SUMMARY
The current surgical trend is to expand the variety of minimally invasive approaches and, in particular, the possible applications of robotic systems in head and neck surgery. This is particularly intriguing in skull base regions. In this paper, we review the current literature and propose personal considerations on the role of robotic techniques in this field. A brief description of our personal preclinical experience on skull base robotic dissection represents the basis for further considerations. We are convinced that the advantages of robotic surgery applied to the posterior cranial fossa are similar to those already clinically experienced in other areas (oropharynx, tongue base), in terms of tremor-free, bimanual, precise dissection: the implementation of instruments for bony work and resolving current drawbacks will definitely increase the applicability of such a system in forthcoming years.
KEY WORDS: DaVinci robotic system, Skull base, Endoscopic, TORS, Robotic surgery
RIASSUNTO
Il trend attuale, in ogni ambito chirurgico, è quello che porta verso una sempre minore invasività delle procedure; nel distretto testa-collo una delle più recenti tendenze è quella di verificare l'applicabilità clinica delle nuove tecnologie robotiche. Questa evoluzione si presenta particolarmente interessante ed intrigante soprattutto in ambito di chirurgia della base cranica. In questo lavoro presentiamo una revisione delle recente letteratura sull'argomento e proponiamo le nostre personali considerazioni sul ruolo delle tecniche robotiche nel campo della chirurgia di base cranica. Una breve descrizione della nostra esperienza dissettoria eseguita utilizzando il DaVinci a livello di base cranica posteriore rappresenta lo spunto per ulteriori considerazioni. Siamo del tutto convinti che gli stessi vantaggi offerti dalla chirurgia robotica a livello orofaringeo – dissezione bimanuale, precisa e priva di tremore – si applicheranno anche a livello della base cranica posteriore e media. In tale contesto lo sviluppo e l'implementazione di strumenti per la gestione dell'osso e la risoluzione dei limiti attuali incrementerà l'applicabilità clinica di questi sistemi nel prossimo futuro.
Introduction
Skull base surgery is technically demanding. Since its first curative attempts dating back to the last part of the 19th century 1 2, things have changed dramatically 3. Pioneering experiences required extensive disassembling of the bony structures of the face and the head in order to reach the target areas. The introduction of microscopic techniques increased the ability to dissect in complex regions, and offered the surgeon two-hand work and a 3D environment. Notwithstanding, major work on the bony skeleton is still required. Introduction of endoscopy through natural orifices, and more recently also through surgical corridors, has revolutionized the way skull base lesions can be accessed and managed. What has really been improved with the introduction of the endoscope is the vision of the target and surrounding regions. The conal vision offered by every external approach, including a microscopic one, has been substituted by a panoramic and dynamic vision offered by the endoscope. The ability to look around the corner and to go close to the target areas are probably the most important advantages offered by endoscopic techniques. Given the fact that surgery is a matter of obtaining a visible surgical field, it is easy to understand the value of this philosophical, rather than technical, innovation. In other words, the endoscope has completely modified the point of view of surgical procedures. Over the last 10 years, as testimony to this "conceptual revolution", an enormous number of papers on an endoscopic, transnasal and non, approach to the skull base has been reported 4-8. Many centres worldwide have gained remarkable experience in this field and new limits are being reached and surpassed day by day, while new corridors are proposed and investigated continuously. What is true at the time of writing can be surpassed at the time of reading. If not yet, it will certainly be so with time.
