Abstract
We studied whether CB1 blockade with rimonabant has an anti-inflammatory effect in obese mice, and whether this effect depends on weight loss and/or diet consumption. High-fat diet (HFD)–induced obese mice were treated orally with rimonabant (HFD-R) or vehicle (HFD-V) for 4 weeks. Paired-feeding was conducted in 2 additional groups of obese mice to achieve either the same body weight (HFD-BW) or the same HFD intake (HFD-DI) as HFD-R. All these groups of mice were maintained on HFD throughout, with mice on normal diet throughout as lean controls. Rimonabant treatment of obese mice induced marked diet-intake reduction and weight loss during the first week, which was followed by maintenance of low body weight but not diet-intake reduction. Lower HFD intake was required to reach the same degree of weight loss in HFD-BW. HFD-DI had similar weight loss initially, but then started to gain weight, reaching a higher body weight than HFD-R. Despite the same degree of weight loss, HFD-R had less fat mass and lower adipogenic gene expression than HFD-BW. Compared to HFD-V or HFD-DI, HFD-R had reduced inflammation in adipose tissue (AT) and/or liver indicated primarily by lower monocyte chemoattractant protein–1 (MCP-1) levels. However, MCP-1 levels were not significantly different between HFD-R and HFD-BW. In vitro incubation of rimonabant with AT explants did not change MCP-1 levels. Thus, rimonabant induced weight loss in obese mice by diet intake–dependent and –independent fashions. Rimonabant decreased inflammation in obese mice, possibly through a primary effect on weight reduction.
Keywords: Rimonabant, chemokine, inflammation, obesity
Introduction
Obesity is associated with chronic inflammation, evidenced by increased levels of chemokines/cytokines such as monocyte chemoattractant protein–1 (MCP-1), regulated on activation normal T cell expressed and secreted (RANTES), and tumor necrosis factor–α (TNF-α) in adipose tissue (AT), liver, and/or blood, and increased accumulation and activation of macrophages/dendritic cells (DCs) and T cells in AT and liver.[1–3] In contrast, obesity is associated with decreased levels of adiponectin, a molecule secreted exclusively by AT with anti-inflammatory properties.[4] Chronic inflammation plays a critical role in the pathogenesis of cardiovascular disease (CVD) and diabetes,[5,6] and therefore may be an important link between obesity and the related disorders of diabetes and CVD. Various modalities of weight loss used to treat obesity have been shown to reduce levels of proinflammatory molecules and increase levels of adiponectin in obese subjects,[7,8] indicating that weight loss may exert beneficial effects by reducing the underlying inflammation.
Recently, a role of the endocannabinoid system in food intake and energy metabolism has been established. Endocannabinoid system overactivity in both brain and peripheral tissues is now considered to contribute to the metabolic syndrome.[9] Pharmacological blockade of the cannabinoid receptor CB1 has shed light on the treatment of obesity and metabolic syndrome by acting on the brain and peripheral tissues.[10,11] For example, blockade of CB1 receptor with rimonabant, a potent and selective CB1 receptor antagonist, induces a transient reduction of food intake and a marked but sustained reduction of body weight, with improvement of metabolic abnormalities in mice with diet-induced obesity.[12,13] Rimonabant also increases adiponectin levels and decreases insulin hypersecretion in obese animals.[12,14] In clinical studies, rimonabant induced a greater weight loss and produced greater improvements in metabolic parameters in obese patients compared with placebo.[11] Nevertheless, the effect of rimonabant on inflammation associated with diet-induced obesity has not been well studied. Previous studies have shown that rimonabant reduced the expression of some inflammatory molecules in AT of obese animals.[13,15] However, since these studies did not include appropriate paired-feeding controls, it is not clear whether the consequence of rimonabant treatment is due to its direct effect or mediated through weight loss induction.
In the present study, using a mouse model of diet-induced obesity, we examined the effect of CB1 receptor blockade with rimonabant on obesity-linked inflammation in AT, liver, and blood, and also compared the effect of rimonabant with the effect of weight loss or diet alone by using body weight– and diet intake–matched controls.
Methods and Procedures
Animals and rimonabant treatment
Obesity was induced in male C57BL/6J (Jackson Laboratory, Bar Harbor, ME) mice by feeding high-fat diet (HFD, 21% w/w fat; Dyets Inc., Bethlehem, PA), with mice fed normal diet (ND; 4.5% w/w fat, PicoLab Rodent Chow 5053) used as lean controls.[1] After 6 months, obese mice were treated with either rimonabant in 0.1% Tween 80 (10 mg/kg/day; HFD-R group) or with 0.1% Tween 80 alone (vehicle controls; HFD-V group) by daily oral gavage for an additional 4 weeks with HFD ad libitum. Pair-feeding was conducted in 2 additional groups of obese mice: body weight–matched controls (HFD-BW) received a limited amount of HFD to keep the same body weight as in HFD-R mice; diet intake–matched controls (HFD-DI) were fed the same amount of HFD as consumed by HFD-R mice. HFD-BW, HFD-DI, and lean mice (ND-V) were also administered vehicle by daily oral gavage for the last 4 weeks. Body weight and food intake were recorded daily during rimonabant treatment and other interventions. Pair-feeding was conducted 1 day behind rimonabant treatment. Whole body composition was examined 2 days before the end of treatment by using a PIXI-mus Small Animal Densitometer (LUNAR, Madison, WI). After 4 weeks of treatment, mice were sacrificed under anesthesia, and perigonadal fat pads, liver, and fasting plasma were collected. All animal studies were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Quantification of mRNA and protein
Total RNA was isolated from AT or liver using Trizol reagent (Invitrogen). mRNA quantity of chemokines and leukocyte markers was determined by RNase protection assay (RPA).[1] mRNA levels of adipogenic genes and adiponectin in AT, and of CD11c in the liver were examined by quantitative reverse transcription polymerase chain reaction (RT-QPCR) using predesigned primers and probes (for adiponectin and CD11c) or SYBR Green Reagent with a melting curve (for adipogenic genes) (Applied Biosystems, Foster City, CA).
Plasma MCP-1 levels were examined by ELISA kit (R&D Systems, Minneapolis, MN).
