic abnormality and HPV test result, especially for women testing negative by HPV and cytology for whom reassurance against cancer is the critical factor for deciding their screening interval. Finally, each successive screen, if effective20-21, should lower the subsequent population risk because those with previously evident, clinically relevant disease have already been identified and treated, and therefore no longer contribute to the overall risk. Therefore, extended follow-up of very large numbers of HPV-negative/Pap-negative women provides an opportunity to evaluate how much further their cancer risks decrease after their return in three years for their second visit, which has not been investigated previously to our knowledge.
In 2003, Kaiser Permanente Northern California (KPNC), a large health maintenance organization, adopted a cervical cancer-screening program based on co-testing, with extended screening intervals for women who test HPV-negative with normal cytology. The KPNC experience serves as a large-scale “demonstration project” of what could realistically be achieved in routine clinical practice, where providers receive no special training and do not require any special qualifications to participate and that no provider, provider group, patient or patient group is excluded. We determined cervical cancer risk for 331,818 women aged 30 and older who enrolled in co-testing at KPNC between 2003 and 2005. As of December 31, 2009, 1,476 women had diagnoses of CIN2, 747 CIN3, and 87 cervical cancers. We believe this is the first opportunity to determine CIN3+ and actual cervical cancer risks for each possible cytologic abnormality and HPV test result in a very large sample of a diverse US population undergoing co-testing. We also evaluated cervical cancer risk after the second screening visit in 195,975 women who were HPV-negative/Pap-negative at enrollment, to determine whether the second co-test provided additional reassurance against cancer. Our principal objectives were to determine the safety of three-year screening intervals for HPV-negative/Pap-negative women and the value of adding HPV testing to cytology screening to earlier identify women at high risk of CIN3+ or cervical cancer over 3-5 years.
Methods
Screening Tests
Conventional Pap tests were reported according to the 2001 Bethesda System22 (in order of increasing severity): no intraepithelial lesion or malignancy (NILM or Pap-negative or normal Pap test); atypical squamous-cells of undetermined significance (ASC-US); low-grade squamous intraepithelial lesion (LSIL); atypical glandular cells of undetermined significance or not otherwise specified (AGUS/NOS); atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H); high-grade squamous intraepithelial lesion (HSIL); or squamous-cell carcinoma (SCC). “Pap-positive” means ASC-US or more severe cytology. We grouped ASC-H, HSIL, and SCC into a single high-risk Pap category because each is individually uncommon and their CIN3+ risks were similarly increased. Conventional Pap slides were manually reviewed following processing by the BD FocalPoint Slide Profiler (BD Diagnostics, Burlington, NC, USA) primary screening and directed quality control system, in accordance with FDA-approved protocols.As an important methodologic point, we note that a meta-analysis23 and two large randomized clinical trials24-25 have failed to show any clinical performance advantage of liquid-based cytology over conventional Pap smears for detection of CIN3+. Hybrid Capture 2 (HC2; Qiagen, Germantown, MD, USA) was used to test for the pool of carcinogenic HPV types according to manufacturer’s instructions. HC2 is known to have high inter-operator reliability.26 All cytology and HPV testing was done at the Regional Laboratory of the Northern California Kaiser Permanente Medical Care Program by a stable staff of around 30 cytotechnicians, laboratory technicians, clinical scientists, and pathologists.
Case Definitions
Histologic findings were classified (in order of increasing severity) as no lesion found, CIN1, CIN2, CIN3, adenocarcinoma in situ (AIS), squamous cell cancer, or adenocarcinoma. The suffix ‘+’ means the indicated histology or more severe. CIN2 or CIN3 histology sufficed to refer a woman for treatment by a loop electrosurgical excision procedure (LEEP). All reported cervical cancers were verified by chart review by Dr. Kinney and Dr. J. Thomas Cox (University of California Santa Barbara). Abnormal biopsies were reviewed and signed out by a stable team of approximately 60 pathologists in the 12 Pathology departments of KPNC.
We primarily focused on CIN3+ or cancer rather than CIN2+ because CIN2 is unreliably determined by pathologists27-28, often regresses29-30, and may simply reflect uncertainty between acute HPV infection (CIN1) and CIN331. However, our findings did not appreciably change when we used CIN2+ as our endpoint (data not shown).
Diagnostic Procedure
KPNC has its own management guidelines for patients that are fairly consistent with the 2004 interim guidelines for HPV testing15 and 2006 ASCCP guidelines16, and KPNC clinicians generally adhere well to their guidelines. . Women with LSIL or more severe cytology, regardless of the HPV test result, were sent to colposcopy. Women with ASC-US who tested HPV-positive were sent to colposcopy while those who tested HPV-negative were asked to return for a one-year follow-up. Women who were HPV-negative/Pap-negative were asked to return for screening in three years. Through 2005, HPV-positive/Pap-negative women were generally monitored annually for cytologic evidence of disease. After 2005, women with consecutive HPV-positive results were offered colposcopy. We had access to information about past history of an abonormal Pap test or abnormal biopsy predating the co-testing era at KPNC, but we could not independently verify the completeness of the information.
KPNC uses a computerized patient follow up system which reviews lab results daily and sets alarm flags for appropriate follow intervals for each abnormal result. If, for example, a biopsy has not been recorded after an abnormal Pap test within a given time period, an alarm is sent to the personnel responsible for the screening system at the appropriate facility. If this condition is not reset by receipt of a biopsy within a given time period, escalating alarms follow to the practitioner, then the Chief of Obstetrics and Gynecology, and finally the Physician-in-Chief of the facility. If a woman loses her job, leaves northern California, or changes her health insurance from KPNC, her future records are unknown to KPNC.
Population
KPNC membership is demographically similar to the US Census–enumerated population in the Bay Area Metropolitan Statistical Area, except for lacking representation of extremes in income.32-33. In a study of the KPNC population including the women in this study, of the 48.8% of women who self-reported their race/ethnicity in KPNC, 62.1% were Caucasian, 12.4% were Asian/Pacific Islander, 12.2% were Hispanic, and 8.4% were African-American.34 The KPNC population is considered a well-screened population, and their risk of cervical cancer has historically been lower than the national average35 (most cancer cases in the US are in areas where screening services are unavailable36). Over 90% of eligible women enrolled in co-testing.34
Statistical Analysis
Using SAS version 9.037, we estimated the cumulative incidence of the outcomes CIN2+, CIN3+, or cervical cancer for each possible combination of HPV test and Pap smear result. We estimated cumulative incidence from enrollment for all women, and after the first return visit for women who co-tested HPV-negative/Pap-negative at enrollment. We defined cumulative incidence to include prevalence at enrollment (plotted at time zero on each figure) and the incidence after enrollment. At “screening visits” only HPV test and Pap smear samples were collected. At “biopsy visits”, colposcopically directed biopsies were taken. The prevalence at enrollment was defined as the ratio of the number of women diagnosed with each outcome on the biopsy visit immediately succeeding their enrollment screening visit to the total number of enrolled women. To estimate post-enrollment incidence, we used Weibull survival models38 which make smoother and more accurate estimates than non-parametric methods analogous to Kaplan-Meier39. Separate Weibull models were fit for each co-test result. We also used Weibull regression models to examine whether age and prior history of abnormal Pap smears or CIN2+ biopsies affect cumulative incidence from enrollment and, among women who tested HPV-negative/Pap-negative at enrollment, from the second screening visit. Supplemental Appendix 1 gives additional detail and justification of statistical methods.