Obviously every coin has two faces, and traditional endoscopic techniques are associated with flaws and limitations. Typically, detractors of this technique underline 2 main concerns. The first one is the lack of 3D vision, and the second is the difficulty of bimanual work. If the first concern was true until some years ago, with the introduction of the 3D endoscope, endosurgeons now have the possibility to work in a true 3D environment. In this respect, previous experiences, including ours 9-12, demonstrate significant advantages with the use of 3D techniques. If some limitations still remain, they will undoubtedly be resolved with time. With regard to the second concern, the introduction of a typical neurosurgical bimanual dissection technique, especially due to the work of the Pittsburgh group, has completely resolved the problem. In this respect, we perfectly agree with Kupferman who states that the ideal surgical technique should offer the surgeon the distinct advantage of 3D vision and bimanual surgical dissection 13, possibly guided by a navigation system. Based on this, we maintain that a standard setting for endoscopic skull base surgery should offer all these opportunities to the surgeon and patient. Grounded on these considerations, we present herein the current state of art regarding the potential application of a robotic technique at the level of the skull base, trying to answer questions about the future role of such technology in this exciting area. Our preclinical experience on this topic – briefly described below – represents the basis for a critical evaluation of the data presented in the current literature.
Technique
The DaVinci system is placed cranially at the head of the cadaver. As previously described 14, the endoscope and the trocars are placed transnasally and paramandibularly, respectively. With this setting an extraordinary and familiar view of the middle and posterior ventral skull base is gained. Bony work – creation of an operative sphenoclival window – is done by traditional endoscopic techniques, given the actual absence of dedicated "robotic" instruments for bone. At this point, the dura of the middle and posterior cranial fossa is exposed and, once opened, a delicate dissection of these areas is performed. Pituitary and basilar tip regions are easily dissected and a pituitary transposition is performed, thus exposing and identifying the noble structures. Oculomotor nerves, superior cerebellar and posterior cerebral arteries, posterior communicating arteries (Figs. 1, 2), abducens nerves and the pituitary vascularization can be visualized through the sphenoclival window. As a whole, during dissection, conflict between instruments is minimal and can be reduced with good placement of trocars.
Fig. 1.
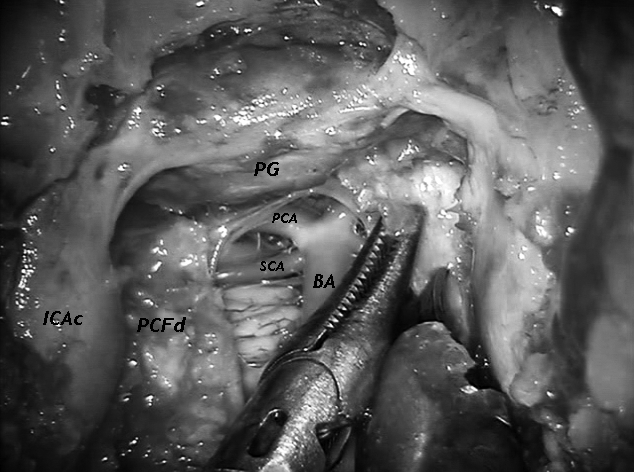
Endoscopic view of the initial phase of robotic dissection-namely the opening of the posterior cranial fossa dura. The 0° scope is placed transnasally. BA-basilar artery, PG-pituitary gland, PCA-posterior cerebral artery, SCA-superior cerebellar artery, ICAc-cavernous portion of the internal carotid artery, PCFd-dura of the posterior cranial fossa.
Fig. 2.
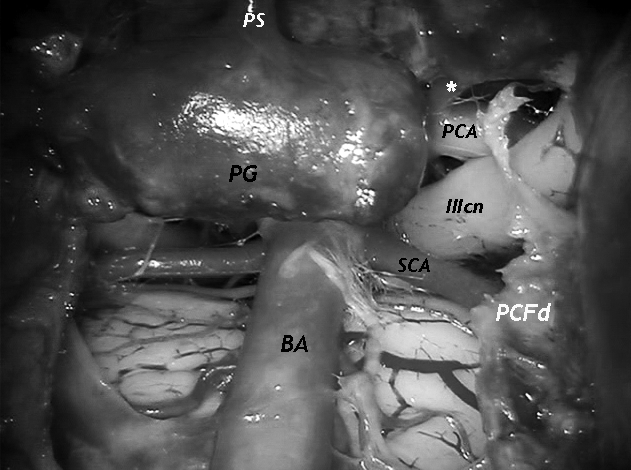
Close vision of the pituitary and upper basilar tip region. Vision obtained with a 0° scope, placed transnasally. PG-pituitary gland, PS-pituitary stalk, BA-basilar artery, SCA-superior cerebellar artery, PCA-posterior cerebral artery, white asterisk-posterior communicating artery, IIIcn-oculomotor nerve, PCFd-dura of the posterior cranial fossa.