Flow cytometric analysis of T cells and macrophages in AT
Collagenase digestion was performed to fractionate mouse AT into adipocytes and stromal/vascular cells (S/Vs).[1] Flow cytometric analysis was conducted to quantify T cells and marcrophages/DCs in S/V cells after staining with fluorescein isothiocyanate (FITC)–, phycoerythrin(PE)–, or PE-Cy5–conjugated anti-mouse CD3, CD11b, CD11c (BD Pharmingen), and/or F4/80 (eBioscience, Inc., San Diego, CA).[16]
Measurement of hepatic triglyceride (TG) content
A piece of liver tissue was homogenized in Folch reagent (2:1 chloroform:methanol), and total lipid was extracted from liver homogenate and dried. The lipid extract was then dissolved in isopropanol, and TG content was measured with a Triglyceride Test Kit (Wako Chemicals USA, Inc. Richmond, VA). Hepatic TG content was normalized to liver wet weight.
Statistical analysis
Values are presented as mean±SEM. GraphPad Prism 4 (GraphPad Software Inc, San Diego, CA) and Instate 3 were used for statistical analyses. Student’s t-test (for comparison between 2 groups) or one-way ANOVA followed by Bonferroni multiple comparison test (for comparisons of ≥3 groups) was used for statistical analyses. Spearman correlation coefficents were computed to examine correlations. All differences between groups were based on mean values but were only considered significant when P<0.05.
Results
Effect of rimonabant on diet intake and body weight
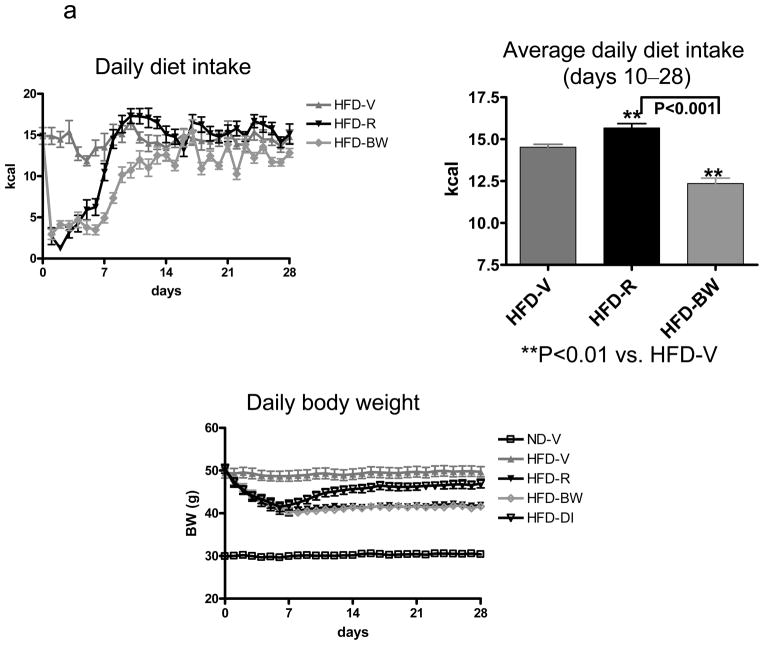
Consistent with previous reports,[13,17] rimonabant treatment of obese mice (HFD-R) markedly inhibited diet intake and induced weight loss during the first week as compared with vehicle-treated obese controls (HFD-V) (Figure 1a). Subsequently, HFD-R mice gradually increased diet intake, reaching even higher intake than HFD-V mice by day 10 through the end of treatment (Figure 1a). However, the body weight of HFD-R mice remained lower than that of HFD-V controls (Figure 1a). To achieve the same body weight as HFD-R mice, a lower amount of HFD was required for mice treated with vehicle only (HFD-BW) (Figure 1a). Mice fed the same amount of HFD as consumed by HFD-R mice (HFD-DI) had similar weight loss during the first week, but then started to gain weight, reaching a higher body weight than HFD-R mice by the end of the intervention (Figure 1a).
Figure 1. Effect of rimonabant on diet intake and physical characteristics.
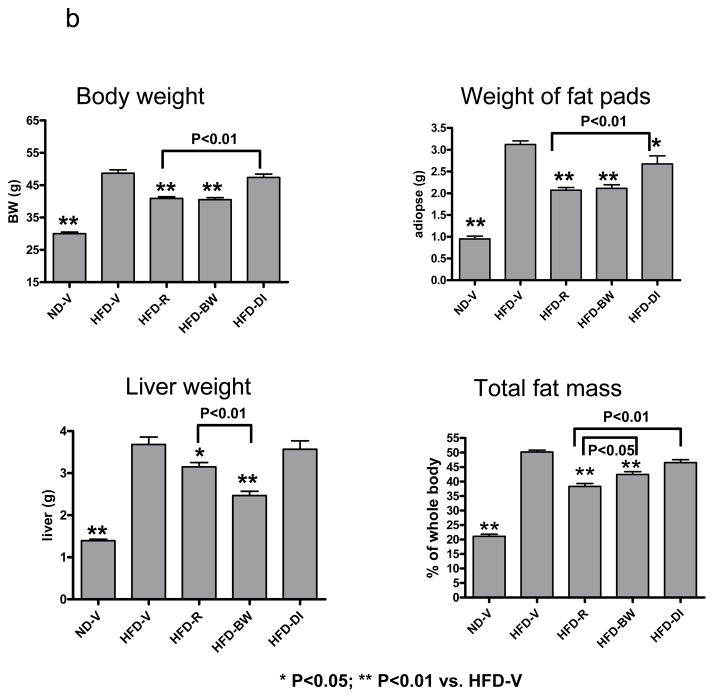
Mice with diet-induced obesity were treated with rimonabant in 0.1% Tween 80 (10 mg/kg/day; HFD-R) or 0.1% Tween 80 vehicle alone (HFD-V) by daily oral gavage for 4 weeks and received HFD ad libitum; pair-feeding was conducted in 2 separate groups of obese mice to achieve either the same body weight (HFD-BW) by adjusting HFD intake or the same HFD consumption (HFD-DI) as HFD-R; lean mice (ND-V), HFD-BW, and HFD-DI mice were also administered 0.1% Tween 80 alone by daily oral gavage during this period; n=12–19 mice/group. (a) Daily diet intake during the whole period of treatment and average daily diet intake for the period of stable body weight (days 10–28 after starting treatment) of HFD-V, HFD-R, and HFD-BW mice; diet intake by HFD-DI mice was the same as HFD-R mice. Daily body weight of each group of mice is shown during the whole period of treatment. (b) Body weight, weights of perigonadal fat pads and livers, and total fat mass expressed as percentage of total body mass at the end of treatment.