Role of the funding source
The funding sources did not review or approve the study design and were not involved in data collection, analysis, interpretation, or in writing the paper. The Intramural Research Program of the US National Institutes of Health/National Cancer Institute reviewed the final manuscript for publication. HAK and PEC had access to the raw data and had responsibility to submit the manuscript for publication. The KPNC institutional review board (IRB) approved use of the data, and the National Institutes of Health Office of Human Subjects Research deemed this study exempt from IRB review.
Results
Distribution of enrollment co-test results and sensitivity
Table 1 shows raw data for the entire 2003-2009 follow-up. At enrollment, testing HPV positive was slightly more common than having abnormal cytology (ASC-US+) (5.1% vs. 3.8%, p<0.0001) and HPV testing had slightly less specificity than cytology (95.5% vs. 96.5%, p<0.0001). However, far higher percentages of disease outcomes (sensitivities) were found in the enrollment HPV-positive women than in Pap-positive women: CIN2 (78% vs. 53%, p<0.0001), CIN3/AIS (84% vs. 53%, p<0.0001), AIS (80% vs. 40%, p<0.0001), total cancers (69% vs. 51%, p=0.02), and adenocarcinoma (78% vs. 15%, p<0.0001). Comparing discordant co-test results (HPV-negative/Pap-positive vs. HPV-positive/Pap-negative) provides information about the relative importance of HPV testing vs. cytology. Far higher percentages of disease outcomes were found in HPV-positive/Pap-negative women than HPV-negative/Pap-positive women: CIN2 (29% vs. 4.3%, p<0.0001), CIN3/AIS (35% vs. 3.6%, p<0.0001), AIS (44% vs. 4%, p<0.0001), total cancers (29% vs. 10%, p=0.004), and especially adenocarcinoma (63% vs. 0%, p<0.0001).
Table 1.
Distribution of worst histologic diagnosis in 331,818 women over 2003-2009 by enrollment HPV test and Pap smear.
| Histology diagnosis | no biopsy or <CIN2 | CIN2 | CIN3/AIS | AIS | Squamous Carcinoma |
Adeno- Carcinoma |
Total Cancers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | |
| Total | 331818 | 100.00% | 329508 | 100.00% | 1476 | 100% | 747 | 100% | 70 | 100% | 49 | 100% | 27 | 100% | 87 | 100% |
| Baseline HPV | ||||||||||||||||
| Negative | 315061 | 94.95% | 314589 | 95.47% | 322 | 22% | 123 | 16% | 14 | 20% | 18 | 37% | 6 | 22% | 27 | 31% |
| Positive | 16757 | 5.05% | 14919 | 4.53% | 1154 | 78% | 624 | 84% | 56 | 80% | 31 | 63% | 21 | 78% | 60 | 69% |
| Baseline Pap | ||||||||||||||||
| Pap− | 319177 | 96.19% | 318093 | 96.54% | 687 | 47% | 354 | 47% | 42 | 60% | 15 | 31% | 23 | 85% | 43 | 49% |
| Total Pap+ | 12641 | 3.81% | 11415 | 3.46% | 789 | 53% | 393 | 53% | 28 | 40% | 34 | 69% | 4 | 15% | 44 | 51% |
| ASCUS | 8517 | 2.57% | 8106 | 2.46% | 283 | 19% | 123 | 16% | 12 | 17% | 4 | 8% | 1 | 4% | 5 | 6% |
| LSIL | 2527 | 0.76% | 2208 | 0.67% | 253 | 17% | 61 | 8% | 1 | 1% | 4 | 8% | 0 | 0% | 5 | 6% |
| AGUS, NOS | 764 | 0.23% | 705 | 0.21% | 26 | 2% | 27 | 4% | 7 | 10% | 1 | 2% | 2 | 7% | 6 | 7% |
| ASC-H, HSIL+ | 833 | 0.25% | 396 | 0.12% | 227 | 15% | 182 | 24% | 8 | 11% | 25 | 51% | 1 | 4% | 28 | 32% |
| Baseline HPV/Pap | ||||||||||||||||
| HPV−/Pap− | 306969 | 92.51% | 306597 | 93.05% | 258 | 17% | 96 | 13% | 11 | 16% | 10 | 20% | 6 | 22% | 18 | 21% |
| HPV−/Pap+ | 8092 | 2.44% | 7992 | 2.43% | 64 | 4% | 27 | 4% | 3 | 4% | 8 | 16% | 0 | 0% | 9 | 10% |
| HPV+/Pap− | 12208 | 3.68% | 11496 | 3.49% | 429 | 29% | 258 | 35% | 31 | 44% | 5 | 10% | 17 | 63% | 25 | 29% |
| HPV+/Pap+ | 4549 | 1.37% | 3423 | 1.04% | 725 | 49% | 366 | 49% | 25 | 36% | 26 | 53% | 4 | 15% | 35 | 40% |
| Baseline HPV/ASC-US | ||||||||||||||||
| HPV-/ASC-US | 6496 | 1.96% | 6455 | 1.96% | 25 | 2% | 14 | 2% | 1 | 1% | 2 | 4% | 0 | 0% | 2 | 2% |
| HPV+/ASC-US | 2021 | 0.61% | 1651 | 0.50% | 258 | 17% | 109 | 15% | 11 | 16% | 2 | 4% | 1 | 4% | 3 | 3% |
Abbreviations: Cervical Intraepthelial Neoplasia Grade 2 (CIN2), Cervical Intraepthelial Neoplasia Grade 3 or adenocarcinoma in situ (CIN3/AIS). Total Cancers includes squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma, and cervical cancer of unknown histology. CIN3/AIS includes 13 histologies that were either CIN3 or AIS, but precisely which is unknown. Conventional Pap tests were reported according to the 2001 Bethesda System17 (in order of increasing severity): no intraepithelial lesion or malignancy (NILM or Pap-negative or normal Pap test); atypical squamous-cells of undetermined significance (ASC-US); low-grade squamous intraepithelial lesion (LSIL); atypical glandular cells of undetermined significance or not otherwise specified (AGUS/NOS); atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H); high-grade squamous intraepithelial lesion (HSIL); or squamous-cell carcinoma (SCC). “Pap-positive” means ASC-US or more severe cytology. We grouped ASC-H, HSIL, and SCC into a single high-risk Pap category because each is individually uncommon and their CIN3+ risks were similarly increasedb.