Discussion
Current endoscopic transnasal techniques, regardless of the use of 2- or 3D endoscopes, represent a valid alternative to traditional external approaches and are probably the gold standard in selected cases of skull base pathologies, especially when dealing with ventral skull base lesions 5 15-19. However, limits and disadvantages still exist. Thus, following the evolving course of minimal invasiveness, since the pioneering work of the Philadelphia group 20-22, an increasing interest in robotic technologies is growing. In clinical settings, robotic procedures in the head and neck area are performed, placing the scope and the arms transorally. At present, these procedures are now being applied outside the region of the first experiences, namely the oropharynx 23-25. Nonetheless, up to now no clinical series regarding ventral skull base lesion management have been reported, with the unique exception of a blended solution on cranio-cervical junction 26. As a corollary, a small series on parapharyngeal lesion management has been recently reported by the "Penn" group 27. On the other hand, there is significantly more consistent preclinical literature on this topic 13 28-33, thus witnessing a growing "robotic" interest in skull base regions. Historically, the first attempts to approach skull base using of a robotic system were pioneered by the "Penn" and the "MD Anderson" groups 28 29. However, one obvious question is whether the robotic technique is the natural evolution of "traditional" endoscopic techniques for skull base regions? Certainly current robotic systems undoubtedly offer the surgeon an excellent view of the surgical field and an incredibly precise and intuitive manoeuvrability of the arms. The strengths of the robotic system are wellknown and their description is outside the scope of this paper. Notwithstanding, the daVinci system presents several limits, especially when dealing with the skull base regions. This is simply related to the fact that the system has been conceived and built to work in large spaces and not in small corridors. In this respect, when dealing with narrow areas, such as the skull base, the arms of the system should work parallel to one another to avoid conflict. Yet with current instrumentation this is not possible and a small amount of conflict is present, especially in the traditional transoral setting. For this reason, and to overcome these limits, different solutions and settings have been proposed in preliminary cadaveric experiences 14 28 29. Among the solutions suggested, we maintain that the combination of the transnasal placement of the scope and the transcervical placement of the trocars offers the best option, at the lowest biological price, with current instrumentation. By placing the endoscope transnasally, we obtain a more familiar, panoramic and orientating view of the surgical field, while, with the traditional transoral placement of the scope – 30° upfaced – we obtain a down-to-up vision, truly unfamiliar and not particularly orientating. This viewpoint makes working less comfortable and less safe in the upper regions. Compared to trocars, if they are placed paramandibularly, it is possible to gain a greater manoeuvrability of instruments in the upper regions. In contrast, transoral placement of the trocars needs complete palatal splitting, if superior work is to be done. Previous experience including our own shows similar conclusions in this respect 14 29. We are aware that our setting 14, like the previous ones 28 29, takes advantage of another portal for robotic arms to achieve an improved position of the arms, and that all these solutions sacrifice the minimally invasive nature of the procedure. However, it is not particularly different from that observed in robotic transabdominal and transthoracic procedures where different portals are used to gain greater manoeuvrability and efficacy. With this setting, once the bony structures have been removed, a delicate dissection of the dural layers of the middle and posterior ventral skull base and intracranial structures is possible, thus demonstrating a technical feasibility in preclinical models. A precise and tremor-free dissection of the pituitary region and the prepontine cistern is performed. Obviously, the system presents significant limitations that preclude current application in clinical settings for skull base pathologies.