After treatment for 4 weeks, HFD-R mice had lower body weight, smaller perigonadal fat pads, smaller livers, and less total fat mass than HFD-V controls (Figure 1b). Body weight was also lower, fat pads were smaller, and total fat mass was lower in HFD-R than HFD-DI, although both groups consumed the same amount of HFD. Perigonadal fat pad weights were comparable between HFD-R and HFD-BW, whereas HFD-R mice had larger livers than HFD-BW mice (Figure 1b). Notably, although HFD-R and HFD-BW mice had comparable total body mass, HFD-R mice had significantly less fat mass than HFD-BW mice (Figure 1b). These data suggest that rimonabant had an additional effect on fat mass reduction compared to weight loss or diet alone.
Effect of rimonabant on expression of adipogenic genes
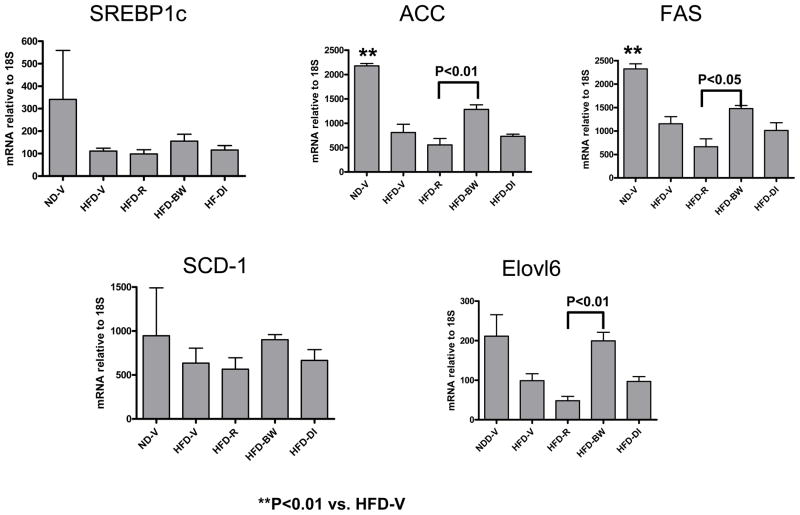
Examination of adipogenic gene expression showed that mRNA of SREBP1c, a regulator of lipogenic molecules, tended to be lower in AT of obese mice than in AT of lean mice (Figure 2), consistent with previous studies.[18] The target genes of SREBP1c, including ACC, FAS, SCD-1, and Elovl 6, were decreased in AT of obese mice compared to lean mice (Figure 2). Weight loss in HFD-BW mice tended to increase these adipogenic genes compared to HFD-V mice. HFD-R mice, which had the same degree of weight loss as HFD-BW, had lower levels of the adipogenic genes in AT than did HFD-BW (Figure 2), suggesting that rimonabant may reduce fat mass by inhibiting expression of adipogenic genes.
Figure 2. Effect of rimonabant on AT expression of adipogenic genes.