Enrollment co-tests and risks of CIN3+ and cancer
Figure 1A and Supplemental Table 1 show that prevalent CIN3+ risks at enrollment were similar in HPV-positive and Pap-positive women (2.1% vs. 2.7%). However, enrollment HPV testing distinguished future risk of CIN3+ and cancer more clearly than enrollment cytology. CIN3+ risk was less in all HPV-negative women than in all Pap-negative women, not only for 3 years (0.063% vs. 0.17%, p=0.001), but also for 5 years (0.17% vs. 0.36%, p=0.02). HPV-negative women also experienced half the 5-year risk of invasive cervical cancer of Pap-negative women (3.8 vs. 7.5/100,000/year; p=0.3). Conversely, CIN3+ risk was greater in HPV-positive women than Pap-positive women, not only for 3 years (5.0% vs. 3.8%, p=0.046), but also for 5 years (7.6% vs. 4.7%, p=0.001)).
Figure 1. Value of HPV testing versus Pap smears (Panel A) and Value added by Pap smears to HPV testing (Panel B).
NOTE: Data are shown on cumulative incidence of CIN3+ by enrollment HPV test versus Pap smear. “Pap−“ means Pap-negative (NILM). “Pap+” means any abnormality (non-NILM). In Panel A, HPV test results are in blue, Pap smear test results are in black, positive test results are solid lines, and negative test results are dashed lines. Prevalent CIN3+ is plotted at time 0 (enrollment) and incident CIN3+ is plotted from that point. The HPV test more clearly separated high-risk women from low-risk women than the Pap smear because both (1) HPV+ women at enrollment had higher CIN3+ risk than the Pap+ women after 3 years (5.0% vs. 3.8%, p=0.046) and 5-years (7.6% vs. 4.7%, p=0.001), and (2) HPV− women at enrollment had lower CIN3+ risk than Pap− women at enrollment after 3-years (0.063% vs. 0.17%, p=0.001) and after 5-years (0.17% vs. 0.36%, p=0.02). In Panel B, HPV+ is in blue, HPV− is in black, Pap+ is solid and Pap− is dashed. Pap+ strongly modified risks for the HPV+ at three-years (10.0% vs. 3.1%, p<0.001) and five-years (12.1% vs. 5.9%, p<0.001), but not for the HPV− either at three-years (0.52% vs. 0.047%, p<0.001) or five-years (0.86% vs. 0.16%, p<0.001), although risks are statistically distinguishable. Comparing to Panel A, also being Pap-did not reduce CIN3+ risk from just being HPV− either at three-years (0.047% vs. 0.063%, p=0.6) or five-years (0.16% vs. 0.17%, p=0.8). See supplemental table 1 for 95% confidence intervals for all risk estimates. The number of women in each group are: HPV-positive: 16,757; HPV-negative: 315,061; Pap-positive: 12,641; Pap-negative: 319,177; HPV-positive/Pap-positive: 4,549; HPV-positive/Pap-negative: 12,208; HPV-negative/Pap-positive: 8,092; HPV-negative/Pap-negative: 306,969.
Figure 1B (and Supplemental Table 1) shows that Pap smears further distinguished risk of CIN3 and cancer among HPV-positive women, but not HPV-negative women. Abnormal cytology substantially increased the CIN3+ risk for all HPV-positive women not only for 3 years (10% vs. 3.1%, p<0.0001) but also for 5 years (12% vs. 5.9%, p<0.0001). In contrast, although statistically significant, abnormal cytology did not increase CIN3+ risk for HPV-negative women to a substantial level, neither for 3 years (0.52% vs. 0.047%) nor for 5 years (0.86% vs. 0.16%). Furthermore, normal cytology did not further reduce the already low cancer risk of HPV-negative women (3.8 and 3.2 per 100,000 women per year, p=0.8).
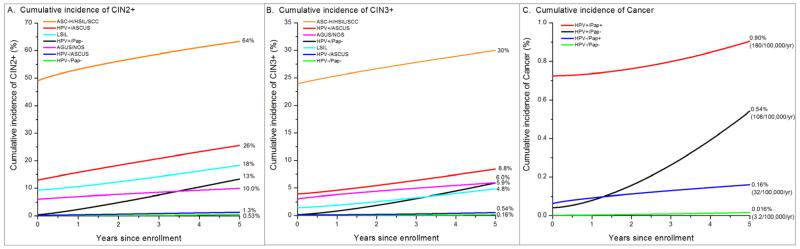
Figure 2 and Supplemental Table 1 combine finely categorized enrollment co-test results to estimate risk of CIN2+, CIN3+, and cancer. The risks conferred by co-testing results clustered into high, low, and medium risk categories. By far the highest 3-year risks of CIN2+ (59%), CIN3+ (28%), and cancer (4.1%) were in the small group (0.25%) of women with ASC-H, HSIL or SCC Pap smears, 81% of whom were HPV-positive. Conversely, the 94.5% of women with HPV-negative/ASC-US or HPV-negative/Pap-negative co-tests had by far the lowest 5-year risks of CIN2+, CIN3+, and cancer. The remaining 5.25% of co-test results had intermediate risk: LSIL or AGUS/NOS (regardless of HPV results), HPV-positive/Pap-negative, HPV-positive/ASC-US. HPV-positive/Pap-negative women, in spite of having by far the lowest prevalent disease risks at enrollment of any HPV-positive group, accrued 5-year risk of CIN2+, CIN3+, and cancer post-enrollment as quickly as women with ASC-H/HSIL/SCC (Figure 2).
Figure 2. Five-year cumulative risks of CIN2+ (Panel A), CIN3+ (Panel B), and Cervical Cancer (Panel C) by enrollment HPV test and finely-categorized enrollment Pap smears.
