SUMMARY
Sensorineural hearing loss of immune-mediated origin may be present as a symptom in systemic autoimmune diseases or may occur as a primary disorder without other organ involvement (auto-immune inner ear disease). The diagnosis of auto-immune inner ear disease is still predicated on clinical features and to date specific diagnostic tests are not available. We report a case of right-sided sudden hearing loss in a female patient in which the clinical manifestations, in addition to ANA positivity and hypocomplementaemia allowed us to hypothesize an auto-immune inner ear disease. The immunosuppressive treatment with cyclosporine-A was capable of a recovery of the hearing that, however, occurred progressively with normalization of the hearing function after 1 year of treatment. cyclosporine-A could be proposed as a therapeutic option in case of auto-immune inner ear disease allowing the suspension of corticosteroids that, at high dose, expose patients to potentially serious adverse events.
KEY WORDS: Sudden sensorineural hearing loss, Cyclosporine-A, Immune dysregulation
RIASSUNTO
L'ipoacusia neurosensoriale di origine immuno-mediata può rappresentare uno dei sintomi di malattie autoimmuni sistemiche o può manifestarsi come disturbo primario, senza il coinvolgimento di altri organi (malattia auto-immune dell'orecchio interno). La diagnosi di malattia auto-immune dell'orecchio interno è ancora basata sulle caratteristiche cliniche e, al momento, non sono disponibili test diagnostici accurati. Segnaliamo il caso di una paziente affetta da perdita improvvisa dell'udito in orecchio destro in cui, le manifestazioni cliniche, il riscontro di ANA-positività e di ipocomplementemia ha permesso di ipotizzare una malattia auto-immune dell'orecchio interno. Il trattamento immunosoppressivo con ciclosporina A è stato in grado di indurre un recupero dell'udito che, tuttavia, si è verificato in maniera progressiva nel tempo con la normalizzazione della funzione uditiva dopo 1 anno di trattamento. Un trattamento a bassi dosaggi e a lungo termine con ciclosporina A potrebbe essere proposto come opzione terapeutica in caso di malattia auto-immune dell'orecchio interno permettendo la sospensione della terapia steroidea potenzialmente gravata da effetti collaterali anche gravi.
Introduction
Sudden sensorineural hearing loss (SSHL) is frequent: the overall incidence ranges from 5 to 20 per 100,000 persons per year 1. SSHL is a syndrome of unknown aetiology often accompanied by vertigo and tinnitus. Sensorineural hearing loss of immune-mediated origin may be present as a symptom in systemic autoimmune diseases (Cogan's syndrome, Behçet's disease, Wegener's granulomatosis, mixed cryoglobulinaemia, systemic sclerosis, systemic lupus erythematosus, giant cell arteritis, panarteritis nodosa, relapsing polychondritis) or may occur as a primary disorder without other organ involvement (auto-immune inner ear disease) (AIED). Although AIED is usually bilateral and rapidly progressive, in some cases it can present suddenly and unilaterally 2. The diagnosis of AIED is still predicated on clinical features, while indirectly on positive therapeutic response to corticosteroids 3. To date specific diagnostic tests are not available 4. In AIED, an immune dysregulation has been hypothesized as pathogenetic mechanism due to the presence, in some patients, of ANA 5, hypocomplementaemia and autoantibodies against the inner ear neuroepithelium and the endothelial cells 2 in presence of normal inflammation indices.