mRNA levels of selected adipogenic genes in mouse AT after rimonabant treatment or other interventions were examined by RT-QPCR; n=3 samples/group; each sample was pooled from 3 mice.
Effect of rimonabant on metabolic parameters
HFD-R mice had lower HOMA-IR and tended to have lower plasma levels of glucose, insulin, cholesterol, FFA, and TGs than HFD-V or HFD-DI controls, but did not show significant differences in these parameters compared to HFD-BW (Supplemental Figure I).
Effect of rimonabant on AT inflammation
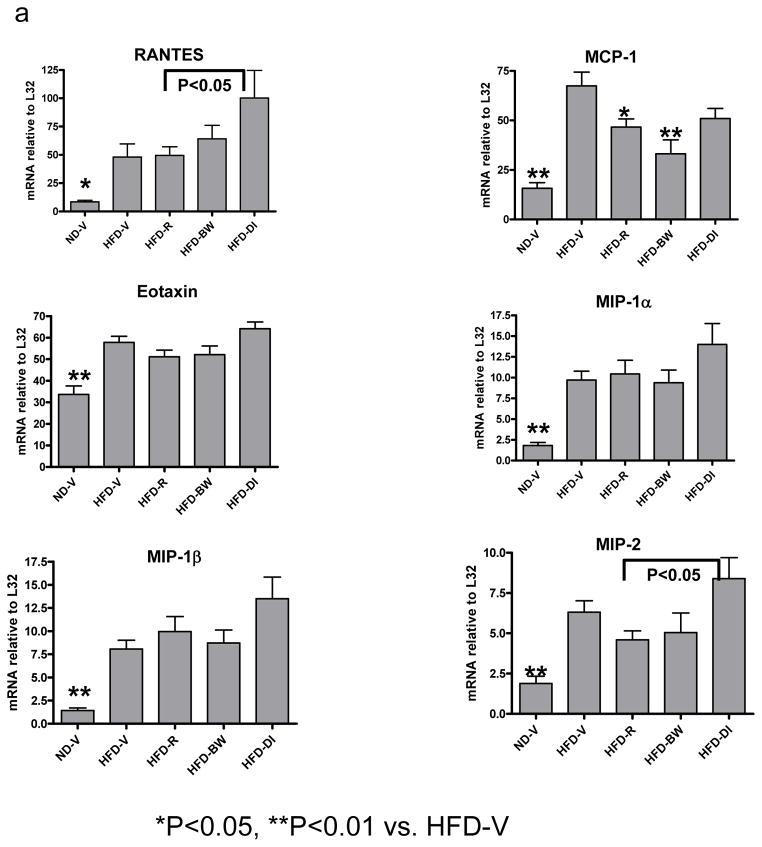
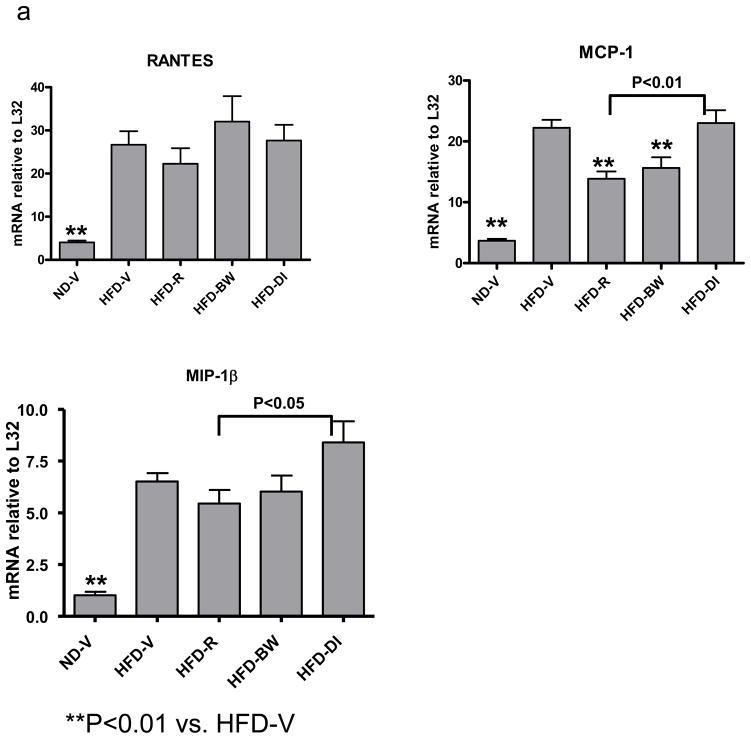
Based on the active roles of chemokines in inflammation, we examined the effect of rimonabant on chemokine expression in AT. Compared to lean ND-V mice, obese HFD-V mice had increased mRNA levels of several chemokines, including RANTES, MCP-1, eotaxin, MIP-1α, MIP-1β, and MIP-2, in AT (Figure 3a). Rimonabant treatment in HFD-R mice significantly decreased AT MCP-1 mRNA levels compared to those in HFD-V controls (Figure 3a). However, AT MCP-1 levels were not statistically different among HFD-R, HFD-BW, and HFD-DI mice (Figure 3a). HFD-R mice also had lower mRNA levels of RANTES and MIP-2 than HFD-DI mice. MIP-2 mRNA tended to be lower in HFD-R and HFD-BW mice than in HFD-V mice, but this difference was not statistically significant (Figure 3a).
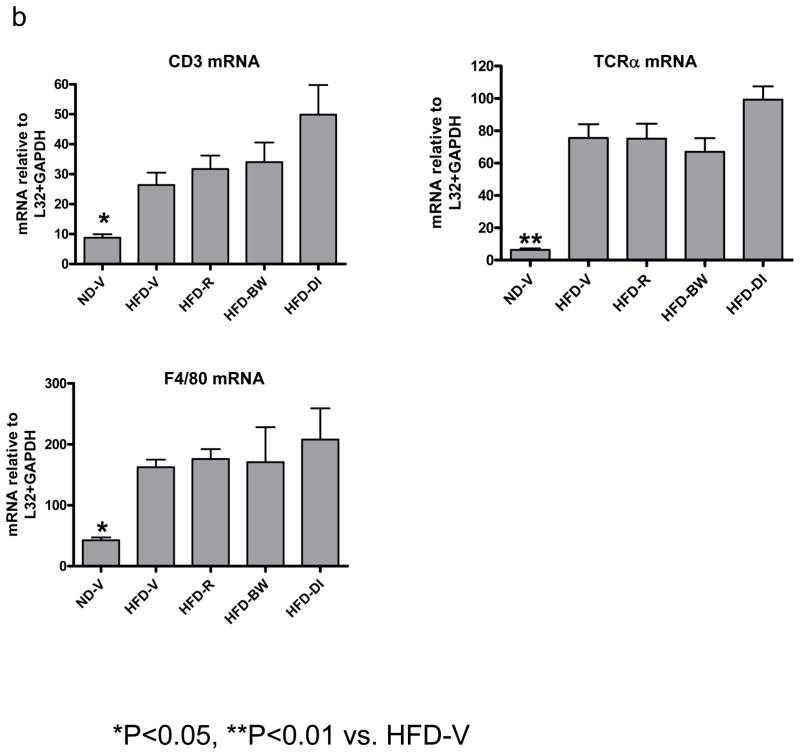
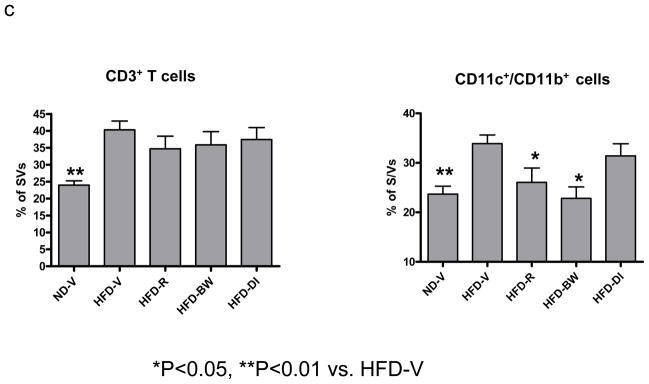
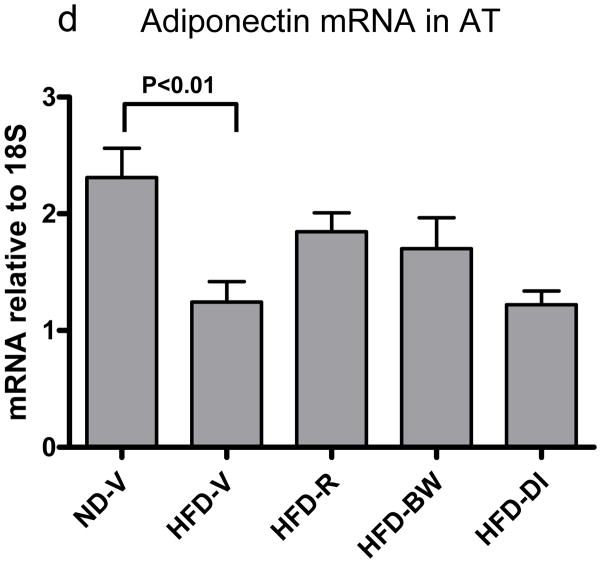
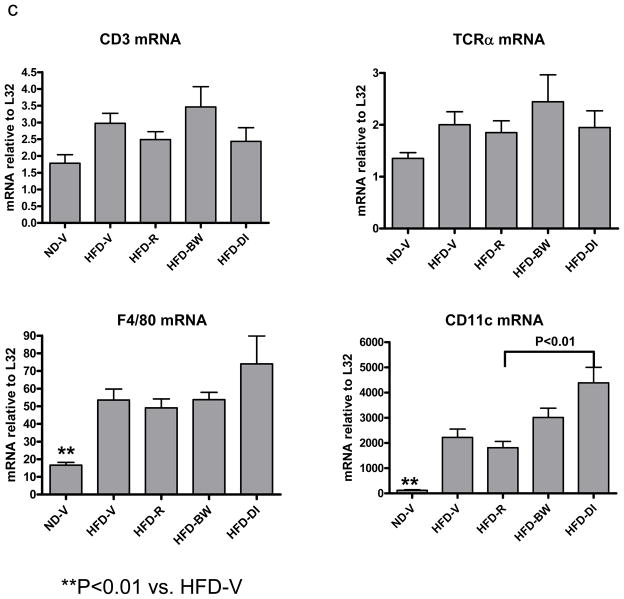
Figure 3. Effect of rimonabant on AT inflammation.