Among the drawbacks of this new technology we must point out the absence of tactile feedback. This is an important limitation of the daVinci system that must be addressed in the future. Certainly with the implementation of instruments for bony work, currently lacking but which will soon be available, the applicability of such systems will be increased in the forthcoming years. As a consequence, a fully robotic skull base surgery will require the development of new tools for the daVinci robotic arms. In this respect, we strongly call for close collaboration with the manufacturer. From an anatomical viewpoint, with the current setting and instrumentation, the intercarotic space at the level of the clivus represents a critical factor that impacts the ability to dissect the posterior cranial fossa and pituitary regions. A wide bony corridor is necessary and the more space is available, the easier and more delicate the dissection is. However, it must be stressed that this kind of problem is also present with traditional endoscopic transnasal procedures.
Lastly, but of minor significance, with current instrumentation a conflict between the scope and the piriform aperture may be present during the nasal time. In fact, the current scope completely fills the piriform aperture. In spite of this, we maintain that it is a false problem for mainly two reasons. Firstly, with technical evolutions smaller lenses will become available, and secondly the piriform aperture can be widened as necessary without any discomfort for the patient.
Finally, some general considerations on the economic aspects are warranted. Costs remain an impediment to the establishment of robotic programs, as evaluated by others 34. These costs include purchase, annual maintenance and cost per case. Obviously centres with high volume robotic procedures for other varying specialities are more likely to expand into otolaryngology. In this respect, by expanding patient access to minimally invasive techniques, increasing the number of treated cases and improving outcomes, all parties involved (patients, surgeons and the healthcare system in general) will benefit from a robotic programme. In this scenario, given the expected clinical benefits, the significant initial costs are justified. Personally, we are strongly convinced that robotic applications in skull base regions will not be an exception to this rule.
In conclusion, based on our clinical experience in both endoscopic transnasal skull base and transoral robotic procedures, we believe that the ability to perform precise, tremor-free, bimanual surgery in confined cavities with instrumentation that exceeds the capabilities of the human hand 13 truly represents a great opportunity for surgeons and patients. In this respect, we are strongly convinced that robotic systems can be considered the natural evolution of traditional endoscopic techniques and that the future will offer the concept of surgical modularity: the combination of different corridors and techniques will create a blended solution in which the target of the therapy is addressed in the best possible manner, and no longer according only to the surgeon's advantage, but rather to the patient's. Clearly, the road to the future must be taken by all of us together in a synergic and multidisciplinary manner, and what has been true in the past is still true today. We must continue to improve our understanding of anatomy, whether endoscopic or traditional, to develop new surgical instruments, describe new surgical approaches and validate new technological solutions. To think that the utmost has been discovered would be a great mistake. Techniques, technologies and instrumentations will change, as will ideas and approaches, but what must not change is the reason for our mission: the patient. Time will certainly judge our personal viewpoint.
Conclusions
Skull base regions, for years considered inaccessible, are now manageable with increasing safety and efficacy. The evolution of this surgery has given birth to a new professional figure: the skull base surgeon. In this particular field, robotic technology represents an exceptional opportunity for both the patient and surgeon. The combination of frameless neuronavigation, modular approaches, intraoperative imaging systems, new materials and robotic surgery will offer incredible opportunities. What is certain is that they will be surpassed by further evolution. In any case, we are strongly convinced that skull base pathologies need a well-coordinated skull base team, and not just a single deus ex machina, that is skilled in using all the technical innovations available for the best interests of the patient.