Case report
In November 2006, a 39-year-old female presented at the Otolaryngology Department of the University of Bari with right-sided hearing loss that had appeared suddenly, 72 hours before admission. The patient's medical history was negative for diabetes, hypertension, dyslipidaemia, thyroid dysfunction and intake of contraceptive drugs. On hospital admittance, the patient reported right-sided hearing loss, fullness and tinnitus, no vertigo or dizziness were reported. Both tympanic membranes were normal, there were no signs of vestibular involvement, and the neuro-otologic examination was completely normal. Hearing test showed a flat, right-sided profound sensorineural hearing loss. Pure tone average (PTA) for the frequencies from 0.125 to 8 kHz was 94.3 dB on the right side and 14.3 dB HL on the left side. Maximum speech discrimination score was 10% at 100 dB HL on the right and 100% at 30 dB HL on the left side. Acoustic reflexes were evoked on the left side; on the right side, ipsilateral reflexes were absent and contra-lateral reflexes presented an increased threshold at 0.5 and 1 kHz. Reflex decay test was normal bilaterally. Auditory brainstem responses were absent on the right side and normal on the left side. Transient evoked otoacoustic emissions and distortion product otoacoustic emission input/output function at 2, 4 and 6 kHz were absent on the right side and normal on the left. The bithermal caloric test showed a right vestibular hyporeflexia of 34% according to Jonkee's formula, while vestibular evoked myogenic potentials (VEMPs) were normal bilaterally.
Laboratory tests showed a slightly reduced C3 fraction (0.84 g/l, ref. values 0.90-1.8 g/l) and ANA (1:160, homogeneous pattern). Total cholesterol, ESR, CRP, α2- globulins, blood fibrinogen, circulating immune complexes, anti-dsDNA, p-ANCA, c-ANCA, ENA, ACA, anti-β2 glycoprotein were normal. The patient was initially treated with prednisone 55 mg/day, xanthinol nicotinate 2 g/day, ascorbic acid 1 g/day, vitamin E 200 mg/day, pentoxifillin (600 mg/day), enoxaparin (4000 UI/day) and carbogen (95% O2 5% CO2).
Ten days after this treatment, a slight recovery in the hearing threshold (PTA 83.5 dB HL) and of the speech discrimination (50% at 100 dB) was scored. On the basis of the immunologic work-up at the Allergology and Clinical Immunology Section of the University of Bari, the patient started treatment with Cyclosporine-A (CsA) (Sandimmun Neoral® Novartis Farma SpA, Varese, Italy) 125 mg/ day in association with steroid, haemorheologic treatment already established. The choice of this immunosuppressive therapy, at low dosage, was necessary due both to the severity of symptoms and the presence of immune dysregulation confirmed by ANA positivity and hypocomplementaemia.
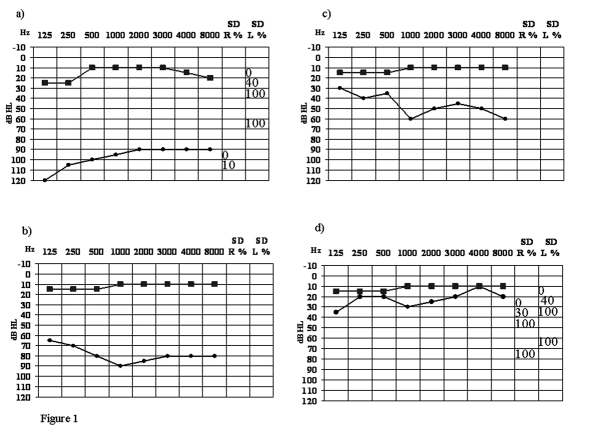
Twelve days after hospitalization the patient was discharged with a PTA of 80.7 dB HL and a speech discrimination of 50% at 100 dB. Regression of fullness and improvement of tinnitus were observed. Two weeks after discharge the patient presented partial recovery of the right hearing that continued over time, with normalization of the hearing function after 1 year of treatment (Fig. 1). During the first year, bimonthly clinical and laboratory follow-up was introduced, while thereafter four-monthly follow-up was carried out. All these evaluations, by monitoring the response to the new treatment protocol and detecting any side-effects that might stem from the inclusion of CsA, revealed normal renal and hepatic function and absence of gastrointestinal side-effects.
Fig. 1.
Hearing test and speech discrimination in right (●) and left (■) ear at: a) hospital admission; b) hospital discharge; c) 15 days after discharge; d) 20 months after discharge.