(a) mRNA levels of chemokines or (b) T cell (CD3, TCRα) and macrophage/DC markers (F4/80) in AT examined by RPA at 4 weeks after rimonabant treatment or other interventions; n=7–14 mice/group. (c) T cells and CD11c+/CD11b+ cells examined by flow cytometry in stromal/vascular cells (S/Vs) of mouse AT; n=9–11 samples/group. (d) Adiponectin mRNA quantitated in mouse AT by RT-QPCR; n=9–17 mice/group.
To determine whether rimonabant had direct effects on chemokine expression in AT, obese mice were administered either vehicle only (HFD-EV) or rimonabant (HFD-ER) once. After 6 hours, mice were sacrificed, and AT explants were harvested and cultured ex vivo in the absence (for HFD-EV group) or presence (for HFD-ER) of 100 nM rimonabant for 24 hours. After the culture, HFD-ER had a significant decrease in mRNA levels of MIP-2, but not other chemokines tested in AT explants, compared with HFD-EV (Supplemental Figure II).
Because obesity is associated with leukocyte accumulation in AT, which may be mediated by chemokines and contribute to AT inflammation,[1,2] we examined effects of rimonabant on leukocyte accumulation in mouse AT. Compared to ND-V mice, HFD-V mice had increased mRNA levels of CD3 (a total T cell marker), TCRα (an αβ T cell marker), and F4/80 (a macrophage/DC marker) in AT (Figure 3b). Compared to HFD-V controls, HFD-R mice did not have significantly different mRNA levels of T cell or macrophage/DC markers (Figure 3b). Flow cytometric analysis confirmed that HFD-R mice did not have significant differences in total T cells (Figure 3c) or αβ T cells (data not shown) in AT as compared to HFD-V, HFD-BW, or HFD-DI groups. The number of F4/80+/CD11b+ cells in AT was not significantly different among HFD-R, HFD-V, HFD-BW, and HFD-DI groups (data not shown). However, the proportion of CD11c+/CD11b+ leukocytes, a subset of F4/80+/CD11b+cells that were increased in AT of obese mice compared to lean mice,[3] was decreased in S/Vs of AT of HFD-R mice compared to HFD-V controls (Figure 3c). HFD-BW also showed decreased CD11c+/CD11b+ leukocytes in AT compared to HFD-V; no significant difference was observed in CD11c+/CD11b+ leukocytes in AT of HFD-R and HFD-BW (Figure 3c).
Adiponectin mRNA in AT was lower in HFD-V than ND-V mice and tended to be higher in HFD-R and HFD-BW mice than HFD-V or HFD-DI mice (Figure 3d). No significant difference in AT adiponectin mRNA was observed between HFD-R and HFD-BW mice (Figure 3d).
Effect of rimonabant on liver inflammation
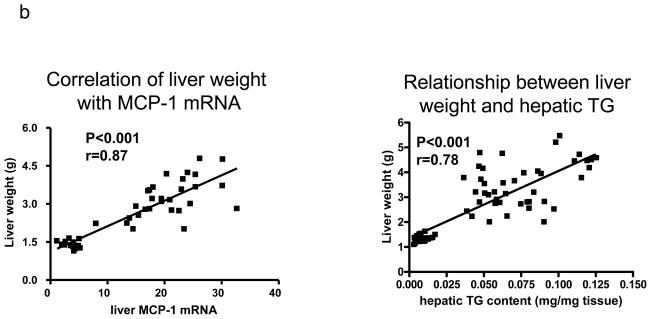
HFD-V mice had higher mRNA levels of RANTES, MCP-1, and MIP-1β in the liver than ND-V mice (Figure 4a). Rimonabant-treated obese mice (HFD-R) had lower hepatic MCP-1 mRNA levels than HFD-V or HFD-DI controls (Figure 4a). However, hepatic MCP-1 mRNA levels were not significantly different between HFD-R and HFD-BW mice (Figure 4a). Without rimonabant treatment, hepatic MCP-1 mRNA levels were highly positively correlated with liver weights, which largely represented hepatic steatosis as reflected by TG content in the liver (Figure 4b). However, rimonabant treatment changed this relationship. For example, although the liver weights were not significantly different between HFD-R and HFD-DI (Figure 1b), HFD-R mice had lower mRNA levels of MCP-1 (and MIP-1β) in the liver than did HFD-DI (Figure 4a). In addition, although HFD-R mice had larger livers than HFD-BW mice (Figure 1b), hepatic MCP-1 mRNA level was not higher in HFD-R mice than HFD-BW mice (Figure 4a). These data suggest that rimonabant may have a direct inhibitory effect on chemokine expression in the liver.
Figure 4. Effect of rimonabant on liver inflammation.
(a) mRNA levels of chemokines in mouse liver assayed by RPA; n=7–15 mice/group. (b) Relationship of liver weight with MCP-1 mRNA in the liver from mice without rimonabant treatment (n=45) and relationship of liver weight with hepatic TG content (n=66). (c) mRNA levels of T cell (CD3, TCRα) and macrophage/DC (F4/80, CD11c) markers in mouse liver examined by RPA or RT-QPCR; n=7–15 mice/group.