NOTE: Prevalent disease is plotted at time 0 (enrollment) and incident disease is plotted from that point. Although only 0.25% of women, women with ASC-H/HSIL/SCC Pap smears had by far the highest 3-year risks of CIN2+ (59%), CIN3+ (28%), and cancer (4.1%). Versus women with LSIL, women with HPV+/ASC-US had higher 3-year risk of CIN2+ (21% vs. 14%, p=0.01) and CIN3+ (6.4% vs. 3.2%, p=0.03). The majority (73%) of enrollment HPV+ women were HPV+/Pap−, and although they had by far the lowest disease risks at enrollment of any HPV+ group (CIN2+: 0.36%, CIN3+: 0.16%, Cancer: 0.04%), they accrued 5-year risks post-enrollment (CIN2+: 13%, CIN3+: 5.9%, Cancer: 0.5%) akin to women with ASC-H/HSIL/SCC (CIN2+: 14%, CIN3+: 6.1%, Cancer: 1.7%) or with HPV+/ASC-US (CIN2+: 13%, CIN3+: 4.5%, Cancer: 0%) (p=0.8 vs. ASC-H/HSIL/SCC, p=0.7 vs. HPV+/ASC-US). HPV+/Pap− women had higher 5-year cancer risk than HPV−/Pap+ women (0.54% vs. 0.16%, p=0.2). HPV−/ASC-US and HPV−/Pap− women had the smallest, and nearly indistinguishable, 5-year risks of CIN2+ (1.3% vs. 0.54%, p=0.07) and CIN3+ (0.54% vs. 0.16%, p=0.08). The 5-year cervical cancer risk in the enrollment HPV−/Pap− was 0.016% (3.2 per 100,000 women per year), only slightly smaller than the 0.019% (3.8/100,000/year) risk in the enrollment HPV− overall (p=0.8). By comparison, the 5-year cervical cancer risk in the Pap− was 0.037% (7.5/100,000/year) (p=0.3 vs. HPV− cancer risk). On the per 100,000 women per year scale, the 5-year cancer risks are: HPV−/Pap−: 3.2; HPV−/Pap+: 32; HPV+/Pap−: 108; HPV+/Pap+: 180. See supplemental table 1 for 95% confidence intervals for all risk estimates. The number of women in each group are: ASC-H/HSIL/SCC: 833; HPV+/ASC-US: 2,021; AGUS/NOS: 764; HPV-positive/Pap-negative: 12,208; LSIL: 2,527; HPV-negative/ASC-US: 6,496; HPV-negative/Pap-negative: 306,969.
Supplemental Table 2 shows that some CIN3+ risks were strongly modified by previous history of abnormalities and by age. Most notably, the already low CIN3+ risk in enrollment HPV-negative/Pap-negative women was further reduced by 80% for women with no history of a previous abnormal Pap smear (p=0.0002) and by 78% for women aged 50 and older compared to women aged 30-34 (p <0.0001).
Risks of CIN3+ and cancer at return co-test among women HPV-negative/Pap-negative at enrollment
Cervical cancer screening is a repetitive process, in which normal women are re-screened after a specified interval. Table 2, Figure 3, and Supplemental Table 3 show raw data and disease risks for the 195,975 women who were HPV-negative/Pap-negative at enrollment and returned for a second co-test (median of 2.9 years to return). The percentage of testing HPV positive at the second screen was half the percentage at enrollment (2.8% vs. 5.1%, p<0.0001, Table 1). The increase in the fraction Pap-positive (4.3% vs. 3.8%, p<0.0001) was almost entirely due to an increase in HPV-negative/ASC-US (2.8% vs. 2.0%, p<0.0001), the lowest-risk Pap-positive co-test. Most importantly, the distribution of the second co-test results was down-staged in severity from the distribution of enrollment co-tests: the total fraction HPV-negative/Pap-negative or HPV-negative/ASC-US increased (96.8% vs. 94.5%, p<0.0001), the fraction HPV-positive/Pap-negative was halved (1.7% vs. 3.7%, p<0.0001), and the fraction HPV-positive/ASC-US, LSIL, AGUS/NOS, or ASC-H/HSIL/SCC decreased (1.5% vs.1.9%, p<0.0001).
Table 2.
Distribution of worst histologic diagnosis in 195,975 women since their second HPV test and Pap smear. These women were HPV negative with normal cytology at enrollment and returned for a second screening visit.
| Histology diagnosis | no biopsy or <CIN2 | CIN2 | CIN3/AIS | AIS | Squamous Carcinoma |
Adeno- Carcinoma |
Total Cancers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | % | # | % | n | % | n | % | # | % | n | % | n | % | n | % | |
| Total | 195975 | 100.00% | 195629 | 100.00% | 244 | 100% | 89 | 100% | 10 | 100% | 7 | 100% | 5 | 100% | 13 | 100% |
| 2nd visit HPV | ||||||||||||||||
| Negative | 190594 | 97.25% | 190523 | 97.39% | 49 | 20% | 16 | 18% | 4 | 40% | 2 | 29% | 3 | 60% | 6 | 46% |
| Positive | 5381 | 2.75% | 5106 | 2.61% | 195 | 80% | 73 | 82% | 6 | 60% | 5 | 71% | 2 | 40% | 7 | 54% |
| 2nd visit Pap | ||||||||||||||||
| Pap− | 187522 | 95.69% | 187423 | 95.81% | 67 | 27% | 27 | 30% | 7 | 70% | 1 | 14% | 3 | 60% | 5 | 38% |
| Total Pap+ | 8453 | 4.31% | 8206 | 4.19% | 177 | 73% | 62 | 70% | 3 | 30% | 6 | 86% | 2 | 40% | 8 | 62% |
| ASCUS | 6698 | 3.42% | 6586 | 3.37% | 92 | 38% | 19 | 21% | 1 | 10% | 1 | 14% | 0 | 0% | 1 | 8% |
| LSIL | 933 | 0.48% | 864 | 0.44% | 55 | 23% | 13 | 15% | 0 | 0% | 1 | 14% | 0 | 0% | 1 | 8% |
| AGUS, NOS | 531 | 0.27% | 517 | 0.26% | 4 | 2% | 7 | 8% | 1 | 10% | 1 | 14% | 2 | 40% | 3 | 23% |
| ASC-H, HSIL+ | 291 | 0.15% | 239 | 0.12% | 26 | 11% | 23 | 26% | 1 | 10% | 3 | 43% | 0 | 0% | 3 | 23% |
| 2nd visit HPV/Pap | ||||||||||||||||
| HPV−/Pap− | 184197 | 93.99% | 184157 | 94.14% | 24 | 10% | 12 | 13% | 4 | 40% | 1 | 14% | 2 | 40% | 4 | 31% |
| HPV−/Pap+ | 6397 | 3.26% | 6366 | 3.25% | 25 | 10% | 4 | 4% | 0 | 0% | 1 | 14% | 1 | 20% | 2 | 15% |
| HPV+/Pap− | 3325 | 1.70% | 3266 | 1.67% | 43 | 18% | 15 | 17% | 3 | 30% | 0 | 0% | 1 | 20% | 1 | 8% |
| HPV+/Pap+ | 2056 | 1.05% | 1840 | 0.94% | 152 | 62% | 58 | 65% | 3 | 30% | 5 | 71% | 1 | 20% | 6 | 46% |
| 2nd visit HPV/ASCUS | ||||||||||||||||
| HPV−/ASCUS | 5428 | 2.77% | 5416 | 2.77% | 10 | 4% | 1 | 1% | 0 | 0% | 1 | 14% | 0 | 0% | 1 | 8% |
| HPV+/ASCUS | 1270 | 0.65% | 1170 | 0.60% | 82 | 34% | 18 | 20% | 1 | 10% | 0 | 0% | 0 | 0% | 0 | 0% |
Abbreviations: Cervical Intraepthelial Neoplasia Grade 2 (CIN2), Cervical Intraepthelial Neoplasia Grade 3 or adenocarcinoma in situ (CIN3/AIS). Total Cancers includes squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma, and cervical cancer of unknown histology. CIN3/AIS includes 1 histology that was either CIN3 or AIS, but precisely which is unknown. Conventional Pap tests were reported according to the 2001 Bethesda System (in order of increasing severity): no intraepithelial lesion or malignancy (NILM or Pap-negative or normal Pap test); atypical squamous-cells of undetermined significance (ASC-US); low-grade squamous intraepithelial lesion (LSIL); atypical glandular cells of undetermined significance or not otherwise specified (AGUS/NOS); atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H); high-grade squamous intraepithelial lesion (HSIL); or squamous-cell carcinoma (SCC). “Pap-positive” means ASC-US or more severe cytology. We grouped ASC-H, HSIL, and SCC into a single high-risk Pap category because each is individually uncommon and their CIN3+ risks were similarly increased.