References
- 1.Caton R, Paul FT. Notes of a case of acromegaly treated by operation. Br Med J. 1893;2:1421–1421. doi: 10.1136/bmj.2.1722.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horsley V. On the technique of operation on the nervous system. Br Med J. 1906;2:411–411. [Google Scholar]
- 3.Donald PJ. History of the skull base surgery. In: Donald PJ, editor. Surgery of the Skull Base. Philadelphia, PA: Lippincott- Raven; 1998. [Google Scholar]
- 4.Kassam AB, Snyderman C, Gardner P, et al. The expanded endonasal approach: a fully endoscopic transnasal approach and resection of the odontoid process: technical case report. Neurosurgery. 2005;57((Suppl)):E213–E213. doi: 10.1227/01.neu.0000163687.64774.e4. [DOI] [PubMed] [Google Scholar]
- 5.Frank G, Pasquini E, Doglietto F, et al. The endoscopic extended transsphendoidal approach for craniopharyngiomas. Neurosurgery. 2006;59((Suppl 1)):ONS75–ONS83. doi: 10.1227/01.NEU.0000219897.98238.A3. [DOI] [PubMed] [Google Scholar]
- 6.Pirris SM, Pollack IF, Snyderman CH, et al. Corridor Surgery: the current paradigm for skull base surgery. Childs Nerv Syst. 2007;23:377–384. doi: 10.1007/s00381-006-0281-6. [DOI] [PubMed] [Google Scholar]
- 7.Castelnuovo P, Dallan I, Battaglia P, et al. Endoscopic Endonasal Skull Base Surgery: past, present and future. Eur Arch Otorhinolaryngol. 2010;267:649–663. doi: 10.1007/s00405-009-1196-0. [DOI] [PubMed] [Google Scholar]
- 8.Moe KS, Kim LJ, Bergeron CM. Transorbital endoscopic repair of cerebrospinal fluid leaks. Laryngoscope. 2011;121:13–30. doi: 10.1002/lary.21280. [DOI] [PubMed] [Google Scholar]
- 9.Brown SM, Tabaee A, Singh A, et al. Three dimensional endoscopic sinus surgery: feasibility and technical aspects. Otolaryngol Head Neck Surg. 2008;138:400–402. doi: 10.1016/j.otohns.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Tabaee A, Anand VK, Fraser JF, et al. Three dimensional endoscopic pituitary surgery. Neurosurgery. 2009;64:288–293. doi: 10.1227/01.NEU.0000338069.51023.3C. [DOI] [PubMed] [Google Scholar]
- 11.Wasserzug O, Margalit N, Weizman N, et al. Utility of a three-dimensional endoscopic system in skull base surgery. Skull Base. 2010;20:223–228. doi: 10.1055/s-0030-1247630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castelnuovo P, Battaglia P, Turri-Zanoni M, et al. Trans-nasal skull base reconstruction using a 3-D endoscope: our first impressions. Skull Base. 2011 doi: 10.1055/s-0032-1311692. (accepted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kupferman M, DeMonte F, Holsinger FC, et al. Transantral robotic access to the pituitary gland. Otolaryngol Head Neck Surg. 2009;141:413–415. doi: 10.1016/j.otohns.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Dallan I, Castelnuovo P, Montevecchi F, et al. Combined transoral transnasal robotic-assisted nasopharyngectomy: a cadaveric fesibility study. Eur Arch Otorhinolaryngol. 2011 Mar 18; doi: 10.1007/s00405-011-1550-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Jho HD, Ha HG. Endoscopic endonasal skull base surgery: Part 3 – The clivus and posterior fossa. Minim Invasive Neurosurg. 2004;47:16–23. doi: 10.1055/s-2004-818347. [DOI] [PubMed] [Google Scholar]
- 16.Solares CA, Fakhri S, Batra PS, et al. Transnasal endoscopic resection of lesions of the clivus: a preliminary report. Laryngoscope. 2005;115:1917–1922. doi: 10.1097/01.mlg.0000172070.93173.92. [DOI] [PubMed] [Google Scholar]
- 17.Stamm AC, Pignatari SS, Vellutini E. Transnasal endoscopic surgical approaches to the clivus. Otolaryngol Clin North Am. 2006;39:639–656. doi: 10.1016/j.otc.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Fraser JF, Nyquist GG, Moore N, et al. Endoscopic endonasal minimal access approach to the clivus: case series and technical nuances. Neurosurgery. 2010;67((Suppl Operative)):ONS150–ONS158. doi: 10.1227/01.NEU.0000383130.80179.41. [DOI] [PubMed] [Google Scholar]