HFD-V mice had higher mRNA levels of F4/80 and CD11c, and a trend towards higher CD3 and TCRα mRNA in the liver than ND-V mice. Rimonabant-treated mice did not show different levels of F4/80 and T cell markers in the liver compared to HFD-V, HFD-BW, or HFD-DI mice. However, HFD-R mice had lower CD11c mRNA in the liver than HFD-DI, and a trend towards lower CD11c mRNA than HFD-V controls (Figure 4c).
Effect of rimonabant on plasma levels of MCP-1
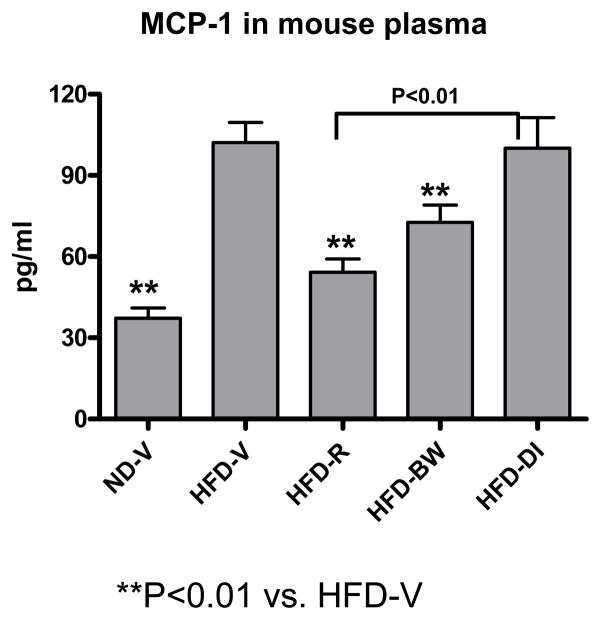
Plasma MCP-1 levels were higher in HFD-V than ND-V mice. HFD-R mice had significantly lower plasma MCP-1 levels than HFD-V or HFD-DI mice (Figure 5). HFD-R mice also had a trend towards lower plasma MCP-1 levels than HFD-BW, but this difference was not statistically significant (Figure 5).
Figure 5. Effect of rimonabant on plasma MCP-1 levels.
MCP-1 protein levels examined in mouse plasma by ELISA; n=9–19 mice/group.
Discussion
Our study confirmed that rimonabant suppressed diet intake transiently but induced persistent weight loss in mice with diet-induced obesity. We also made the novel observation that rimonabant treatment markedly reduced fat mass in obese mice, with more fat mass reduction than in vehicle, body weight– or diet intake–matched controls. Furthermore, we discovered that rimonabant-treated obese mice had decreased inflammation in AT, liver, and blood, primarily indicated by lower MCP-1. The decreases in inflammation with rimonabant treatment may contribute to its beneficial effects on metabolic parameters associated with diet-induced obesity.
Although anti-obesity and metabolic effects of rimonabant have been documented,[10–13,15,19] most of the previous studies did not include all the appropriate controls. In addition to lean and obese vehicle controls, our study included 2 additional control groups: body weight–matched controls, in which diet intake was adjusted to maintain the same degree of weight loss as in rimonabant-treated mice, and diet intake–matched controls, which received the same amount of HFD as rimonabant-treated mice. These control groups enabled us to evaluate the effects of rimonabant beyond its effects on weight loss and diet intake. Our study showed that early weight loss with rimonabant treatment closely correlated with inhibition of calorie intake, whereas the maintenance of lower body weight afterwards was independent of diet intake. Lower diet intake required for body weight–matched controls and greater weight gain in the diet intake–matched controls also support an additional anti-obesity effect of rimonabant besides calorie intake inhibition. One potential explanation for the calorie intake–independent anti-obesity effect of rimonabant was an increase in whole body energy expenditure mediated via both peripheral and central endocannabinoid systems.[13,20]
Rimonabant treatment of obese mice decreased fat mass as compared to obese vehicle or diet intake–matched controls. Of note, rimonabant-treated mice also had less fat mass than body weight–matched controls, although both groups had comparable body weight and weight loss. It is unclear which type of AT, visceral or subcutaneous, was reduced more with rimonabant treatment in our study. Previous studies showed that obesity in both animals and humans correlated with CB1-mediated overactivity of the endocannabinoid system in visceral AT.[21–23] Rimonabant treatment was associated with greaterloss of visceral than subcutaneous AT in obese humans,[24] and with reduced visceral fat in obese rats compared to “pair-fed” controls (corresponding to our diet intake–matched controls, and also with greater body mass than rimonabant-treated mice).[25] Consistently, we found smaller perigonadal fat pads (part of intra-abdominal fat), in rimonabant-treated mice than diet intake–matched controls. Rimonabant may decrease fat mass through increasing whole body energy expenditure and enhancing lipolysis in AT.[13],[20] The increased energy expenditure may also explain our observation of the inhibitory effect of rimonabant on adipogenesis as supported by lower expression of adipogenic genes in AT of rimonabant-treated mice than body weight–matched controls, which may also contribute to fat mass reduction in rimonabant-treated mice. Consistently, Jourdan et al recently also reported that rimonabant treatment of obese mice downregulated expression of several adipogenic genes in AT, particularly in visceral fat.[26] These data, along with previous studies,[22],[27–29] suggest that endocannabinoids may stimulate adipogenesis through CB1 receptors and that CB1 receptor blockade can increase energy expenditure and induce weight loss–independent reduction of adipogenesis.
Obesity is associated with AT inflammation characterized by increased chemokines/cytokines and increased leukocyte accumulation in AT.[1,3,30] Rimonabant treatment of obese mice decreased inflammation in AT primarily by decreasing MCP-1, a chemokine critically involved in leukocyte migration.[31] Indeed, AT CD11c+ cells, which are increased and show proinflammatory characteristics in obesity,[3,32] were reduced with rimonabant treatment. Jourdan et al found that rimonabant treatment of obese mice also decreased TNF-α expression in AT, particularly in subcutaneous fat, as compared with obese controls.[26] Based on the important role of inflammation in the development of obesity-linked metabolic abnormalities,[1,30] the reduced inflammation in rimonabant-treated obese mice may contribute to the improvement in insulin resistance observed in these mice.