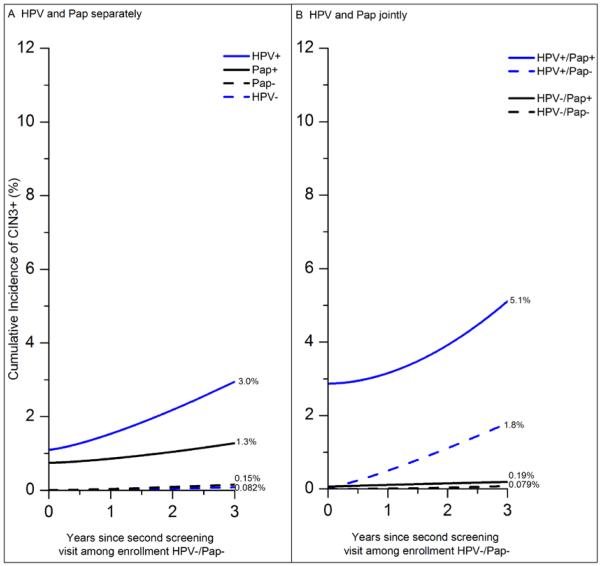
Figure 3. Cumulative incidence of CIN3+ after second visit among 195,975 women with an enrollment HPV-negative/Pap-negative co-test, by second HPV test and second Pap test separately (Panel A) and jointly (Panel B).
NOTE: Data are shown on cumulative incidence of CIN3+ based on HPV test and Pap smear at the second screen, among women HPV−/Pap− at enrollment, for HPV test and Pap smear separately (Panel A), and jointly (Panel B). “Pap−“ means Pap-negative (NILM). “Pap+” means any abnormality (non-NILM). Prevalent CIN3+ is plotted at time 0 (second visit) and incident CIN3+ is plotted from that point. The y-axis goes to 12%, the maximum risk observed based on enrollment co-tests (Figure 1), to emphasize that CIN3+ risks for women HPV+ or Pap+ after enrollment HPV−/Pap− are markedly decreased from women HPV+ or Pap+ at enrollment. In Panel A, (1) women HPV+ at their return visit had higher 3-year CIN3+ risk than women Pap+ at their return visit (3.0% vs. 1.3%, p=0.2) and (2) women HPV− at their return visit had half the 3-year CIN3+ risk of women Pap− at their return visit (0.082% vs. 0.15%, p=0.3). Neither is statistically significant since there were only 102 CIN3+ at or after the second screening visit among women HPV−/Pap− at enrollment. However, comparing to enrollment co-test CIN3+ risks in Figure 1, the CIN3+ risks for women HPV+ or Pap+ have decreased markedly. In particular, the second HPV test (or Pap smear) does not separate 3-year CIN3+ risks as clearly as at enrollment (HPV+: 5.0% vs. 3.0%, p= 0.09; Pap+: 3.8% vs. 1.3%, p=0.04). Furthermore, comparing to Figure 1, the second HPV− test did not reduce 3-year CIN3+ risk accrued by the first HPV− result (0.082% vs. 0.063%, p=0.6); similarly, the second Pap− did not reduce the 3-year CIN3+ risk accrued by the first Pap− result (0.15% vs. 0.17%, p=0.8). In Panel B, being Pap+ modified risks more for the HPV+ (5.1% vs. 1.8%, p=0.4) than for the HPV− (0.19% vs. 0.079%, p=0.4), although neither is statistically significant. Comparing to figure 2, the 3-year risks were greater for HPV+/Pap+, HPV+/Pap−, HPV−/Pap+ at enrollment than at the second co-test (10.0% vs 5.1%, p=0.3; 3.1% vs. 1.8%, p=0.3; 0.52% vs. 0.19%, p=0.4), although none were statistically significant. However, comparing to Figure 2, risks were similar for HPV−/Pap− women at enrollment vs. women HPV−/Pap− again at their return visit (0.047% vs. 0.079%, p=0.5). Finally, being Pap− did not reduce 3-year CIN3+ risk from just being HPV− at second co-test after enrollment HPV−/Pap− (0.079% vs. 0.082%, p=0.9). See supplemental table 3 for 95% confidence intervals for all risk estimates. The number of women in each group are: HPV-positive: 5,381; HPV-negative: 190,594; Pap-positive: 8,453; Pap− negative: 187,522; HPV-positive/Pap-positive: 2,056; HPV-positive/Pap-negative: 3,325; HPV-negative/Pap-positive: 6,397; HPV-negative/Pap-negative: 184,197.
Moreover, the risks of CIN3+ in the years after positive screening tests at the second visit were lower than following positive screening tests at enrollment (Figure 3 and Figure 1). Women who were HPV-positive at the second co-test after enrollment HPV-negative/Pap-negative had notably reduced 3-year CIN3+ risk than women who were HPV-positive at enrollment (3.0% vs. 5.0%, p=0.09); a similar pattern was observed for Pap-positive women (1.3% vs. 3.8%, p=0.04). The 3-year risks were also markedly lower for HPV-positive/Pap-positive, HPV-positive/Pap-negative, and HPV-negative/Pap-positive co-tests at second co-test after enrollment HPV-negative/Pap-negative than the respective risk of the same screening combinations at enrollment (5.1% vs. 10.1%, p=0.3; 1.8% vs. 3.1%, p=0.3; 0.19% vs. 0.52%, p=0.4).
However, at the return visit after an enrollment negative cotest (Figure 3b vs. Figure 1b), 3-year CIN3+ risks were not lower for women testing HPV-negative again (0.082% vs. 0.063%, p=0.6), Pap-negative again (0.15% vs. 0.17%, p=0.8), or HPV-negative/Pap-negative again (0.079% vs. 0.047%, p=0.5). The risk of cancer in the subsequent three years was also no lower among women who re-tested HPV-negative/Pap-negative (2.7 vs. 3.0 per 100,000 women per year, p=0.9) (Supplemental Table 3).