- 19.Holzmann D, Reisch R, Krayenbuhl N, et al. The transnasal transclival approach for clivus chordoma. Minim Invasive Neurosurg. 2010;53:211–217. doi: 10.1055/s-0030-1267929. [DOI] [PubMed] [Google Scholar]
- 20.Hockstein NG, Weinstein GS, O'Malley BW., jr Maintenance of hemostasis in transoral robotic surgery. ORL J Otorhinolaryngol Relat Spec. 2005;67:220–224. doi: 10.1159/000088012. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein GS, O'Malley BW, jr, Hockstein NG. Transoral robotic surgery: Supraglottic laryngectomy in a canine model. Laryngoscope. 2005;115:1315–1319. doi: 10.1097/01.MLG.0000170848.76045.47. [DOI] [PubMed] [Google Scholar]
- 22.O'Malley BW, jr, Weinstein GS, Snyder W, et al. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope. 2006;116:1465–1472. doi: 10.1097/01.mlg.0000227184.90514.1a. [DOI] [PubMed] [Google Scholar]
- 23.Park YM, Kim WS, Byeon HK, et al. Feasibility of transoral robotic hypopharyngectomy for early stage hypopharyngeal carcinoma. Oral Oncol. 2010;46:597–602. doi: 10.1016/j.oraloncology.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Wei WI, Ho WK. Transoral robotic resection of recurrent nasopharyngeal carcinoma. Laryngoscope. 2010;120:2011–2014. doi: 10.1002/lary.21059. [DOI] [PubMed] [Google Scholar]
- 25.Alon EE, Kasperbauer JL, Olsen KD. Feasibility of transoral robotic-assisted supraglottic laryngectomy. Head Neck 2011. doi: 10.1002/hed.21719. doi: 10.1002/hed.21719 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Lee JYK, Lega B, Bhowmick D, et al. DaVinci robot-assisted transoral odontoidectomy for basilar invagination. ORL J Relat Spec. 2010;72:91–95. doi: 10.1159/000278256. [DOI] [PubMed] [Google Scholar]
- 27.O'Malley BW, jr, Quon H, Leonhardt FD, et al. Transoral robotic surgery for parapharyngeal space tumors. ORL J Otorhinolaryngol Relat Spec. 2010;72:332–336. doi: 10.1159/000320596. [DOI] [PubMed] [Google Scholar]
- 28.Hanna EY, Holsinger C, DeMonte F, et al. Robotic endoscopic surgery of the skull base: a novel surgical approach. Arch Otolaryngol Head Neck Surg. 2007;133:1209–1214. doi: 10.1001/archotol.133.12.1209. [DOI] [PubMed] [Google Scholar]
- 29.O'Malley BW, jr, Weinstein GS. Robotic Skull Base Surgery. Arch Otolaryngol Head Neck Surg. 2007;133:1215–1219. doi: 10.1001/archotol.133.12.1215. [DOI] [PubMed] [Google Scholar]
- 30.O'Malley BW, jr, Weinstein GS. Robotic anterior and midline skull base surgery: preclinical investigations. Int J Radiat Oncol Biol Phys. 2007;69((Suppl)):S125–S128. doi: 10.1016/j.ijrobp.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Lee JYK, O'Malley BW, jr, Newman JG, et al. Transoral robotic surgery of craniocervical junction and atlantoaxial spine: a cadaveric study. J Neurosurg Spine. 2010;12:13–18. doi: 10.3171/2009.7.SPINE08928. [DOI] [PubMed] [Google Scholar]
- 32.Lee JYK, O'Malley BW, jr, Newman JG, et al. Transoral robotic surgery of the skull base: a cadaver and feasibility study. ORL J Relat Spec. 2010;72:181–187. doi: 10.1159/000276937. [DOI] [PubMed] [Google Scholar]
- 33.Kupferman ME, DeMonte F, Levine N, et al. Feasibility of a robotic surgical approach to reconstruct the skull base. Skull Base. 2011;21:79–82. doi: 10.1055/s-0030-1261258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein GS, O'Malley BW, jr, Desai SC, et al. Transoral robotic surgery: does the ends justify the means? Curr Opin Otolaryngol Head Neck Surg. 2009;17:126–131. doi: 10.1097/MOO.0b013e32832924f5. [DOI] [PubMed] [Google Scholar]