Despite reduced AT inflammation and improved insulin resistance compared to obese vehicle controls or diet intake–matched controls, rimonabant-treated mice did not show significant changes in these parameters compared to body weight–matched controls, suggesting that rimonabant may exert beneficial effects on AT inflammation and insulin resistance primarily through induction of weight loss. However, another possibility is that the favorable effects of rimonabant, if any, were masked by the greater intake of the proinflammatory western HFD (as compared to body weight–matched controls or, at some time points, obese vehicle controls). In support of the latter, MIP-2 was significantly reduced in AT explants by ex vivo treatment of rimonabant compared to vehicle alone and reduced in AT of rimonabant-treated mice compared to diet intake–matched controls, but was not significantly different in AT of rimonabant-treated mice compared to vehicle-treated or body weight–matched controls. In addition, in the study from Jourdan et al, it appeared that rimonabant was also fat type–specific in attenuating AT inflammation; treatment of obese mice with rimonabant completely normalized TNF-α expression in subcutaneous fat, whereas inflammation remained high in visceral fat.[26] As we did not examine inflammatory markers in subcutaneous fat, it is unclear how rimonabant treatment affected these markers in subcutaneous fat in our mouse models.
In addition to the effects on AT, HFD also induces fatty liver and liver inflammation,[1,3] which contributes substantially to obesity-linked metabolic dysfunctions. Compared to obese vehicle controls, rimonabant-treated mice had smaller livers and lower hepatic MCP-1 mRNA. Based on the highly positive correlation of liver weight with TG content, these data suggested that CB1 receptor blockade in obese mice ameliorated fatty liver and also reduced liver inflammation. However, it was unexpected that rimonabant-treated mice had larger livers than body weight–matched controls. One potential cause is the greater intake of HFD, resulting in more severe fatty liver. Most of the previous studies indicated that CB1 receptor played an important role in the development of HFD-induced hepatic steatosis. For example, Osei-Hyiaman et al showed a critical role of hepatic CB1 receptor in diet-induced steatosis.[33] Gary-Bobo et al found that CB1 blockade with rimonabant reduced hepatic steatosis and TNF-α level in obese rats compared with vehicle controls and “pair-fed” controls[34] (equivalent to our diet-intake controls). Recently, Tam et al reported that in mice with genetic or diet-induced obesity, a novel, peripherally restricted CB1 receptor antagonist caused weight-independent improvements in glucose homeostasis and fatty liver.[35] However, Koolman et al found that rimonabant treatment in mice did not change hepatic TG accumulation and lipogenic gene expression.[36] The reasons for these differences are unclear. Previous studies indicated that insulin levels or extent of insulin resistance affected endocannabinoid levels.[37,38] Differences in animal species (mouse[33,35,36] [as in our current study] vs. rat[34]), mouse background (C57BL/6J[35,36] [as in our current study] vs. C57BL/6J × C57BL/6N[33]) and diet composition (type of fat and fat content) and duration[33,34,36] could all influence insulin levels and extent of insulin resistance, thereby potentially affecting in vivo endocannabinoid levels, which may impact effects of the CB1 receptor antagonist, thereby leading to the observed discrepancies in these studies.[33–36] In addition to the comparisons between rimonabant treatment with obese controls or with (diet-intake) “pair-fed” controls, our current study also made comparisons between rimonabant treatment and body weight–matched controls.
Of interest, our study showed that, without rimonabant treatment, liver weight, which was highly associated with hepatic TG content, seemed to be a major determinant of hepatic MCP-1 expression, since MCP-1 mRNA levels were highly positively correlated with liver weights in all mice without rimonabant treatment. However, although liver weight and TG content (data not shown) were comparable between rimonabant-treated mice and diet intake–matched controls, rimonabant-treated mice had lower mRNA levels of MCP-1 (and MIP-1β) in the liver than diet intake–matched controls, suggesting that rimonabant may have a direct inhibitory effect on chemokine expression in the liver. Additional supporting evidence is that MCP-1 mRNA levels in the liver of rimonabant-treated mice were not higher than those of body weight–matched controls, although rimonabant-treated mice had larger livers than body weight–matched controls. Consistent with its effect on AT and liver inflammation, rimonabant treatment also lowered plasma MCP-1 levels compared to those in obese vehicle-treated mice or diet intake–matched controls. As circulating MCP-1 levels have been shown to be associated with atherosclerotic disease and obesity-related metabolic dysfunctions,[39,40] the reduced plasma MCP-1 levels with rimonabant treatment may also contribute to the favorable effects of rimonabant on the risks for these obesity-related disorders.
In conclusion, by comparing control groups that were lean, obese receiving vehicle only, or obese receiving vehicle and matched for body weight or diet intake, we showed that blockade of the CB1 receptor with rimonabant induced weight loss in obese mice in diet intake–dependent and –independent fashions. Furthermore, we found that rimonabant had weight loss– and diet intake–independent effects on fat mass reduction, at least partially via inhibition of adipogenesis. We also demonstrated that rimonabant treatment reduced inflammation in obese mice particularly as assessed by MCP-1 levels in AT, liver and plasma. Considering the contribution of inflammation to the development of diabetes and CVD, we postulate that pharmacological blockade of CB1 receptor may reduce risks for these disorders in obese individuals by lowering the underlying inflammation.
In previous studies, exogenous and endogenous cannabinoids attenuated experimental autoimmune hepatitis by suppressing cytokine levels,[41]and cannabinoids appeared to suppress immune and inflammation functions through cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells.[42,43]In contrast, our current study and other studies demonstrated that CB1 receptor blockade with rimonabant reduced obesity-associated inflammation.[13,44] Compared with previous studies on obesity-related inflammation, our study was the first to systematically examine the effect of CB1 receptor blockade on obesity-related inflammation, including that in AT, liver, and plasma, and to compare the effect of rimonabant treatment with body weight–matched controls. The comparable anti-inflammatory effects of rimonabant with those of weight loss in body weight–matched controls indicated that rimonabant may exert anti-inflammaory effects primarily through induction of weight loss. Alternatively, the potential favorable direct effect of the CB1 receptor blocker on obesity-linked inflammation may be counteracted by continuous consumption of proinflammatory western HFD.
Supplementary Material
Acknowledgments
This work was supported by a research grant from Sanofi Aventis (to C.M.B. and H.W.), and partially by grants from NIH (R01HL098839 to H.W., R01DK078847 to C.M.B.) and a research award from the American Heart Association (to H.W.). We thank Kerrie Jara for editorial assistance.