Discussion
The KPNC co-testing program provided us with an unparalleled opportunity to evaluate the real-world clinical effectiveness of concurrent HPV testing with cytology in a large and diverse U.S. screening population followed for several years. Women testing HPV-negative had extremely low risk of CIN3+ or cancer over five years, regardless of whether they had normal cytology or minor abnormalities. Although HPV-positive women with cytologic abnormalities had the highest risks, HPV-positive women with normal cytology accrued substantial risk of CIN3+ and cancer over 5 years. Co-testing was less able to identify women at high risk at their second visit in approximately 3 years after an HPV-negative/Pap-negative enrollment test. Their co-test results were down-staged to safer co-tests and the CIN3+ risk for each possible second co-test result was generally diminished from the risk associated with that co-test result at enrollment.
Enrollment HPV-negative/Pap-negative women had an extremely low cervical cancer risk of 3.2/100,000 women/year over five years. This risk is comparable to the risk of vulvar cancer in northern California in women aged 30 and older (3.1/100,000 women/year; SEER 2003-2007), another potentially preventable cancer that is too rare to justify organized efforts at prevention. This finding supports lengthening the screening interval for HPV-negative/Pap-negative women to five years, as has already been done in many European countries40. The 80% lower risks of CIN3+ among HPV-negative/Pap-negative women aged 50 or older (vs. aged 30-34), or for HPV-negative/Pap-negative women with no history of previous abnormal cytology, raise the possibility that some subgroups of HPV-negative/Pap-negative women could be safely screened at an even longer interval.
A single HPV-negative test sufficed to reassure a woman of extremely low risk of CIN3+ or cancer for five years. We found that negative cytology provided no extra reassurance against cancer beyond that conferred by an HPV-negative test result. The practically equal 5-year risks of CIN3+ or cancer for HPV-negative/Pap-negative women and HPV-negative/ASC-US women provides powerful evidence that women with HPV-negative/ASC-US could be safely screened in routine clinical practice at the same extended interval as HPV-negative/Pap-negative women.41-43 These findings are consonant with the biological fact that carcinogenic HPV is necessary to cause almost every cervical cancer. Finally, the low yields of LSIL or worse Pap smear (0.5%) and ASC-H/HSIL/SCC Pap smears (0.05%) in HPV-negative women may not be sufficient to justify Pap smear tests for HPV-negative women. Our findings strongly suggest that primary HPV testing, with HPV-positive tests triaged by cytology (or other tests with high specificity), a strategy which might preserve nearly all the safety of co-testing while reducing the number of Pap tests by 95% in our population, could be more efficient than co-testing (as has been suggested by others13, 40,5, 44).
Although cytologic abnormalities indicated prevalent disease, HPV testing predicted future disease much better than cytology. For example, HPV-positive/Pap-negative women, the majority of HPV-positive women (73%), had low prevalent disease risk at enrollment (in part because these women were rarely sent for immediate colposcopy), yet accrued considerable incident disease risks over five years that were comparable to women with ASC-H/HSIL/SCC cytology. Importantly, 17 of the 27 adenocarcinomas were found in HPV-positive/Pap-negative women. Follow-up of HPV-positive/Pap-negative women must strike the difficult balance of being stringent yet avoiding excessive early intervention45-46. Thus, it is imperative to develop new biomarkers (such as HPV genotyping43, 47 } or p16INK4a/Ki-67 immunocytochemistry48) to better triage HPV-positive/Pap-negative women into finer risk categories49, especially risk of adenocarcinoma, whose incidence is on the rise in the U.S.50
We expected that the ability of co-testing to separate high-risk women from low-risk women would diminish at the second co-test after an enrollment HPV-negative/Pap-negative co-test because there are always fewer high-risk women with prevalent CIN3+ at the second and subsequent screens. Therefore, women HPV-positive at the second co-test approximately three years after an enrollment HPV-negative/Pap-negative had lower CIN3+ and cancer risks than women testing HPV-positive at enrollment. In contrast, a second consecutive HPV-negative/Pap-negative test (at a median of 2.9 years after the first) appeared to offer no detectable additional reassurance against CIN3 or cervical cancer than the first HPV-negative/Pap-negative co-test. These findings suggest the futility of the common practice of yearly HPV testing for HPV-negative women “just to be sure”18 and support guidelines15-16 that HPV-negative/Pap-negative women should not be re-screened before at least 3 years.
Although KPNC practices are not typical in many ways, because KPNC serves a large and diverse population using providers without special qualifications or special training to participate, we believe that the KPNC experience serves as a large-scale “demonstration project” of what could realistically be achieved in real-life clinical practice. Consequently, non-compliance by clinicians or patients to protocols, imperfect diagnostic testing, and other imperfections are real-life complexities that should be appropriately reflected in our risk estimates. The value of our study is to reassure women, clinicians, and screening guidelines committees that the benefits of HPV testing seen in clinical trials and research cohorts, which might not represent routine clinical practice in a general population, are indeed observed in general practice with all its attendant complexities.
However, the KPNC experience may be difficult to apply to substantially different screening programs. For example, most women at KPNC with abnormal cytology were referred for colposcopy at enrollment, but 73% of HPV-positive women had normal cytology and few of them were referred for colposcopy at enrollment. Thus, an alternate screening program that sends more HPV-positive/Pap-negative women to colposcopy would likely observe greater CIN3+ risk at enrollment than we did in KPNC. For another example, although we observed low 5-year cancer rates in HPV-negative/Pap-negative women, a program that actually implements a 5-year screening interval for these women would observe a slightly higher cancer rate than that observed in KPNC because the second co-test at 3 years, while having little effect, still excised the few CIN2 or CIN3 lesions that could have progressed to cancer in two years. For a final example, the cancer risk for all HPV-negative women in a primary HPV testing program where all HPV-negative women have extended screening intervals should be somewhat higher than the cancer risk in all HPV-negative women in KPNC, because KPNC referred the 0.5% of HPV-negative women with LSIL or worse abnormalities for colposcopy where excision of CIN2 or CIN3 lesions may have prevented a few future cancers.
In summary, our findings demonstrate that adding HPV testing to cytology screening promoted earlier identification of the women at high risk of cervical cancer (especially adenocarcinoma) and allowed safe 3-year screening intervals for HPV-negative/Pap-negative women that reduced the burden of screening on patients and clinicians. Furthermore, our findings suggest that 5-year screening intervals for HPV-negative/Pap-negative women may be safe and that HPV testing without adjunctive cytology may be sufficiently sensitive for primary cervical cancer screening. The results of co-testing in 330,000 women over five years at KPNC definitively demonstrates that concurrent HPV testing and cytology can be feasibly implemented in routine clinical practice to provide powerful prevention of cervical cancer.