The clinical improvement associated with the absence of inflammation, normalization of complement level and absence of ANA allowed gradual steroid tapering and limited reduction of the CsA daily dosage. From May 2008 to November 2008, the patient was on a maintenance protocol consisting of CsA (100 mg/day) and salicylic acid (100 mg/day). At follow-up 24 months after hospitalisation, hearing was normal on the right side (PTA 20.8 dB HL) with normal speech discrimination (100% at 40 dB) (Fig. 1). Acoustic reflexes were present upon ipsi-lateral and contra-lateral stimulation with normal thresholds and otoacustic emissions reappeared on the right side. Bithermal caloric stimulation and VEMPs showed normal vestibular function. Thereafter, with gradual tapering, CsA was discontinued on May 2009 and at the last follow-up, in November 2009, the patient presented the same PTA and speech discrimination as in the evaluation made 24 months after hospitalization (Fig. 1).
Discussion
No guidelines have been established for the treatment of AIED. High doses of steroids are usually the only treatment (generally prednisone 1 mg/kg/day) able to partially restore ear function. Immunosuppressive agents (methotrexate, cyclophosphamide or azathioprine) are of poor efficacy 3. In our case, after careful assessment of the risks and benefits of immunosuppressive therapy, prompt treatment with steroids and CsA, in addition to haemoreological agents, led to recovery of hearing that, however, occurred progressively. The clinical improvement started 15 days after hospital discharge and progressed over one year. The excellent response to CsA, in the form of recovery of the right hearing, together with normalisation of C3 and disappearance of ANA, was then, maintained over the years. CsA, by inhibiting T-lymphocyte synthesis of various cytokines (in particular IL-2, IL-4) and inducing a reduction in the CD40 ligand expression, is responsible for the inhibition of the T and B cell interaction. This mechanism induces a reduction in antibody production 6. In our case, the CsA, at a low dosage, was found to be an efficient form of treatment that led to the reduction and subsequent suspension of the steroid dosage. No adverse drug reactions were recorded during the treatment. In addition, the beneficial effect of the CsA treatment was maintained for five months after suspension.
Conclusions
In the light of these findings, CsA could be proposed, at low dosage, as a therapeutic option in case of SSHL showing laboratory signs of immune dysregulation, although it is rare to find clear signs of immunological changes. In fact, the use of CsA resulted effective also in the case of idiopathic sensorineural hearing loss 7. This molecule showed a good safety and efficacy profile allowing suspension of corticosteroids that, at a high dose, expose patients to potentially severe adverse events. CsA may be assigned as long-term treatment, considering the chance of a late recovery by scrupulous monitoring of the renal and hepatic function.
References
- 1.Ramunni A, Quaranta N, Saliani MT, et al. Does a reduction of adhesion molecules by LDL-apheresis have a role in the treatment of sudden hearing loss? Ther Apher Dial. 2006;10:282–286. doi: 10.1111/j.1744-9987.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruckenstein MJ. Autoimmune inner ear disease. Curr Opin Otolaryngol Head Neck Surg. 2004;12:426–430. doi: 10.1097/01.moo.0000136101.95662.aa. [DOI] [PubMed] [Google Scholar]
- 3.Broughton SS, Meyerhoff WE, Cohen SB. Immune-mediated inner ear disease: 10-year experience. Semin Arthritis Rheum. 2004;34:544–548. doi: 10.1016/j.semarthrit.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Bovo R, Aimoni C, Martini A. Immune-mediated inner ear disease. Acta Otolaryngol. 2006;126:1012–1021. doi: 10.1080/00016480600606723. [DOI] [PubMed] [Google Scholar]
- 5.García-Berrocal JR, Ramírez-Camacho R, Trinidad A, et al. Autoimmune hearing loss: improving diagnostic performance. Acta Otorinolaringol Esp. 2007;58:138–142. doi: 10.1016/s2173-5735(07)70321-6. [DOI] [PubMed] [Google Scholar]
- 6.Ho S, Clipstone N, Timmermann L, et al. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80:40–45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 7.McClelland L, Powell RJ, Birchall J, et al. Role of cyclosporin in steroid-responsive sudden sensorineural hearing loss. Acta Otolaryngol. 2005;125:1356–1360. doi: 10.1080/00016480510012372. [DOI] [PubMed] [Google Scholar]