Footnotes
Disclosure
Drs. Ballantyne and Wu received a research grant from Sanofi Aventis to support this study; Dr. Ballantyne has also received research/grant support and honoraria from Sanofi-Synthelabo. Drs. Wang and Perrard, Mr. Perrard, Mr. Mansoori, and Dr. Smith declare no potential conflict of interest.
References
- 1.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu H, Perrard X-YD, Wang Q, et al. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2009 Nov 12; doi: 10.1161/ATVBAHA.109.198044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 7.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 8.Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 9.Jesudason D, Wittert G. Endocannabinoid system in food intake and metabolic regulation. Curr Opin Lipidol. 2008;19:344–348. doi: 10.1097/MOL.0b013e328304b62b. [DOI] [PubMed] [Google Scholar]
- 10.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 11.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 12.Bensaid M, Gary-Bobo M, Esclangon A, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- 13.Jbilo O, Ravinet-Trillou C, Arnone M, et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005;19:1567–1569. doi: 10.1096/fj.04-3177fje. [DOI] [PubMed] [Google Scholar]
- 14.Getty-Kaushik L, Richard AM, Deeney JT, Krawczyk S, Shirihai O, Corkey BE. The CB1 Antagonist Rimonabant Decreases Insulin Hypersecretion in Rat Pancreatic Islets. Obesity (Silver Spring) 2009;17:1856–1860. doi: 10.1038/oby.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schafer A, Pfrang J, Neumuller J, Fiedler S, Ertl G, Bauersachs J. The cannabinoid receptor-1 antagonist rimonabant inhibits platelet activation and reduces pro-inflammatory chemokines and leukocytes in Zucker rats. Br J Pharmacol. 2008;154:1047–1054. doi: 10.1038/bjp.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Rodgers JR, Perrard X-YD, et al. Deficiency of CD11b or CD11d results in reduced staphylococcal enterotoxin-induced T cell response and T cell phenotypic changes. J Immunol. 2004;173:297–306. doi: 10.4049/jimmunol.173.1.297. [DOI] [PubMed] [Google Scholar]
- 17.Poirier B, Bidouard JP, Cadrouvele C, et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7:65–72. doi: 10.1111/j.1463-1326.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 18.Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci U S A. 2000;97:11371–11376. doi: 10.1073/pnas.97.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cota D, Sandoval DA, Olivieri M, et al. Food Intake-independent effects of CB1 antagonism on glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1641–1645. doi: 10.1038/oby.2009.84. [DOI] [PubMed] [Google Scholar]
- 20.Verty AN, Allen AM, Oldfield BJ. The effects of rimonabant on brown adipose tissue in rat: implications for energy expenditure. Obesity (Silver Spring) 2009;17:254–261. doi: 10.1038/oby.2008.509. [DOI] [PubMed] [Google Scholar]
- 21.Starowicz KM, Cristino L, Matias I, et al. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 2008;16:553–565. doi: 10.1038/oby.2007.106. [DOI] [PubMed] [Google Scholar]
- 22.Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 23.Cote M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007;31:692–699. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- 24.Despres JP, Ross R, Boka G, Almeras N, Lemieux I. Effect of rimonabant on the high-triglyceride/low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: the ADAGIO-Lipids trial. Arterioscler Thromb Vasc Biol. 2009;29:416–423. doi: 10.1161/ATVBAHA.108.176362. [DOI] [PubMed] [Google Scholar]
- 25.Herling AW, Kilp S, Juretschke HP, Neumann-Haefelin C, Gerl M, Kramer W. Reversal of visceral adiposity in candy-diet fed female Wistar rats by the CB1 receptor antagonist rimonabant. Int J Obes (Lond) 2008;32:1363–1372. doi: 10.1038/ijo.2008.105. [DOI] [PubMed] [Google Scholar]
- 26.Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 59:926–934. doi: 10.2337/db09-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Marzo V, Despres JP. CB1 antagonists for obesity--what lessons have we learned from rimonabant? Nat Rev Endocrinol. 2009;5:633–638. doi: 10.1038/nrendo.2009.197. [DOI] [PubMed] [Google Scholar]
- 28.Pagano C, Pilon C, Calcagno A, et al. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab. 2007;92:4810–4819. doi: 10.1210/jc.2007-0768. [DOI] [PubMed] [Google Scholar]
- 29.Bellocchio L, Cervino C, Vicennati V, Pasquali R, Pagotto U. Cannabinoid type 1 receptor: another arrow in the adipocytes’ bow. J Neuroendocrinol. 2008;20 (Suppl 1):130–138. doi: 10.1111/j.1365-2826.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- 30.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunn MD, Nelken NA, Liao X, Williams LT. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol. 1997;158:376–383. [PubMed] [Google Scholar]
- 32.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osei-Hyiaman D, Liu J, Zhou L, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gary-Bobo M, Elachouri G, Gallas JF, et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122–129. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- 35.Tam JVV, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010 doi: 10.1172/JCI42551. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koolman AH, Bloks VW, Oosterveer MH, et al. Metabolic responses to long-term pharmacological inhibition of CB1-receptor activity in mice in relation to dietary fat composition. Int J Obes (Lond) 34:374–384. doi: 10.1038/ijo.2009.219. [DOI] [PubMed] [Google Scholar]
- 37.D’Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR. The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes. 2008;57:1262–1268. doi: 10.2337/db07-1186. [DOI] [PubMed] [Google Scholar]
- 38.Di Marzo V, Verrijken A, Hakkarainen A, et al. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. Eur J Endocrinol. 2009;161:715–722. doi: 10.1530/EJE-09-0643. [DOI] [PubMed] [Google Scholar]
- 39.Hoogeveen RC, Morrison A, Boerwinkle E, et al. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities study. Atherosclerosis. 2005;183:301–307. doi: 10.1016/j.atherosclerosis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 2006;30:1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- 41.Hegde VL, Hegde S, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol Pharmacol. 2008;74:20–33. doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashton JC. Cannabinoids for the treatment of inflammation. Curr Opin Investig Drugs. 2007;8:373–384. [PubMed] [Google Scholar]
- 43.Maresz K, Pryce G, Ponomarev ED, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- 44.Croci T, Zarini E. Effect of the cannabinoid CB1 receptor antagonist rimonabant on nociceptive responses and adjuvant-induced arthritis in obese and lean rats. Br J Pharmacol. 2007;150:559–566. doi: 10.1038/sj.bjp.0707138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.