Supplementary Material
Research in Context.
Systematic Review
Overwhelming evidence from long-term prospective cohorts and randomized clinical trials demonstrates that incorporation of HPV testing into cervical cancer screening programs could reduce cervical cancer incidence in women 30 and older4-5, 8, 10 and even cervical cancer mortality12. Cohorts and trials suggest that women’s risk of CIN3 or cancer (CIN3+) following a negative HPV test (HPV-negative) is very low for five years.3, 10, 13,14, although these studies were too small to reliably estimate risk of cancer itself. This evidence was the basis for regulatory and clinical guideline approval15-16 of routine HPV testing in conjunction with cervical cytology (co-testing) for cervical cancer screening of women 30 and older. In particular, guidelines discourage screening of women testing HPV-negative with normal cytology (Pap-negative) before three years to avoid detection of new HPV infections because their cancer risk is likely extremely low. Although very promising, co-testing has not been widely adopted in the US. In a recent survey, only 19% of US clinicians would recommend the 3-year screening interval for women testing HPV-negative with normal cytology18. These facts suggested to us that there remains serious concern about the safety of co-testing guidelines against cancer in real-life clinical practice. To address this issue, we collaborated with a health care provider that serves a population large enough to directly estimate cancer risks for even the lowest-risk women undergoing co-testing.
Interpretation
Previous clinical trials and research cohorts showed that HPV testing can prevent CIN3+ in tightly-controlled, research settings, but it remained unclear if HPV testing could prevent cancer in a real-life clinical setting. We found that women testing HPV-negative had extremely low risk of developing cervical cancer over five years, so low that it was similar to their risk of developing vulvar cancer, a cancer that is considered too rare to justify screening. Therefore, the three-year screening interval for women testing HPV-negative with a normal Pap test is safe in routine clinical practice. Furthermore, testing HPV-positive identified more women at enrollment who developed cervical cancer, and especially cervical adenocarcinoma, an uncommon but particularly lethal form of cervical cancer whose precursors are poorly-identified by Pap tests. The earlier identification of these women by HPV testing can facilitate earlier treatment and closer monitoring of high-risk women. In particular, HPV-positive women with normal cytology accrued substantial risk of cancer over five years and require stringent follow-up.
Acknowledgements
This research was supported, in part, by the Intramural Research Program of the NIH/National Cancer Institute, and by the American Cancer Society (Dr. Kinney). The authors acknowledge the support of the Women’s Health Research Institute at Kaiser Permanente Northern California. We thank David Check (US National Cancer Institute) for help in preparing the figures. We thank J. Thomas Cox (University of California, Santa Barbara) for assisting with the chart-review to verify all reported cervical cancers.
Funding: Intramural Research Program of the U.S. National Cancer Institute/NIH/DHHS, and the American Cancer Society (Dr. Kinney)
Abbreviations
- Pap-positive
(atypical squamous cells of undetermined significance [ASC-US] or more severe cytology)
- Pap-negative
(NILM cytology)
- CIN2+
(cervical intraepithelial neoplasia grade 2 or worse)
- CIN3+
(cervical intraepithelial neoplasia grade 3 or worse)
- SCC
(squamous cell carcinoma)
Footnotes
Conflicts of Interest: Dr.s Schiffman and Castle report working with Qiagen, Inc. on independent evaluations of non-commercial uses of CareHPV (a low-cost HPV test for low-resource regions) for which they have received research reagents and technical aid from Qiagen for free. No other authors report any conflicts of interest.
Author Contributions: HAK, WKK, and PEC contributed to study design. HAK, WKK, BF, MS, SW, and PEC contributed to drafting. BF, TL, NEP, and FD collected, arranged, cleaned, and managed the data. HAK, LC, and PEC contributed to the statistical analysis. All authors contributed to revision of the manuscript.
References
- 1.Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003 Dec 6;362(9399):1871–6. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman M, Herrero R, Hildesheim A, Sherman ME, Bratti M, Wacholder S, et al. HPV DNA testing in cervical cancer screening: results from women in a high-risk province of Costa Rica. JAMA. 2000 Jan 5;283(1):87–93. doi: 10.1001/jama.283.1.87. [DOI] [PubMed] [Google Scholar]
- 3.Sherman ME, Lorincz AT, Scott DR, Wacholder S, Castle PE, Glass AG, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst. 2003 Jan 1;95(1):46–52. doi: 10.1093/jnci/95.1.46. [DOI] [PubMed] [Google Scholar]
- 4.Anttila A, Kotaniemi-Talonen L, Leinonen M, Hakama M, Laurila P, Tarkkanen J, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ. 2010;340:c1804. doi: 10.1136/bmj.c1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007 Nov 24;370(9601):1764–72. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 6.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007 Oct 18;357(16):1579–88. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 7.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007 Oct 18;357(16):1589–97. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 8.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Palma P Dalla, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010 Mar;11(3):249–57. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 9.Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Palma P Dalla, Del Mistro A, et al. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst. 2008 Apr 2;100(7):492–501. doi: 10.1093/jnci/djn065. [DOI] [PubMed] [Google Scholar]
- 10.Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010 Oct 6;102(19):1478–88. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Relationship of atypical glandular cell cytology, age, and human papillomavirus detection to cervical and endometrial cancer risks. Obstet Gynecol. 2010 Feb;115(2 Pt 1):243–8. doi: 10.1097/AOG.0b013e3181c799a3. [DOI] [PubMed] [Google Scholar]
- 12.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009 Apr 2;360(14):1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 13.Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754. doi: 10.1136/bmj.a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesher D, Szarewski A, Cadman L, Cubie H, Kitchener H, Luesley D, et al. Long-term follow-up of cervical disease in women screened by cytology and HPV testing: results from the HART study. Br J Cancer. 2010 Apr 27;102(9):1405–10. doi: 10.1038/sj.bjc.6605619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright TC, Jr., Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004 Feb;103(2):304–9. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 16.Wright TC, Jr., Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007 Oct;197(4):346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010 Mar 3;102(5):315–24. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saraiya M, Berkowitz Z, Yabroff KR, Wideroff L, Kobrin S, Benard V. Cervical cancer screening with both human papillomavirus and Papanicolaou testing vs Papanicolaou testing alone: what screening intervals are physicians recommending? Arch Intern Med. 2010 Jun 14;170(11):977–85. doi: 10.1001/archinternmed.2010.134. [DOI] [PubMed] [Google Scholar]
- 19.Katki HA, Wacholder S, Solomon D, Castle PE, Schiffman M. Risk estimation for the next generation of prevention programmes for cervical cancer. Lancet Oncol. 2009 Nov;10(11):1022–3. doi: 10.1016/S1470-2045(09)70253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaem-Maghami S, De-Silva D, Tipples M, Lam S, Perryman K, Soutter W. Determinants of success in treating cervical intraepithelial neoplasia. BJOG. 2011 May;118(6):679–84. doi: 10.1111/j.1471-0528.2010.02770.x. [DOI] [PubMed] [Google Scholar]
- 21.Ghaem-Maghami S, Sagi S, Majeed G, Soutter WP. Incomplete excision of cervical intraepithelial neoplasia and risk of treatment failure: a meta-analysis. Lancet Oncol. 2007 Nov;8(11):985–93. doi: 10.1016/S1470-2045(07)70283-8. [DOI] [PubMed] [Google Scholar]
- 22.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002 Apr 24;287(16):2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 23.Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008 Jan;111(1):167–77. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 24.Ronco G, Cuzick J, Pierotti P, Cariaggi MP, Palma P Dalla, Naldoni C, et al. Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening: randomised controlled trial. BMJ. 2007 Jul 7;335(7609):28. doi: 10.1136/bmj.39196.740995.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siebers AG, Klinkhamer PJ, Grefte JM, Massuger LF, Vedder JE, Beijers-Broos A, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: a randomized controlled trial. JAMA. 2009 Oct 28;302(16):1757–64. doi: 10.1001/jama.2009.1569. [DOI] [PubMed] [Google Scholar]
- 26.Castle PE, Wheeler CM, Solomon D, Schiffman M, Peyton CL. Interlaboratory reliability of Hybrid Capture 2. Am J Clin Pathol. 2004 Aug;122(2):238–45. doi: 10.1309/BA43-HMCA-J26V-WQH3. [DOI] [PubMed] [Google Scholar]
- 27.Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001 Mar 21;285(11):1500–5. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 28.Castle PE, Stoler MH, Solomon D, Schiffman M. The relationship of community biopsy-diagnosed cervical intraepithelial neoplasia grade 2 to the quality control pathology-reviewed diagnoses: an ALTS report. Am J Clin Pathol. 2007 May;127(5):805–15. doi: 10.1309/PT3PNC1QL2F4D2VL. [DOI] [PubMed] [Google Scholar]
- 29.Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009 Jan;113(1):18–25. doi: 10.1097/AOG.0b013e31818f5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moscicki AB, Ma Y, Wibbelsman C, Darragh TM, Powers A, Farhat S, et al. Rate of and risks for regression of cervical intraepithelial neoplasia 2 in adolescents and young women. Obstet Gynecol. 2010 Dec;116(6):1373–80. doi: 10.1097/AOG.0b013e3181fe777f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007 Sep 8;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 32.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992 May;82(5):703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manos MM, Kinney WK, Hurley LB, Sherman ME, Shieh-Ngai J, Kurman RJ, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999 May 5;281(17):1605–10. doi: 10.1001/jama.281.17.1605. [DOI] [PubMed] [Google Scholar]
- 34.Castle PE, Fetterman B, Cox J Thomas, Shaber R, Poitras N, Lorey T, et al. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstet Gynecol. 2010 Jul;116(1):76–84. doi: 10.1097/AOG.0b013e3181e3e719. [DOI] [PubMed] [Google Scholar]
- 35.Sung HY, Kearney KA, Miller M, Kinney W, Sawaya GF, Hiatt RA. Papanicolaou smear history and diagnosis of invasive cervical carcinoma among members of a large prepaid health plan. Cancer. 2000 May 15;88(10):2283–9. [PubMed] [Google Scholar]
- 36.Horner MJ, Altekruse SF, Zou Z, Wideroff L, Katki HA, Stinchcomb DG. U.S. geographic distribution of prevaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol Biomarkers Prev. 2011 Apr;20(4):591–9. doi: 10.1158/1055-9965.EPI-10-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SAS, version 9.0. SAS Institute; Cary, NC: 2009. [Google Scholar]
- 38.Lawless JF. Statistical models and methods for lifetime data. 2nd ed Wiley-Interscience; Hoboken, N.J.: 2003. [Google Scholar]
- 39.Turnbull B. The empirical distribution function with arbitrarily grouped, censored and truncated data. J Roy Stat Soc B. 1976;38:290–5. [Google Scholar]
- 40.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst. 2009 Jan 21;101(2):88–99. doi: 10.1093/jnci/djn444. [DOI] [PubMed] [Google Scholar]
- 41.Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001 Feb 21;93(4):293–9. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- 42.Arbyn M, Martin-Hirsch P, Buntinx F, Van Ranst M, Paraskevaidis E, Dillner J. Triage of women with equivocal or low-grade cervical cytology results: a meta-analysis of the HPV test positivity rate. J Cell Mol Med. 2009 Apr;13(4):648–59. doi: 10.1111/j.1582-4934.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rijkaart DC, Berkhof J, van Kemenade FJ, Rozendaal L, Verheijen RH, Bulk S, et al. Comparison of HPV and cytology triage algorithms for women with borderline or mild dyskaryosis in population-based cervical screening (VUSA-screen study) Int J Cancer. 2010 May 1;126(9):2175–81. doi: 10.1002/ijc.24891. [DOI] [PubMed] [Google Scholar]
- 44.Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008 Aug 19;26(Suppl 10):K29–41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Kitchener HC, Almonte M, Thomson C, Wheeler P, Sargent A, Stoykova B, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009 Jul;10(7):672–82. doi: 10.1016/S1470-2045(09)70156-1. [DOI] [PubMed] [Google Scholar]
- 46.Sasieni P, Castle PE, Cuzick J. Further analysis of the ARTISTIC trial. Lancet Oncol. 2009 Sep;10(9):841–2. doi: 10.1016/S1470-2045(09)70246-3. author reply 2-3. [DOI] [PubMed] [Google Scholar]
- 47.Hesselink AT, Heideman DA, Steenbergen RD, Coupe VM, Overmeer RM, Rijkaart D, et al. Combined Promoter Methylation Analysis of CADM1 and MAL: An Objective Triage Tool for High-Risk Human Papillomavirus DNA-Positive Women. Clin Cancer Res. 2011 Apr 15;17(8):2459–65. doi: 10.1158/1078-0432.CCR-10-2548. [DOI] [PubMed] [Google Scholar]
- 48.Carozzi F, Confortini M, Palma P Dalla, Del Mistro A, Gillio-Tos A, De Marco L, et al. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2008 Oct;9(10):937–45. doi: 10.1016/S1470-2045(08)70208-0. [DOI] [PubMed] [Google Scholar]
- 49.Castle PE, Katki HA. Benefits and risks of HPV testing in cervical cancer screening. Lancet Oncol. 2010 Mar;11(3):214–5. doi: 10.1016/S1470-2045(09)70385-7. [DOI] [PubMed] [Google Scholar]
- 50.Watson M, Saraiya M, Benard V, Coughlin SS, Flowers L, Cokkinides V, et al. Burden of cervical cancer in the United States, 1998-2003. Cancer. 2008 Nov 15;113(10 Suppl):2855–64. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.